Back to Journals » Journal of Inflammation Research » Volume 14
Gasdermin D in Different Subcellular Locations Predicts Diverse Progression, Immune Microenvironment and Prognosis in Colorectal Cancer
Authors Wang J , Kang Y , Li Y, Sun L, Zhang J, Qian S, Luo K, Jiang Y, Sun L, Xu F
Received 9 September 2021
Accepted for publication 2 November 2021
Published 25 November 2021 Volume 2021:14 Pages 6223—6235
DOI https://doi.org/10.2147/JIR.S338584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jiahui Wang,1,2,* Yixin Kang,1,2,* Yuxuan Li,1,2,* Liang Sun,1,2 Jun Zhang,1,2 Senmi Qian,1,2 Ke Luo,1 Yi Jiang,3 Lichao Sun,4 Fangying Xu1,2
1Department of Pathology and Pathophysiology, and Department of General Surgery of The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Key Laboratory of Disease Proteomics of Zhejiang Province, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 3Department of Statistics, School of Mathematical Sciences, Anhui University, Hefei, Anhui, People’s Republic of China; 4State Key Laboratory of Molecular Oncology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fangying Xu
Department of Pathology and Pathophysiology, and Department of General Surgery of The Second Affiliated Hospital, Key Laboratory of Disease Proteomics of Zhejiang Province, Zhejiang University School of Medicine, 866 Yuhangtang Road, Hangzhou, 310058, Zhejiang, People’s Republic of China
Tel +86— 571-88208198
Fax +86-571-88208197
Email [email protected]
Lichao Sun
State Key Laboratory of Molecular Oncology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 Panjiayuan South Lane, Beijing, 100021, People’s Republic of China
Email [email protected]
Background: Pyroptosis is a type of cell death that causes an immune reaction. Gasdermin D (GSDMD), as an executor of pyroptosis, has become an attractive target in cancer research. However, the clinical significance of GSDMD expression in different subcellular locations remains unclear.
Methods: GSDMD was detected by immunohistochemistry in 178 cases of colorectal cancer with follow-up information. General data and information on systemic inflammatory indicators were collected from case records, and the clinicopathological parameters were reviewed by microscopy. CD3+, CD4+, and CD8+ T lymphocytes, CD20+ B lymphocytes, and CD68+ macrophages were detected by immunohistochemistry. Univariate survival analysis (Kaplan–Meier method, Log rank test) and a multivariate Cox proportional hazard model were used to analyze the impact of GSDMD on overall survival.
Results: Survival analysis showed that high expression of cytoplasmic GSDMD was an independent favorable indicator for prognosis (P=0.027) and improved the efficacy of chemotherapy (P=0.012). Positive cytoplasmic GSDMD expression indicated lower probability of distant metastasis (P=0.024), yet nuclear GSDMD expression predicted deeper infiltration depth (P=0.007). Membranous GSDMD expression positively correlated with CD68+ macrophages in tumor center (P=0.002) and CD8+ lymphocytes in tumor invasive front (P=0.007). However, nuclear GSDMD was negatively related to CD68+ macrophages in tumor invasive front (P< 0.001) and CD8+ lymphocytes in tumor center (P=0.069). Cytoplasmic GSDMD was associated with more CD3+ lymphocytes both in tumor center (P=0.066) and tumor invasive front (P=0.008). Moreover, positive membranous GSDMD indicated a lower neutrophil-to-lymphocyte ratio (P=0.013).
Conclusion: GSDMD subcellular localization patterns are related to CRC progression and immune reaction, and should be investigated in future studies.
