Back to Journals » Journal of Inflammation Research » Volume 14
Functional Mechanism of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Animal Models with Alzheimer’s Disease: Inhibition of Neuroinflammation
Received 2 July 2021
Accepted for publication 18 August 2021
Published 17 September 2021 Volume 2021:14 Pages 4761—4775
DOI https://doi.org/10.2147/JIR.S327538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Chuan Qin,1,* Yongning Li,2,* Kewei Wang1
1Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences & Comparative Medical Center, Peking Union Medical College, Beijing, 100021, People’s Republic of China; 2Department of International Medical Service & Department of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kewei Wang 5 Panjiayuan Nanli St., Beijing, 100021, People’s Republic of China
Tel +010-67779915
Email [email protected]
Abstract: The transplantation of bone marrow-derived mesenchymal stem cells (BMMSCs) alleviates neuropathology and improves cognitive deficits in animal models with Alzheimer’s disease. However, the underlying mechanisms remain to be determined. Available data demonstrate transplanted BMMSCs can inhibit neuroinflammation, which may be related to microglial M1/M2 polarization and is regulated by the secretion of autocrine and paracrine cytokines. BMMSCs also mitigate Aβ plaques and Tau tangles in the brain, which may be associated with the recruitment of peripheral blood monocytes and the subsequent comprehensive effects. The therapeutic effects of stem cells involve potential mechanisms such as immunomodulation, apoptosis, and proliferation. BMMSC-mediated functional reconstruction through dynamic remodeling develops a novel balance. Herein, present review recapitulates the molecular basis of BMMSC-assisted biological processes and summarizes the possible mechanisms related to the interaction between BMMSCs and microglia. The transplanted BMMSCs can suppress neuroinflammation that plays a key role in the pathogenesis of Alzheimer’s disease.
Keywords: Alzheimer’s disease, bone marrow-derived mesenchymal stem cells, microglia, immunomodulation, apoptosis
Graphical Abstract:
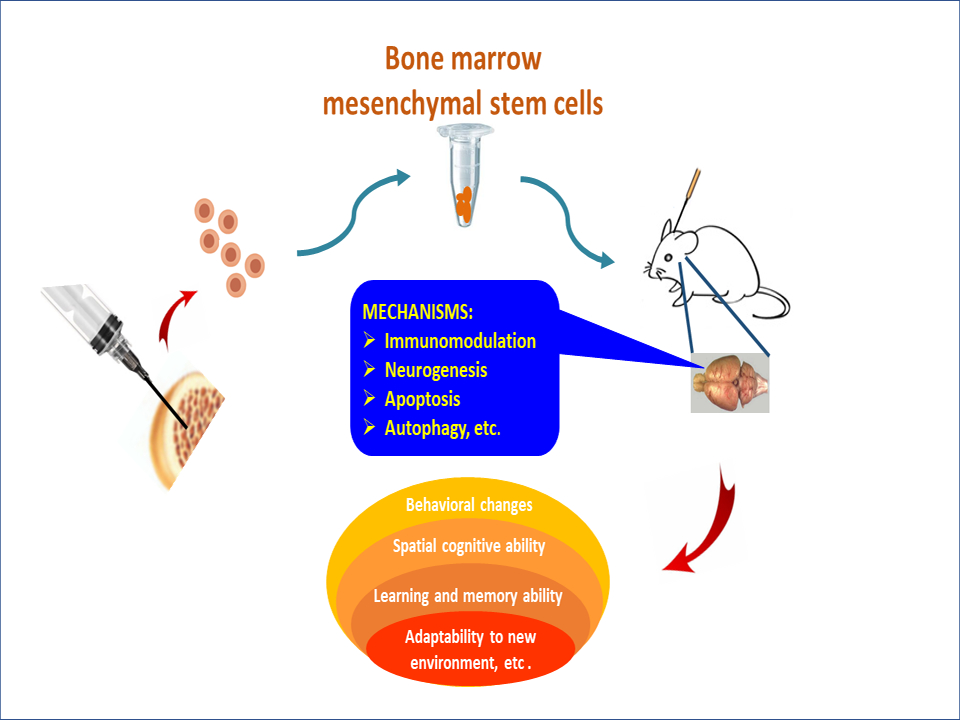
Highlights
- Alzheimer’s disease is characterized by the accumulation of aberrant amyloid-beta (Aβ) peptides and Tau aggregates in pathological tissues.
