Back to Journals » Journal of Inflammation Research » Volume 16
Ge-Gen Decoction Exerts an Anti-Primary Dysmenorrhea Effect in Rats by Inactivating the HSP90/NLRP3/NF-κB/COX-2 Pathway
Received 7 December 2022
Accepted for publication 5 April 2023
Published 15 April 2023 Volume 2023:16 Pages 1571—1580
DOI https://doi.org/10.2147/JIR.S400545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Yazhen Xie,1 Jianqiang Qian2
1Department of Gynaecology, Taicang Traditional Chinese Medicine Hospital, Affiliated to Nanjing University of Chinese Medicine, Taicang, People’s Republic of China; 2Department of Traditional Chinese Medicine, Taicang Traditional Chinese Medicine Hospital, Affiliated to Nanjing University of Chinese Medicine, Taicang, People’s Republic of China
Correspondence: Yazhen Xie, Department of Gynaecology, Taicang Traditional Chinese Medicine Hospital, Affiliated to Nanjing University of Chinese Medicine, 140 Renmin South Road, Taicang, Jiangsu Province, People’s Republic of China, Tel +86 512 5372 8661, Email [email protected]
Objective: Although Ge-Gen decoction (GGD) has beneficial effects on primary dysmenorrhea (PD), the underlying mechanisms remain poorly understood. Our previous proteomic data revealed decreased level of heat shock protein 90 (HSP90) in uterine tissues of rats with PD after GGD treatment. However, the potential role of HSP90 in the anti-PD effect of GGD and the underlying mechanisms remain unexplored. This study investigated the potential role and mechanism of HSP90 in the anti-PD effect of GGD using a PD rat model.
Methods: Wistar female rats were used to investigate the potential role of HSP90 in the anti-PD effect of GGD. The rat PD model was established by injecting estradiol benzoate and oxytocin. GGD, Terazosin (an agonist of HSP90) or GGD combined with Terazosin were orally administered to the PD rats. The expression levels of protein and cytokines, including HSP90, nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), nuclear factor kappa B (NF-κB) and cyclooxygenase-2 (COX-2) in the uterine tissue of rats in each group were detected by immunohistochemical assay or Western blot.
Results: GGD ameliorated the writhing response, suppressed the protein levels of HSP90 and inflammation-associated proteins, including NLRP3, NF-κB, and COX-2 in uterine tissues of rats with PD. Terazosin attenuated the anti-PD effect of GGD and reversed the effects of GGD on the protein levels of NLRP3, NF-κB and COX-2 in uterine tissues.
Conclusion: GGD exerts an anti-PD effect and suppresses levels of HSP90 and some inflammation associated proteins in uterine tissues of rats.
Keywords: primary dysmenorrhea, heat-shock protein 90, nucleotide-binding oligomerization domain-like receptor protein 3, nuclear factor kappa B, cyclooxygenase-2
Introduction
Primary dysmenorrhea (PD), also known as menstrual cramps or functional painful periods, is one of the most common gynecological disorders in young women with prevalence varies between 45% and 95%.1 Although nonsteroidal anti-inflammatory drugs (NSAIDs) are currently the first-line medication for PD with rapid efficacy, their renal and gastrointestinal side effects cannot be ignored.1,2 Recently, many traditional Chinese medicine compounds were shown to effectively treat PD with the least adverse effects.3–6
Ge-Gen decoction (GGD), containing Pueraria Lobata, Ephedrae, Cinnamon Twig, Paeonia Lactiflora, Ginger, Glycyrrhizae Radix Et Rhizome, and Red Dates (dry weight ratio of 4:1:3:3:2:2:4), is a traditional Chinese medicine compound. A previous ex vivo and in vivo study revealed that GGD possessed a significant spasmolytic effect on uterine tetanic contraction as well as improvement on uterine artery blood velocity which may involve PGF2α and Ca2+ signaling.7 A randomized controlled trial study by Chai et al8 showed that GGD can greatly relieve the severity of menstrual pain of PD patients without obvious adverse effects, and the therapeutic effect of GGD on PD may be related to the regulation of pituitary hypothalamic ovarian hormones, and interfering with the metabolic change. However, the anti-PD mechanisms of GGD remain poorly understood, which limits the application of GGD on a larger scale.