Keywords: gasdermin D, pyroptosis, prognosis, immune microenvironment, colorectal cancer
Introduction
Colorectal cancer (CRC) is the leading cause of cancer death worldwide. It is estimated that among all cancers diagnosed, the incidence of CRC ranks third (10.6%) and its mortality rate ranks second (9.3%).1 Advances in screening and treatment have significantly improved the prognosis of CRC. However, the 5-year survival rate ranges from 90% for patients in the early stage to 14% for those diagnosed with distant metastasis.2 Immunotherapy has unlocked a new way to improve the prognosis of CRC patients with distant metastasis.3
As is well known, both the characteristics of tumor cells and their microenvironment affect the prognosis in cancer. Lymphocytes and tumor-associated macrophages (TAMs) are the most common immune cells within the immune microenvironment (IME). Abundant cytotoxic T lymphocytes may predict a good prognosis. M1 macrophages are favorable but M2 macrophages are unfavorable for patient outcome. IMEs can be simply classified as “hot” or “cold”. “Hot” tumors are characterized by active T-lymphocyte infiltration and “cold” tumors show absence or exclusion of T lymphocytes.4 Pyroptosis, as a perpetrator of cytokine release and inflammation, could possibly be regarded as a converter between “hot” and “cold”.5
Pyroptosis is defined as gasdermin (GSDM)-mediated programmed cell death, accompanied by the destruction of membranes and the release of cellular contents, which then triggers immune response and inflammation.6 The GSDMs family includes six members: GSDMA, GSDMB, GSDMC, GSDMD, GSDME, and pejvakin (PJVK). GSDMD, a mediator of inflammasome-induced pyroptosis, is the first identified and most studied executor of pyroptosis. Human GSDMD is cleaved by Caspase-1/4/5 at the 272FLTD275 site, and then forms a GSDMD N-terminal domain and GSDMD C-terminal domain. GSDMD N-terminal domains bind and perforate cell membranes to induce pyroptosis.7 GSDMD is widely expressed in different tissues and cell types, including the intestinal epithelia.8
GSDMD is linked to worse prognosis in lung adenocarcinoma and osteosarcoma.9,10 Wu et al analyzed the expression of GSDMD in 244 cases of CRC and found that GSDMD was an unfavorable predictor.11 However, they did not distinguish the clinical significance of GSDMD expression in membrane, cytoplasm, and nucleus. Furthermore, the correlation between GSDMD expression and IME in cancer tissue samples has not been determined. IME includes different types of lymphocytes, macrophages, granulocytes, and other cells. The distribution of immune cells is diverse in the tumor invasive front and in the tumor center. Microenvironmental heterogeneity also contributes to heterogeneity arising among cancer cells and affects therapeutic response.12
Cytokines released during pyroptosis elicit a systemic immune response. The amount and percentage of inflammatory cells in peripheral blood are potential pretreatment prognostic markers. For example, when the lymphocyte-to-monocyte ratio (LMR)≤2.83, no benefit of adjuvant 5-FU-based chemotherapy could be found in stage III colon cancer patients.13 The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and prognostic nutritional index (PNI) are all valuable markers in treatment or prognostic evaluation.14,15
In this study, we collected 178 CRC samples with follow-up and clinicopathological data, and evaluated the expression and localization of GSDMD by immunohistochemistry, to explore the effects of GSDMD on cancer progress, immune microenvironment, systemic inflammatory response, and prognosis.
Materials and Methods
Case Materials
All 178 colorectal carcinoma patients were inhabitants of Xiaoshan District, Zhejiang Province, China. The patients had not received chemotherapy or radiotherapy before surgery. Information on follow-up was provided by the Xiaoshan Centre of Disease Control, with a median follow-up period of 26.5 months (range 2–75 months). By the end of follow-up, 149 patients had survived and 29 were deceased. Of the 178 total cases, 98 were males and 80 were females; 100 tumors were located in the colon and 78 in the rectum. The age at diagnosis ranged from 24 to 91 years old, with a mean age of 62.53. There were 43 cases of TNM stage I, 53 cases of TNM stage II, 66 cases of TNM stage III, and 16 cases of TNM stage IV. Eighty-four patients underwent postoperative 5-Fu based chemotherapy. This retrospective study was approved by the Ethics Committee of Zhejiang University School of Medicine. Informed consent was waived for this study’s retrospective feature and the anonymized processing of patient data. The study complied with the Declaration of Helsinki.