- Neuroinflammation plays an important role in the pathogenesis of Alzheimer’s disease, which can be alleviated by the transplantation of bone marrow-derived mesenchymal stem cells (BMMSCs).
- The functional activity of transplanted stem cells establishes a new balance through dynamic reconstruction, which lays a theoretical foundation for stem cell therapy.
Alzheimer’s Disease
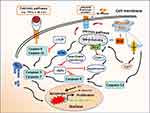
Alzheimer’s disease (AD) is a neurodegenerative disorder.1–3 Clinical manifestations are characterized by memory decline and cognitive deficits, but symptoms may be varied due to the location and severity of neuropathology. Pathological features are reflected by the extracellular deposition of amyloid-beta (Aβ) peptides, neurofibrillary tangles, microglia-driven inflammation, and neuron loss in various areas, such as hippocampus and temporal lobe. Neuronal death is caused by different mechanisms, mainly due to apoptosis/necroptosis/necrosis induced by aberrant Aβ plaques, neurofibrillary Tau tangles, and inflammatory cytokines. Meanwhile, neuronal apoptosis plays an important role in the development of AD, especially in the early stage. The apoptosis cascade may be initiated through intrinsic and extrinsic pathways (Figure 1). The disturbance of intracellular homeostasis triggers intrinsic pathway, leading to apoptotic cell death. The intrinsic pathway can be further divided into mitochondrial failure because of abnormal energy metabolism and/or oxidative stress, and endoplasmic reticulum (ER) stress due to hyperphosphorylated aggregates of the microtubule-associated protein Tau in neurofibrillary tangles.4–6 The extrinsic pathway is activated by the aberrant Aβ proteins of brain plaques. Inflammatory cytokines, such as IL-6, IL-1β, and TNF-α from microglia can cause neuronal apoptosis through the mediation of membrane receptors.7–9 Accumulated Aβ plaques and Tau tangles are hallmarks in the pathogenesis of AD, which are correlated with neuronal degeneration and cognitive impairment in patients with AD. In terms of treatment, there is no cure for Alzheimer’s disease. Owing to uncertain pathomechanism, most treatments are symptom-related or exploratory. Presently, its drug intervention involves the adjustment of neurotransmitter release.10,11 There are two types of medicines, including (i) cholinesterase inhibitors (i.e., donepezil and galantamine). They may improve neuropsychiatric agitation or depression; (ii) memantine, an uncompetitive NMDA antagonist. It can ameliorate memory and awareness in moderate or severe patients with AD. Non-pharmacological therapies are also supplemented for the improvement of patients’ life quality, including health diet, regular exercise, and special care. Nowadays, the exact etiology of AD remains unknown. Extracellular Aβ deposits and intracellular hyperphosphorylated Tau tangles are typical changes in the pathogenesis of AD.3,12 These pathophysiological characteristics are highlighted by neuroinflammation. Inflammation is an essential mechanism to induce hippocampal neuron apoptosis and synaptic deficits, leading to cognitive impairment and memory decline.13,14
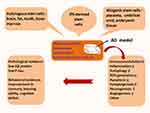
Alzheimer’s Disease and Stem Cell Therapy
Stem cell therapy as a novel strategy has been explored in the treatment of animal models with Alzheimer’s disease (Figure 2). According to the tissue sources of stem cells, therapeutic stem cells are approximately classified into autologous and allogenic categories.15–17 Autologous stem cells are isolated from brain, fat, dental pulp, and bone marrow. In contrast, allogenic stem cells are obtained from placenta, umbilical cord, or embryonic tissue. Some studies have used iPS-derived stem cells.18,19 iPS-derived stem cells are not discussed as an independent tissue source. Comparative studies have been conducted among different tissue