The pathogenesis of PD is still unclear. Uterine inflammation is one of the main pathogenesis of dysmenorrhea.9–11 Pro-inflammatory cytokines, such as interleukin-6 (IL-6), IL-1β, and IL-18, stimulate the release of prostaglandins (PG), leading to excessive contraction of uterine smooth muscles in PD.9–11 The nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome is a core part of inflammation and is implicated in diverse diseases that are worsened by the presence of inflammation.12 Recently, activation of NLRP3 inflammasome was shown to induce deleterious effects in PD mice.11 Previous studies showed that GGD alleviated inflammation in the uterine tissues of rats with PD.7 However, the underlying mechanisms remain unexplored.
To explore the anti-PD mechanisms of GGD, we previously investigated the differentially expressed proteins (DEPs) in the uterine tissues of rats with PD following GGD administration using label-free quantitative proteomics, and found that heat shock protein 90 (HSP90) was decreased in the uterine tissues of PD rats after GGD administration.13 HSP90 is a molecular chaperone that is involved in the activation and expression of disparate client proteins.14 Through its ability to chaperone client proteins, HSP90 is implicated in various human diseases including cancer, inflammation, and diseases associated with protein misfolding.14 However, the role of HSP90 in the development of PD remains unknown. It is demonstrated that HSP90 plays a key role in regulating inflammatory processes via its regulation of the NLRP3 inflammasome and secretion of the pro-inflammatory cytokine IL-1β.15 Excessive and dysregulated inflammation is known to contribute to the progression of PD.9–11 Therefore, it is worthwhile to investigate whether HSP90 mediates the anti-PD effect of GGD through regulation of the inflammatory response.
In this study, we explored the potential role and mechanism of HSP90 in the anti-PD effect of GGD using a PD rat model.
Materials and Methods
Experimental Animals
Female Wistar rats (weighing 200 ± 20 g; 8–10 weeks-of-age) were acquired from Huachuang Sino Co., Ltd. (Taizhou, China). The rats were kept in plastic cages at 22 ± 2 °C on a 12 h light/dark cycle with free access to pellet food and water. Food and water were supplied as required, and cages were changed every week.
GGD Preparation
GGD consists of Pueraria Lobata (20g), Ephedrae (5g), Cinnamon Twig (15g), Paeonia Lactiflora (15g), Ginger (10g), Glycyrrhizae Radix Et Rhizome (10g), and Red Dates (20g). All of the drugs were provided by the pharmacy of Taicang Hospital of Traditional Chinese Medicine. These materials were soaked in 1000 mL water for 30 minutes and then decocted with boiling water twice for 2 h each time. Finally, we mixed these two times filtered solutions together in a water-bath at 60°C and evaporated them to 100 mL. The final extract was bottled and stored at 4°C for further use.
Construction of PD Rats and Treatment
The animal experiment design and schedule are shown in Figure 1. The rat model of PD was established by injecting estradiol benzoate and oxytocin, as described previously.13 The GGD concentration is calculated by mass conversion based on the effective clinical. Previous research by our team set up three concentrations of high (3.6 g/kg), middle (1.8 g/kg), and low (0.9 g/kg) for pre-experiment. The experimental results determined that the concentration of 1.8 g/kg in the GGD animal experiment was appropriate (Figure S1). One week after adaptation, thirty rats were randomly divided into five groups (n = 6 rats/group): sham group, PD group, GGD group, Terazosin group, GGD + Terazosin group. The rats in the GGD group were intragastrically administered GGD (1.8 g/kg) once a day. The rats in the Terazosin group were intragastrically administered Terazosin (0.08g/kg) once a day. The rats in the GGD + Terazosin group were intragastrically administered GGD (1.8 g/kg) and Terazosin (0.08g/kg) once a day. The rats in sham and PD groups were given once daily with the same amount of distilled water. All rats were anesthetized by inhalation of 10% chloral hydrate (Proteinbio Biotechnology Co., Ltd., Nanjing, China) and sacrificed by cervical dislocation. After anesthetic euthanasia, the uteruses were removed, rinsed with phosphate buffered saline (PBS) and measured. The uterine tissues were cryopreserved at −80°C until further analysis. All experiments were approved by the Ethics Committee of Taicang Hospital of Traditional Chinese Medicine (No. 2021-031) and followed the guidelines for the ethical review of laboratory animal welfare issued by Regulations of Jiangsu Laboratory Animal Management.