Clinicopathological Parameters
All archival sections were reviewed and diagnosed by two pathologists. Clinicopathological predictors included histological type, histological grade, tertiary lymphoid structures, vessel invasion, perineural invasion, infiltration depth, lymph node metastasis, distant metastasis and TNM stage.
Systemic Inflammatory Indicators
Preoperative peripheral blood indicators were collected from the records of patients, including concentration of plasma albumin, platelet count, neutrophil count, lymphocyte count, monocyte count, total white blood cell count, neutrophil percentage, lymphocyte percentage, monocyte percentage, and eosinophil percentage. Furthermore, NLR, PLR, LMR and PNI were calculated. NLR was determined as the ratio of the peripheral neutrophil count to the lymphocyte count. PLR was equal to the ratio of the peripheral platelet count to the lymphocyte count. LMR was defined as the lymphocyte-to-monocyte ratio. PNI was calculated by the formula (serum albumin (g/L) + 5×total lymphocyte count×109/L).
Tissue Microarray
We constructed tissue microarrays of 178 CRC tissue samples. Each case had three tissue punches, which were taken from the normal mucosa, tumor center (TC), and tumor invasive front (TIF) of formalin-fixed, paraffin-embedded blocks. The TIF area was determined as a 20× field within the most distal tumor cells. Punches with a diameter of 1cm were transferred into one recipient paraffin block (6×7 punches). Finally, recipient paraffin blocks were cut into 4μm-thick slices and mounted on slides coated with APES (3-aminopropyltriethoxysilane).
Immunohistochemical Staining
Five types of immune cell (CD3+, CD4+, and CD8+ T lymphocytes, CD20+ B lymphocytes, and CD68+ macrophages) and GSDMD were investigated in this study. The GSDMD antibody was a gift from Professor Feng Shao (National Institute of Biological Sciences, Beijing, China). The information on primary antibodies and staining patterns is summarized in Supplementary Table 1. CD3, CD4, CD8, and CD20 were detected in tissue microarrays. CD68 and GSDMD were stained in whole tissue sections. The sections were dewaxed and dehydrated before immunohistochemical staining. Microwave antigen retrieval was carried out in citrate buffer (0.01M, pH 6.0), and the 2-step method was performed (PV-9000 polymer detection system, Zhongshan Jinqiao, Beijing, China). Then the color was developed with 3, 3-diaminobenzidine (DAB) solution and counterstained with hematoxylin. For blank controls, the primary antibodies were replaced with PBS solution (100mM, PH7.4). Distinctly brown granular stain was defined as positive. Missing data were caused by tissue falling off slides. All immunohistochemical stained slides were digitally scanned with NanoZoomer 2.0HT (Hamamatsu, Japan).
As regards the expression of GSDMD in cancer cells, we scored the percentages of positive cells using the following scale: 0 = no staining; 1=less than 5%; 2 = 5–25%; 3 = 26–50%; 4 = 51–75%; 5 = more than 75%. The numbers of immune cells were counted in four hotspots (20×, 545×577 μm2) using a computer-automated method (Image-pro plus 6.0, Media Cybernetics Inc.) and the density of immune cells was defined as average count per high-power field (HPF, 20×).
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics 26.0 (IBM SPSS, Armonk, NY, USA). We used the Chi-square test or Fisher exact test to compare GSDMD expression and other categorical variables. The t-test was used to examine differences in the overall distribution between the two groups. Univariate survival analyses were performed with the “Survfit” function in R, and survival curves were drawn using the Kaplan–Meier method with a Log rank test. Cumulative survival rate was calculated with the life-table method. Multivariate survival analysis was performed using the Cox proportional hazard model and a forward stepwise method was used to bring variables into the model. A significant difference was identified if P value < 0.05. A tendency towards significant difference was identified if 0.05≤ P value <0.1.