sources of stem cells. There are two problems with allogenic stem cells. One is ethical issue and the other is allogeneic immunogenicity. Since these problems cannot be resolved in the short term, allogeneic stem cells may be not suitable for the treatment of AD in the near future. In the clinical treatment of patients with AD, the autologous stem cells derived from brain biopsy may front onto unacceptable attitude and technical challenges. Therefore, the stem cells from autologous bone marrow or fat are preferred. Interestingly, the therapeutic stem cells derived from bone marrow have better results than those isolated from the adipose tissue.20,21 BMMSCs have certain advantages as evidenced in pre-clinical studies, but they are still complicated by various problems, such as heterogeneity, low viability, and poor homing into injured tissue.22,23 Also, therapeutic efficiency is affected by preconditioning, cell viability, and delivery methods.24,25 There are different methods for the transplantation of stem cells. Usually, autologous MSCs are delivered intravenously, intrahippocampally, intracerebroventricularly, or intranasally.26,27 The therapeutic effect of transplanted BMMSCs has been verified in several AD-like models, such as APP mice, DAL mice, or scopolamine-induced rats.26 A lot of evidence shows that BMMSCs can alleviate neuropathology, memory decline, and behavioral deficits. Research data indicate the reduced level of Aβ plaques is beneficial to both young and aged TASTPM mice.22 Cognitive impairment (i.e., learning ability and spatial memory performance) are improved as demonstrated by Morris water maze test, Y-maze alternation test, plus-maze discriminative avoidance task, social recognition test and open-field evaluation, respectively.26,28–30 Moreover, a single transplantation of bone marrow-derived mononuclear cells could obtain a positive result.22,31,32 Today, the technical improvement in the preparation of autologous BMMSCs provides an assurance for their clinical application.
Autocrine and Paracrine Cytokines
Transplanted stem cells have two essential properties, including (i) self-renewal; (ii) trans-differentiation into tissue-specific cell lineages. Following the transplantation of BMMSCs, autocrine and paracrine cytokines are secreted, which are conducive to the adaption of new microenvironment (Figure 3). Nevertheless, cytokine induction and signal transduction are varied due to the tissue sources (eg, placenta, cord blood, and bone marrow) of mesenchymal stem cells.33–35 Those autocrine and paracrine cytokines have special functions as demonstrated in previous studies. In cardiology, the autocrine and paracrine cytokines from transplanted hBMSCs could upregulate the expression of angiogenic factors, such as VEGF-A, HGF, bFGF, Ang1, Ang2, and PDGF-B, which promoted cardiomyogenesis for the repair of myocardial damage.35–39 In hepatology, the transplantation of autologous BMSCs improved liver function in patients with acute liver failure. MSCs secreted a mixture of growth factors (eg, PCNA, SDF-1, HGF, VEGF), immunoregulatory factor (eg, IL-10), chemokines, and other constituents.40–43 The therapeutic effects of autologous BMSCs could be maintained more than six months. Further long-term effects are still under observation.44 Currently, there are no data on stem cell therapy for patients with Alzheimer’s disease, although clinical trials using hMSCs has been carried out for the therapeutic purposes.45 The favorable effects of BMMSCs observed in other organs (eg, heart, liver) should be carefully translated to central nervous system, because the secretion of autocrine and paracrine cytokines can be influenced by local environment. Up to now, most studies on the transplantation of BMMSCs belong to preliminary stage. The comprehensive effects of autocrine and paracrine cytokines are under investigation.