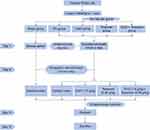 |
Figure 1 Figure of the animal experiment design and schedule. |
Measurement of Writhing Response
The writhing times and the writhing latency of each rat were observed within 30 mins after the injection of oxytocin. Writhing response was evidenced by contractions and indentations on the abdomen, trunk, and posterior of each of the experimental rats. Writhing latency: from intraperitoneal injection of oxytocin to the first writhing reaction.
Western Blotting
Western blotting assays were performed as previously depicted in 13. The antibodies used in this study were as follows: anti-NF-κB (Cat. No. 66535-1-Ig, 1:2000, Proteintech, Wuhan, China), anti-COX-2 (Cat. No. 66351-1-Ig, 1:2500, Proteintech, Wuhan, China), and β-Actin (Cat. No. AC026, 1:10,000, ABclonal, Wuhan, China). β-actin was used as a loading control. Signals were detected by using an ECL substrate (Thermo Scientific) and exposure with the Tanon 5800 imaging system. The intensity of the bands was quantified by Photoshop 2022.
Histopathological Examination
Uterine tissues were washed in phosphate buffer, fixed in 5% formalin at room temperature, dehydrated in a graded concentration of ethanol, and then embedded in paraffin. Tissue sections of 4 μm were stained with hematoxylin and eosin (H&E) to evaluate the histopathological changes of the uterus. Digital images of uterine morphology at 200× magnification were obtained using a light microscope (Olympus CKX53, Tokyo, Japan).
Immunohistochemistry (IHC)
The 4 μm sections of uterine tissues were used for immunolabeling analysis. Briefly, the uterine sections were permeabilized, blocked, and then incubated with primary antibodies against HSP90 (Cat. No. ab203126, 1:10,000, Abcam, USA), NLRP3 (Cat. No. ab263899, 1:2000, Abcam, USA), overnight at 4°C, followed by incubation with a corresponding secondary antibody at ordinary temperature for 1 h. Staining was developed using peroxidase 3,30-diaminobenzidine (DAB) substrate and counterstained with hematoxylin. Images were obtained using an Immunohistochemical scanner (Pannoramic MIDI, 3D histech, Hungary). The relative fluorescence intensity of positive cells was quantified using Image-Pro Plus software. The integrated optical density (IOD) and mean optical density (AOD) were quantified using Image-Pro Plus 6.0 software. AOD = IOD sum/area sum.
Statistical Analysis
The data represent the mean ± standard deviation (SD) from at least three separate experiments. Statistical analyses were carried out using GraphPad Prism (version 9.0, USA). Student’s t-test was used to analyze differences between two groups. When comparisons between multiple groups were carried out, one-way ANOVA followed by SNK tests was employed. Statistical significance was considered when P < 0.05.
Results
GGD Exerts an Anti-PD Effect in Rats
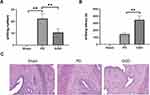
To detect the effects of GGD on PD, we examined writhing response and writhing latency in GGD-pretreated rats after treatment with estradiol benzoate and oxytocin. As shown in Figure 2A, there was no writhing reaction in the sham group, whereas the writhing response numbers significantly increased in the PD group. Compared with the PD group, the writhing response numbers notably decreased (Figure 2A), and the writhing latency significantly increased (Figure 2B) in the GGD group. H&E examination of uterine tissues showed that there was no inflammation or edema in the sham group, whereas a large number of inflammatory cell infiltrations and mild edema were observed in the PD group (Figure 2C). In the GGD group, inflammation and edema were rarely observed (Figure 2C). The above data indicated that GGD exerts an anti-PD effect in rats.
GGD Decreases the Level of HSP90 in Uterine Tissues of Rats with PD
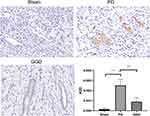
Our previous proteomic data revealed decreased level of HSP90 in uterine tissues of rats with PD after GGD treatment 13. Therefore, uterine tissues were examined by IHC to detect the level of HSP90. The results showed that compared with the sham group, the level of HSP90 in the PD group was increased (Figure 3). Compared with the PD group, the level of HSP90 in the GGD group was decreased (Figure 3). These results suggested that GGD decreases the level of HSP90 in uterine tissues of rats with PD.