Results
Expression Profile of GSDMD
GSDMD was expressed in the membrane, cytoplasm, and nucleus (Figure 1). The number of cases for each score in different locations is listed in Supplementary Table 2. In the following analyses, GSDMD positive was defined as score >0, and GSDMD was negative when score=0. The positive rates of GSDMD in membrane, cytoplasm, and nucleus were 3.93% (7/178), 63.48% (113/178), and 61.24% (109/178) respectively. There was consistency between the expression of cytoplasmic GSDMD and nuclear GSDMD (P=0.001).
 |
Figure 1 Immunohistochemical staining images of GSDMD in membrane (A), cytoplasm (B) and nucleus (C). 400× magnification. |
GSDMD Expression and Clinicopathological Parameters
GSDMD expression was classified as negative or positive. Membranous GSDMD expression showed no significant difference among age, sex, location, histological type, histological grade, tertiary lymphoid structure, vessel invasion, perineural invasion, infiltration depth, lymph node metastasis, distant metastasis, or TNM stage (Table 1). Patients with cytoplasmic GSDMD expression were less likely to have distant metastases (P=0.024) (Table 2).
 |
Table 1 Association Between Membranous GSDMD Expression and Clinicopathological Indicators |
 |
Table 2 Association Between Cytoplasmic GSDMD Expression and Clinicopathological Indicators |
Intriguingly, positive nuclear GSDMD expression was more common in the female (P=0.063) and rectal cancer (P=0.053) groups, and related to low histological grade (P=0.010), presence of perineural infiltration (P=0.006), tumor infiltration beyond serosa (P=0.007), positive lymph node metastasis (P=0.020), and higher TNM stage (P=0.068) (Table 3).
 |
Table 3 Association Between Nuclear GSDMD Expression and Clinicopathological Indicators |
GSDMD Expression and Systemic Inflammatory Indicators
Regardless of the location of GSDMD expression, there was no significant difference between the positive and the negative group in the concentration of plasma albumin, platelet count, total white blood cell count, neutrophil count, lymphocyte count, monocyte count, neutrophil percentage, lymphocyte percentage, or monocyte percentage (Supplementary Tables 3–5).
GSDMD expression in membrane, cytoplasm or nucleus was not linked to NLR, PLR, LMR, or PNI. Furthermore, we grouped NLR, PLR, LMR, and PNI according to the median. The 6 cases with positive membranous GSDMD expression (6/6100%) had a lower NLR level (NLR≤2.8), while 73/155 (47.1%) in the negative membranous GSDMD group had a lower NLR level (P=0.013, Table 1).
GSDMD Expression and Immune Cells in IME
Immune cells infiltrated in the stroma of tumor were counted separately in TC and TIF (Figure 2). In the group with positive expression of membrane GSDMD, there were more CD68+ macrophages in TC (P=0.002) and more CD8+ lymphocytes (P=0.007) in TIF. In the group with positive cytoplasmic GSDMD, there were more CD3+ lymphocytes both in TC (P=0.066) and TIF (P=0.008), but fewer CD4+ lymphocytes in TC (P=0.062). However, when nuclear GSDMD expression was positive, there were fewer CD68+ macrophages in TIF (P<0.001) and fewer CD8+ lymphocytes in TC (P=0.069) (Table 4).