The types of autocrine and paracrine factors are classified as follows: (i) pro-inflammatory cytokines such as IL-1β, IL-6, IL-8 and TNF-α;46,47 (ii) fibrosis-related cytokines FGF, bFGF, TIMP-1, and TIMP-2;34,48 (iii) chemokines CXCL-12, CXCL-10, CCL5 and so forth;36,48,49 (iv) leucocyte chemoattractant factors CINC-1, G-CSF, SCF, GM-CSF or IL-3;50 (v) transcription factors, such as GATA-4, Nkx2.5, and MEF2C;51 (vi) growth factors HGF and IGF-1;23,36 (vii) anti-inflammatory cytokines such as IL-4, IL-10 and IL-11;46,52,53 (viii) other active factors, including Ang-1, Ang-2, PDGF-B, MCP-1, VEGF-A, and OPG.36,54 Gene analysis has proved that certain cytokines such as MIF (GIF, DER6), IL-8 (CXCL8), Serpin E1 (PAI-1), GROα (CXCL1) and IL-6, can be secreted by the most types of stem cells.33 MCP-1, however, is produced in the MSCs from bone marrow and amnion.33,55 CXCL-12 (SDF-1) is only expressed in BMMSCs.33,56 There are significant differences in the expression levels of autocrine and paracrine factors, which have been tested in APP/PS1 transgenic mice or rat model following the transplantation of BMMSCs.23,47,49,57 Now, certain cytokine profiles mediated by transplanted BMMSCs have been clarified, but the overall spectrum and their respective patterns still need to be investigated. For instance, the proteomics of BMMSC-mediated cytokines was characterized by the predominant hybridization signal for IL-6 and the moderate elevation signals for IL-8, TIMP-2, MCP-1, VEGF and OPG.54
The types and levels of autocrine and paracrine cytokines are regulated by various factors, including (i) age. The constitutive secretion of IL-6 in human bone marrow is positively correlated with age.46 The basal secretion of immunoreactive IL-6 and IL-11 is increased when the cell culture time is extended. Moreover, the secretion of IL-6 and IL-11 can be stimulated by IL-1β in a dose-dependent manner;46,58 (ii) gender. The cytokines secreted by human bone marrow are modulated by estrogen status. Women receiving estrogen replacement therapy show a low secretion of IL-6 and IL-11 as compared with those of age-matched controls;46,59 (iii) injection site and delivery method. When MSCs are injected into APP/PS1 mice via the tail vein, there are no significant changes in the expression of IL-10, CCR5, and IFN-γ;21 (iv) the interaction between stem cells and immune cells. The transplanted stem cells can facilitate the shift of microglial M1 to M2 phenotype and thereby decrease the secretion of pro-inflammatory cytokines.21,48,60 The polarization of M1/M2 phenotype can even be elicited by the intranasal delivery of BMMSC-derived exosomes, rather than whole-cell transplantation, which also exerts immunomodulatory and neuroprotective effects in the 3xTg model;61 (vi) the modification of stem cells. The preconditioning MSCs with dimethyloxalylglycine enhance the therapeutic efficiency of Aβ-induced animal models.62 Other preconditioning methods, such as hypoxia, LPS, inflammatory cytokines, vitamin E, electromagnetic stimulation and low-level lasers, can also improve the viability and immunomodulatory activity of MSCs.25,63 Chemokine receptor CXCR4 is involved in the homing processes to injured tissues.64 The expression of CXCR4, CCR2, Nrf2 and HIF-1α is upregulated in the MSCs, which mediates the rescue of learning and memory function in Aβ-injected rats.62 When anti-apoptotic microRNA Let-7f-5p is used in APP/PS1 transgenic mice, the prolonged survival of BMMSCs can increase the therapeutic effect of BMMSCs.65 Paracrine effects are reflected by the secretion of soluble cytokines, such as TGF-β, IL-10, VEGF, BDNF, NGF, and neurotrophin-3 in human MSCs.66 In addition, paracrine cytokines are modulated by local blood supply, metabolic activity, and nutrition.