Up-Regulation of HSP90 Reverses the Anti-PD Effect of GGD
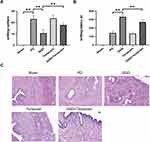
To investigate the role of HSP90 down-regulation in the anti-PD effect of GGD, the level of HSP90 was up-regulated in GGD-treated PD rats using Terazosin, an agonist of HSP90. Compared with the GGD group, the writhing response numbers significantly increased (Figure 4A), and the writhing latency notably decreased (Figure 4B) in the GGD + Terazosin group. H&E examination showed that compared with the GGD group, infiltrations and edema in the GGD + Terazosin group were increased (Figure 4C). The above data indicated that up-regulation of HSP90 reverses the anti-PD effect of GGD.
Up-Regulation of HSP90 Reverses GGD-Reduced NLRP3 Expression in Uterine Tissues of Rats with PD
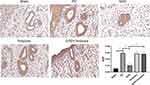
We then investigated the mechanism underlying HSP90-mediated anti-PD effect of GGD. NLRP3 is the key regulator of inflammatory response.12 Our and other previous studies revealed that GGD exerts its protective function on PD by regulating the inflammatory response.7,13 Furthermore, NLRP3 expression was shown to be regulated by HSP90.15,16 Therefore, uterine tissues were examined by IHC to detect the level of NLRP3. The results showed that compared with the sham group, the level of NLRP3 in the PD group was increased (Figure 5). Compared with the PD group, the level of NLRP3 in the GGD group was decreased (Figure 5). Compared with the GGD group, the level of NLRP3 in the GGD + Terazosin group was increased (Figure 5). These results suggested that up-regulation of HSP90 reverses GGD-reduced NLRP3 expression in uterine tissues of rats with PD.
Up-Regulation of HSP90 Reverses GGD-Inactivated NF-κB/COX-2 Axis in Uterine Tissues of Rats with PD
NF-κB/COX-2 axis is an important downstream target of NLRP3-mediated inflammatory response, and plays a critical role in the pathological process of PD.11 Therefore, we explored whether NF-κB/COX-2 axis is involved in HSP90-mediated anti-PD effect of GGD. Western blotting analyses showed that compared with the sham group, the levels of NF-κB and COX-2 in the PD group was increased (Figure 6). Compared with the PD group, the levels of NF-κB and COX-2 in the GGD group was decreased (Figure 6). Compared with the GGD group, the levels of NF-κB and COX-2 in the GGD + Terazosin group was increased (Figure 5). These results suggested that up-regulation of HSP90 reverses GGD-inactivated NF-κB/COX-2 axis in uterine tissues of rats with PD.
Discussion
The beneficial effects of GGD on PD have been observed in animal models or clinical patients. However, the anti-PD mechanism of GGD has not been thoroughly elucidated. The present study investigated the anti-PD mechanism of GGD. Our previous proteomic data revealed that HSP90 was decreased in uterine tissues of rats with PD after GGD treatment.13 Therefore, the role of HSP90 in the anti-PD effect of GGD was first investigated. Our study showed that GGD-reduced HSP90 contributes to the anti-PD effect of GGD. To the best of our knowledge, this is the first report of the central role of HSP90 in the anti-PD effect of GGD. Therefore, we investigated the major molecular pathways that regulated by HSP90 to mediate the anti-PD effect of GGD.
NLRP3 inflammasome, an oligomeric complex comprised the NLRP3, the adaptor ASC, and caspase-1, is the key regulator of inflammation.17,18 Previous studies revealed that NLRP3 inflammasome is activated and expressed in PD, and inactivation NLRP3 inflammasome attenuates PD.11 Moreover, it is demonstrated that NLRP3, the critical component of the inflammasome, is one of the HSP90 clients and the protein stability of NLRP3 is compromised by HSP90 inhibition.15,19 Therefore, we investigated whether HSP90 mediates the anti-PD effect of GGD by regulating NLRP3 expression. Our results showed that NLRP3 expression was obviously increased in the uterine tissues of PD rats, which was significantly decreased after GGD administration. When the level of HSP90 in GGD-treated PD rats was up-regulated by Terazosin, NLRP3 expression was obviously increased. These data indicate that GGD-reduced NLRP3 is attributed to the decreased level of HSP90 in the uterine tissues of PD rats.