 |
Table 4 Difference of Immune Cells in IME Based on GSDMD Expression |
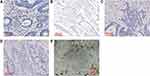 |
Figure 2 Immunohistochemical staining images of CD3+ lymphocytes (A), CD4+ lymphocytes (B), CD8+ lymphocytes (C), CD20+ lymphocytes (D) and CD68+ macrophages (E). 400× magnification. |
Univariate Survival Analysis of GSDMD
First, we compared the overall survival rate of GSDMD within each score. For cytoplasmic GSDMD, the results showed that the group with score 2 had the worst survival (9/24 deceased). In detail, 14 out of 65 patients died in the score 0 group, 4 out of 41 patients died in the score 1 group, 9 out of 24 patients died in the score 2 group, 0 out of 13 patients died in the score 3 group, 1 out of 19 patients died in the score 4 group, and 1 out of 16 patients died in the score 5 group (see Supplementary Fig. 1A). Neither membranous GSDMD nor nuclear GSDMD showed significant differences in survival between any score group (Supplementary Fig. 1B and C). Next, we grouped cytoplasmic GSDMD as low expression (score≤2) and high expression (score>2). At the end of follow-up, there were 27/130 deceased patients in the low-expression group, and 2/48 deceased patients in the high expression group (P=0.006) (Figure 3A). The five-year overall survival rate of the group with low cytoplasmic GSDMD expression was 72%, and that of the group with high cytoplasmic GSDMD expression was 95%. It was therefore evident that high expression of GSDMD was a favorable prognostic marker in CRC.
We also compared the overall survival rate between the negative and positive cytoplasmic GSDMD groups. At the end of follow-up, 14/65 patients were deceased in the negative group and 15/113 patients in the positive group (P=0.1) (Figure 3B).
To explore whether expression of cytoplasmic GSDMD affected the efficacy of chemotherapy, we performed survival analyses on the groups with or without postoperative chemotherapy based on 5-Fu. The results showed that both positive (P=0.072) and high (P=0.012) cytoplasmic GSDMD expression improved the efficacy of chemotherapy (Figure 3C and D). The expression of cytoplasmic GSDMD had no effect on survival in the cases without chemotherapy (P>0.1) (Figure 3E and F).
Multivariate Survival Analysis
Finally, age, sex, histological type, histological grade, vessel infiltration, perineural infiltration, TNM stage, chemotherapy, and cytoplasmic GSDMD expression were included in our multivariate Cox proportional hazard model. The results showed that TNM stage and cytoplasmic GSDMD expression were independent prognostic factors (Table 5). High expression of cytoplasmic GSDMD improved overall survival (RR (95 CI): 0.196 (0.046–0.834), P=0.027).
 |
Table 5 The Results of Multivariate Cox Proportional Hazard Model |
Discussion
Currently, more than 10 types of regulated cell death have been defined. Pyroptosis is a form of regulated lytic cell death that relies on perforation of the plasma membrane mediated by the gasdermin family.16 Initially, pyroptosis was observed in monocytes or macrophages undergoing canonical Caspase 1 activation, but later it was also found in certain epithelial cells.17 NLRP1 (NLR family pyrin domain containing 1), NLRP3, and AIM2 (absent in melanoma 2) can participate in the formation of inflammasome and activate Caspase 1. Caspase-1/4/11 can cleave GSDMD in the central linker region (FLTD in humans or LLSD in mice) to form a GSDMD N-terminal fragment (NT) and GSDMD C-terminal fragment (CT). Functionally, GSDMD-NT forms transmembrane pores in both cellular and organelle membrane (such as mitochondrial or nuclear membrane). GSDMD-NT pores in cellular membrane causes substance exchange and pyroptosis. GSDMD-NT pores in organelle membrane also contribute to pyroptosis or other reactions. For example, when GSDMD-NT targets the mitochondrial membrane, it promotes the production of reactive oxygen species.18 Our results showed that GSDMD was located in cellular membrane, cytoplasm, and nucleus. Among membranous, cytoplasmic, and nuclear GSDMD, the positive rate of membrane GSDMD was the lowest, but that of cytoplasmic GSDMD and nuclear GSDMD was similar.
Membranous GSDMD correlated with lower NLR, more CD68+ macrophages in TC, and more CD8+ lymphocytes in TIF, but was not linked to other clinicopathological parameters in this study. Cytoplasmic GSDMD predicted less distant metastasis, more CD3+ lymphocytes both in TC and TIF, fewer CD4+ lymphocytes in TC, and better prognosis. However, there was no relationship between cytoplasmic GSDMD and systemic inflammatory indicators. Nuclear GSDMD was more common in females, rectal cancer, and cancer with low histological grade, indicating perineural infiltration, deeper degree of infiltration, more lymph node metastasis, higher TNM stage, fewer CD68+ macrophages in TIF, and fewer CD8+ lymphocytes in TC.
In summary, membranous GSDMD is related to systemic inflammatory reaction and tumor immune microenvironment. Cytoplasmic GSDMD tends to recruit CD3+ lymphocytes in TIF, decrease distant metastasis, and improve prognosis, while nuclear GSDMD leads to lower density of macrophages and CD8+ lymphocytes and more aggressive cancer. Function and mechanism studies are needed to confirm the phenomena in our study. Our results only touch the tip of iceberg about the role of GSDMD. Further mechanism studies are necessary to confirm the correlation between GSDMD subcellular location and function, such as downstream signaling pathways activated by GSDMD in different subcellular locations.
GSDMD, as a powerful executor of pyroptosis, is closely associated with immune reaction both in local IME and systemic immune response. Membranous GSDMD does not always mean pyroptosis. Membranes perforated by GSDMD can be repaired by the ESCRT-III machinery.19 In conformation, the GSDMD prepore is short, resembling autoinhibited GSDMD-NT.20 GSDMD pores or prepores mediate the release of cellular contents such as proinflammatory cytokines and endogenous damage-associated molecular patterns (DAMPs) in both tumor cells and immune cells like macrophages and NK cells.21 As linkers between innate immunity and adaptive immunity, these contents play an intricate role in anti-tumor immunity. For instance, the IL-1 family are the most-studied cytokines released by pyrolytic cells. Among them, IL-1β can induce the differentiation of Th1 and Th17 cells.22 IL-18 can not only upregulate the levels of tumor-infiltrating CD8+ T lymphocytes and natural killer cells (NK), but also promote the activity of IFN-γ, which enhances immune response.23 The upregulated tumor-infiltrating cytotoxic T lymphocytes induce tumor cell apoptosis through caspase 3-dependent or other mechanisms, further leading to the release of more cytokines and chemokines to recruit lymphocytes, which forms a positive feedback pathway.24 On the other hand, HMGB1, the DAMP most associated with cancer, can paradoxically trigger antitumor immunity inflammation or immunotolerance by provoking the recruitment of leukocytes or inducing the release of IL-10.25 In general, GSDMD-mediated pyroptosis of tumor cells and their released contents may jointly regulate the recruitment of immune cells in the IME.
In the systemic inflammatory response of CRC, neutrophils can interact with tumor cells and facilitate the invasion and metastasis of tumor cells.14 Some studies have reported that elevated NLR may contribute to a poor immune response to malignancy.26,27 Notably, among all systemic inflammatory indicators, only an association between positive membranous GSDMD and a lower NLR level was found in our study. Our results suggest that membranous GSDMD expression probably promotes immune response.
Recent research has shown that GSDMD is related to prognosis in a variety of cancers. As mentioned above, Wu et al found that GSDMD was associated with poor prognosis in CRC.11 However, some studies also indicate that levels of important molecules involved in the pathway of pyroptosis, such as NLRP1, NLRP3, and AIM2, were lower in CRC with worse prognosis.28,29 Moreover, high GSDMD expression was proved to be linked to longer overall survival and less invasion of cancer cells in breast cancer. However, in adenoid cystic carcinoma (ACC), GSDMD enhanced the invasive capacity of ACC cells, which indicated that high GSDMD expression was tied to poor prognosis.30 As for the mechanism, Wang et al reported that decreased GSDMD expression can promote tumor cell proliferation through activating the extracellular signal-regulated kinase 1/2 (ERK1/2), signal transducer and activator of transcription 3 (STAT3), and phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (AKT) signaling pathway, and regulating cell cycle-related proteins.21 Studies have also shown that lncRNA RP1-85F18.6 can induce the occurrence of cell pyroptosis in CRC through the activation of GSDMD, which has a certain prognostic value.31
From these conflicting prior research results, we found that existing studies do not differentiate between the expression of GSDMD in membrane, cytoplasm, and nucleus. Meaningfully, in our study, we discovered that nuclear GSDMD seemed to be opposite to cytoplasmic GSDMD with regard to cancer progress. High nuclear GSDMD levels promoted CRC invasion and metastasis, and decreased the density of CD68+ macrophages and CD8+ T lymphocytes. Meanwhile patients with high cytoplasmic GSDMD levels had lower risk of distant metastasis, better overall survival, and more CD3+ lymphocytes.