NF-κB is a transcription factor that has an essential role in inflammation.20 NF-κB was reported to play a crucial role in PD via regulation of COX-2, a key enzyme catalyzes prostaglandin biosynthesis.21 COX-2 and its downstream product prostaglandin E2 (PGE2) play a key role in generation of the inflammatory microenvironment in uterine tissues of PD.22,23 The NF-κB/COX-2 signaling axis has been demonstrated to play a pathogenic role in PD.11,21 Recently, MCC950, a potent and specific small-molecule inhibitor of the NLRP3 inflammasome, was shown to alleviate the pain and pathological damage via suppression of the NF-κB/COX-2 signaling axis in uteruses of PD mice,11 indicating that NLRP3 inflammasome is responsible for the activation of NF-κB/COX-2 signaling axis in PD. Therefore, the activation of NF-κB/COX-2 signaling axis was investigated in our study. Consistent with previous study, we observed the activation of NF-κB/COX-2 pathway in the uterine tissues of PD rats. GGD obviously reduced the activation of activation of NF-κB/COX-2 pathway, which was reversed by Terazosin administration. These results indicate that NF-κB/COX-2 signaling axis acts as a downstream of NLRP3 inflammasome in HSP90-mediated anti-PD effect of GGD.
PG content plays an important role in the pathogenesis of PD. Our previous study showed that GGD reduces the content of PG in the uterus of PD rats.13 It is worth further exploring whether HSP90 is involved in GGD-reduced PG content and the underlying mechanism. Furthermore, the uterus of the rat is anatomically different than human uterus. Whether the uterine pain of rat is consistent with that of PD patients is still unknown. The resemblence of uterine pain in humans and rats needs to be investigated.
Conclusion
In conclusion, the current study demonstrates that GGD exerts an anti-PD effect in vivo. GGD downregulated inflammation associated proteins (NLRP3, NF-κB and COX-2) in uterine tissues of PD rats. The HSP90 was also involved in the inflammation regulation of GGD treatment. Our findings suggest that GGD may be useful as an anti-PD medicine owing to its unique anti-inflammatory activity.
Abbreviations
GGD, Ge-Gen decoction; PD, primary dysmenorrhea; NLRP3, nucleotide-binding oligomerization domain-like receptor protein 3; NF-kB, nuclear factor kappa B; COX-2, cyclooxygenase-2; NSAIDs, nonsteroidal anti-inflammatory drugs; DEPs, differentially expressed proteins.
Data Sharing Statement
The data supporting the findings of this study can be obtained from the corresponding author according to reasonable request, and the corresponding author/s can be directly contacted for further inquiry.
Acknowledgments
This work was supported by the “Suzhou Health Youth Backbone Talent Project” under Grant No. Qngg2021041, and the “Suzhou Science and Technology Development Project” under Grant No. SKJY2021014 and No. SKJYD2021179.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferries-Rowe E, Corey E, Archer JS. Primary dysmenorrhea: diagnosis and therapy. Obstet Gynecol. 2020;136(5):1047–1058. doi:10.1097/AOG.0000000000004096
2. Feng X, Wang X. Comparison of the efficacy and safety of non-steroidal anti-inflammatory drugs for patients with primary dysmenorrhea: a network meta-analysis. Mol Pain. 2018;14:1744806918770320. doi:10.1177/1744806918770320
3. Leem J, Jo J, Kwon CY, Lee H, Park KS, Lee JM. Herbal medicine (Hyeolbuchukeo-tang or Xuefu Zhuyu decoction) for treating primary dysmenorrhea: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2019;98(5):e14170. doi:10.1097/MD.0000000000014170
4. Li N, Li J, Gai P. Effects of modified wenjing decoction combined with online publicity and education on the treatment of primary dysmenorrhea of cold coagulation and blood stasis. J Healthc Eng. 2022;2022:1899356.