Some researchers have proposed a theory of the dual mechanisms of pyroptosis. On one hand, pyroptosis may inhibit the occurrence and development of tumors, but as a type of proinflammatory death, it may also create a suitable microenvironment for tumor cell growth, meaning that it has dual mechanisms of promoting and inhibiting tumorigenesis.32 Maybe these dual mechanisms have something to do with the difference between nuclear and cytoplasmic GSDMD expression.
What are the effects of nuclear GSDMD accumulation? Why does GSDMD in cytoplasm and nucleus have opposite functions? Is there something specific that takes place during the nucleocytoplasmic transport of GSDMD? Finding the answers to these questions would be helpful in assessing the value of GSDMD as a candidate target with therapeutic potential in cancer. Our study only focused on the relationship between GSDMD and clinic-pathological parameters, and further mechanism studies will be required.
Wang et al revealed that chemotherapeutic drugs could effectively inhibit tumor proliferation and metastasis by inducing pyroptosis.33 Most studies have found that chemotherapeutic drugs can convert caspase-3-dependent apoptosis into pyroptosis via GSDME. Intriguingly, in our study, GSDMD expression evidently helped patients benefit from chemotherapy. This provides new insights into anticancer treatment. But still little is known about how GSDMD inhibits cancer progression and improves the chemotherapeutic effect. Considerable effort should be expended to identify GSDMD-targeted molecules in pursuit of new drug development.
Conclusion
Our results show that membranous GSDMD expression is closely related to immune response, and that cytoplasmic GSDMD correlates with tumor immune microenvironment and improves patient prognosis; but that expression of GSDMD in the cancer cell nucleus promotes tumor invasion and metastasis. In other words, the function of GSDMD depends on its subcellular location. Our research provides a novel perspective for future study of GSDMD, and also provides new support for further development of GSDMD as a prognostic biomarker and therapeutic target.
Acknowledgments
Thanks for the technical support by the Core Facilities, Zhejiang University School of Medicine. Jiahui Wang, Yixin Kang, and Yuxuan Li are co-first authors for this study.
Funding
This work was supported by the grants of National Natural Science Foundation of China under Grant 81772570; the Open Projects of State Key Laboratory of Molecular Oncology (SKLMO-KF2021-17) and Program of Introducing Talents of Discipline to Universities under Grant B13026.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi:10.1001/jama.2021.0106
3. Dai Y, Zhao W, Yue L, et al. Perspectives on immunotherapy of metastatic colorectal cancer. Front Oncol. 2021;11:659964. doi:10.3389/fonc.2021.659964
4. Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6(7):605–618. doi:10.1016/j.trecan.2020.02.022
5. Legrand AJ, Konstantinou M, Goode EF, Meier P. The diversification of cell death and immunity: memento mori. Mol Cell. 2019;76(2):232–242. doi:10.1016/j.molcel.2019.09.006
6. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.004
7. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi:10.1038/nature15514
8. Saeki N, Sasaki H. Gasdermin superfamily: a novel gene family functioning in epithelial cells. In: Endothelium and Epithelium: Composition, Functions and Pathology. New York: Nova Science Publishers, Inc.; 2012:193–211.
9. Gao J, Qiu X, Xi G, et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in nonsmall cell lung cancer. Oncol Rep. 2018;40(4):1971–1984.
10. Lin R, Wei H, Wang S, et al. Gasdermin D expression and clinicopathologic outcome in primary osteosarcoma patients. Int J Clin Exp Pathol. 2020;13(12):3149–3157.
11. Wu LS, Liu Y, Wang XW, et al. LPS enhances the chemosensitivity of oxaliplatin in HT29 cells via GSDMD-mediated pyroptosis. Cancer Manag Res. 2020;12:10397–10409. doi:10.2147/CMAR.S244374
12. Marusyk A, Janiszewska M, Polyak K. Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell. 2020;37(4):471–484. doi:10.1016/j.ccell.2020.03.007
13. Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110(2):435–440. doi:10.1038/bjc.2013.785
14. Xia LJ, Li W, Zhai JC, Yan CW, Chen JB, Yang H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer. 2020;20(1):208. doi:10.1186/s12885-020-6698-6
15. Yatabe S, Eto K, Haruki K, et al. Signification of systemic immune-inflammation index for prediction of prognosis after resecting in patients with colorectal cancer. Int J Colorectal Dis. 2020;35(8):1549–1555. doi:10.1007/s00384-020-03615-w
16. Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541.
17. Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi:10.1038/nature13683
18. Platnich JM, Chung H, Lau A, et al. Shiga toxin/lipopolysaccharide activates caspase-4 and gasdermin D to trigger mitochondrial reactive oxygen species upstream of the NLRP3 inflammasome. Cell Rep. 2018;25(6):1525–1536 e1527. doi:10.1016/j.celrep.2018.09.071
19. Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science (New York, NY). 2018;362(6417):956–960. doi:10.1126/science.aar7607
20. Xia S, Zhang Z, Magupalli VG, et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593(7860):607–611. doi:10.1038/s41586-021-03478-3
21. Wang WJ, Chen D, Jiang MZ, et al. Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. J Dig Dis. 2018;19(2):74–83. doi:10.1111/1751-2980.12576
22. Ju XL, Yang ZL, Zhang H, Wang Q. Role of pyroptosis in cancer cells and clinical applications. Biochimie. 2021;185:78–86. doi:10.1016/j.biochi.2021.03.007
23. Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev. 2018;281(1):138–153. doi:10.1111/imr.12616
24. Minton K. Pyroptosis heats tumour immunity. Nat Rev Immunol. 2020;20(5):274–275. doi:10.1038/s41577-020-0297-2
25. Pandolfi F, Altamura S, Frosali S, Conti P. Key role of DAMP in inflammation, cancer, and tissue repair. Clin Ther. 2016;38(5):1017–1028. doi:10.1016/j.clinthera.2016.02.028
26. Del Prete M, Giampieri R, Loupakis F, et al. Prognostic clinical factors in pretreated colorectal cancer patients receiving regorafenib: implications for clinical management. Oncotarget. 2015;6(32):33982–33992. doi:10.18632/oncotarget.5053
27. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
28. Markowitz GJ, Yang P, Fu J, et al. Inflammation-dependent IL18 signaling restricts hepatocellular carcinoma growth by enhancing the accumulation and activity of tumor-infiltrating lymphocytes. Cancer Res. 2016;76(8):2394–2405. doi:10.1158/0008-5472.CAN-15-1548
29. Zhou CB, Fang JY. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection. Biochim Biophys Acta Rev Cancer. 2019;1872(1):1–10. doi:10.1016/j.bbcan.2019.05.001
30. Shen X, Zhang Q, He Z, Xiao S, Li H, Huang Z. Overexpression of gasdermin D promotes invasion of adenoid cystic carcinoma. Int J Clin Exp Pathol. 2020;13(7):1802–1811.
31. Ruan J, Wang S, Wang J. Mechanism and regulation of pyroptosis-mediated in cancer cell death. Chem Biol Interact. 2020;323:109052. doi:10.1016/j.cbi.2020.109052
32. Xia X, Wang X, Cheng Z, et al. The role of pyroptosis in cancer: pro-cancer or pro-”host”? Cell Death Dis. 2019;10(9):650. doi:10.1038/s41419-019-1883-8
33. Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi:10.1038/nature22393
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