5. Ma C, Liang N, Gao L, Jia C. Danggui Sini Decoction (herbal medicine) for the treatment of primary dysmenorrhoea: a systematic review and meta-analysis. J Obstet Gynaecol. 2021;41(7):1001–1009. doi:10.1080/01443615.2020.1820461
6. Li G, Zhang Z, Zhou L, et al. Chinese herbal formula Xuefu Zhuyu for primary dysmenorrhea patients (CheruPDYS): a study protocol for a randomized placebo-controlled trial. Trials. 2021;22(1):95. doi:10.1186/s13063-021-05050-w
7. Yang L, Chai CZ, Yue XY, et al. Ge-Gen Decoction attenuates oxytocin-induced uterine contraction and writhing response: potential application in primary dysmenorrhea therapy. Chin J Nat Med. 2016;14(2):124–132. doi:10.1016/S1875-5364(16)60005-5
8. Chai C, Hong F, Yan Y, et al. Effect of traditional Chinese medicine formula GeGen decoction on primary dysmenorrhea: a randomized controlled trial study. J Ethnopharmacol. 2020;261:113053. doi:10.1016/j.jep.2020.113053
9. Yu WY, Ma LX, Zhang Z, et al. Acupuncture for primary dysmenorrhea: a potential mechanism from an anti-inflammatory perspective. Evid Based Complement Alternat Med. 2021;2021:1907009. doi:10.1155/2021/1907009
10. Szmidt MK, Granda D, Sicinska E, Kaluza J. Primary dysmenorrhea in relation to oxidative stress and antioxidant status: a systematic review of case-control studies. Antioxidants. 2020;9(10). doi:10.3390/antiox9100994
11. Tang B, Liu D, Chen L, Liu Y. NLRP3 inflammasome inhibitor MCC950 attenuates primary dysmenorrhea in mice via the NF-kappaB/COX-2/PG pathway. J Inflamm. 2020;17:22. doi:10.1186/s12950-020-00251-7
12. Coll RC, Schroder K, Pelegrin P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 2022;43(8):653–668. doi:10.1016/j.tips.2022.04.003
13. Xie Y, Qian J, Lu Q. The therapeutic effect of ge-gen decoction on a rat model of primary dysmenorrhea: label-free quantitative proteomics and bioinformatic analyses. Biomed Res Int. 2020;2020:5840967. doi:10.1155/2020/5840967
14. Hoter A, El-Sabban ME, Naim HY. The HSP90 family: structure, regulation, function, and implications in health and disease. Int J Mol Sci. 2018;19(9):2560. doi:10.3390/ijms19092560
15. Nizami S, Arunasalam K, Green J, et al. Inhibition of the NLRP3 inflammasome by HSP90 inhibitors. Immunology. 2021;162(1):84–91. doi:10.1111/imm.13267
16. Xu G, Fu S, Zhan X, et al. Echinatin effectively protects against NLRP3 inflammasome-driven diseases by targeting HSP90. JCI Insight. 2021;6(2). doi:10.1172/jci.insight.134601
17. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. doi:10.3390/ijms20133328
18. Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(9):688. doi:10.1038/nrd.2018.149
19. Piippo N, Korhonen E, Hytti M, et al. Hsp90 inhibition as a means to inhibit activation of the NLRP3 inflammasome. Sci Rep. 2018;8(1):6720. doi:10.1038/s41598-018-25123-2
20. Capece D, Verzella D, Flati I, Arboretto P, Cornice J, Franzoso G. NF-kappaB: blending metabolism, immunity, and inflammation. Trends Immunol. 2022;43(9):757–775. doi:10.1016/j.it.2022.07.004
21. Chen Y, Cao Y, Xie Y, et al. Traditional Chinese medicine for the treatment of primary dysmenorrhea: how do Yuanhu painkillers effectively treat dysmenorrhea? Phytomedicine. 2013;20(12):1095–1104. doi:10.1016/j.phymed.2013.05.003
22. Zhang Y, Su N, Liu W, Wang Q, Sun J, Peng Y. Metabolomics study of Guizhi Fuling capsules in rats with cold coagulation dysmenorrhea. Front Pharmacol. 2021;12:764904. doi:10.3389/fphar.2021.764904
23. Hong F, He G, Zhang M, Yu B, Chai C. The establishment of a mouse model of recurrent primary dysmenorrhea. Int J Mol Sci. 2022;23(11):6128. doi:10.3390/ijms23116128
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.