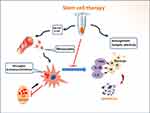
Transplanted BMMSCs Inhibit Neuroinflammation
Transplanted stem cells not only provide cell sources for regeneration but also regulate inflammatory and immune responses.67,68 Immunomodulation is an important function of stem cells that play an inhibitive role as neuroinflammation in an active state. Stem cell treatment suppresses neuroinflammation through different mechanisms, including (i) direct roles. Transfused stem cells secrete anti-inflammatory cytokines such as IL-4, IL-10, and IL-11.46,52,53 Furthermore, anti-inflammatory IL-11 can activate downstream signaling pathways to regulate neurogenesis;69 (ii) indirect roles. Growth factors such as HGF and IGF-1 are upregulated by stem cells.23,36 Neurotrophic factors such as BDNF and NGF are also enhanced.70,71 All these mediators have indirect effects on the inhibition of neuroinflammation; (iii) microglial M1/M2 polarization. In the classic M1 state, the expression of CD86 and the release of pro-inflammatory cytokines are increased, including TNF-α, iNOS, IL-1β, IL-6, IL-12, and IL-23.72,73 In contrast, the M2 phenotype can be determined by candidate markers, such as CD206 and Arg1, which has neuroprotective effects by enhancing anti-inflammatory cytokines and growth factors, such as IL-4, IL-10, IGF-1, and TGF-β. The heterogeneity of microglia is discovered through high-throughput single-cell transcriptomics, showing at least nine transcriptional states that are affected by age and pathological conditions.24 The transcriptomic responses of microglia may be used to identify signaling pathways, particularly focusing on pro- and anti-inflammatory signatures.74,75 The transplantation of stem cells modifies the resident microglia to trigger M1/M2 polarization.76,77 This process prevents M1 microglia from secreting pro-inflammatory cytokines, but stimulates M2 microglia to produce anti-inflammatory cytokines.78 A series of comprehensive effects result in the mitigation of neuroinflammation.
In the lesion of AD, microglia are activated by (a) the direct effect of Aβ peptides; (b) Aβ protein-caused apoptotic bodies; and (c) paracrine cytokines. Transplanted BMMSCs can mediate microglial M1/M2 polarization through autocrine and paracrine factors, which represses microglial M1 activity and decrease the release of pro-inflammatory cytokines. Microglia reside in the parenchyma of central nervous system in activated or quiescent state. The activated microglia are distributed in the area of Aβ deposits.79–81 After phagocytosis, the morphology of microglia is changed from ramified to amoeboid, which is considered to be in an activated state.82 The degree of microglial activation can be measured using frequent markers such as CD68 and IBA-1. For example, hMSC treatment could significantly down-regulate IBA-1 levels in the young and aged brains of APP/PS1 transgenic mice.32 There was a dramatic decline in the panel of cerebral cytokines, such as IFNγ, diverse interleukins (IL-1β, IL-2, IL-5, IL-6, and IL-12p70), KC/GRO, and TNF-α.32 Even, a single injection of hMSC might reduce levels of IL-1, IL-2, IL-12p70, TNF-α and IFNγ in APP/PS1 mice.32,47 The anti-inflammatory role of BMMSCs was also confirmed in the rat model of spinal cord injury.83 BMMSCs could secret anti-inflammatory factors, such as IL-4, IL-10, and IL-11. When the IL-4 and IL-10 were utilized to treat microglia, they had different effects on proliferation and differentiation, suggesting that the type of microglial activators could change cell fate and affected neuronal damage and repair.84,85 Moreover, the transplanted BMMSCs could elevate the expression of Nrf2 and seladin-1 in Aβ-injected AD rats as well as aluminium chloride-induced AD rats.62,86 The function of Nrf2 was to regulate the levels of antioxidant proteins and protect neurons from oxidative damage.5 Seladin-1 inhibited the activation of caspase-3 and mediated neuronal apoptosis, improving neuroprotective effects.87 Moreover, MSCs could release mediators to mediate the gene expression in astrocyte cultures, including intermediate filaments (GFAP, vimentin), pro-inflammatory enzymes (iNOS, COX-2), and receptors (TLR4, CD14, mGluR3, mGluR5).88 Astrocytes participate in the secretion of inflammatory factors. Previous studies demonstrated BMMSCs could decrease the levels of pro-inflammatory genes (IL-1β, TNF-α, IL-6) in astrocytes.37,88 In the lesion, neurons produce Aβ peptides and take part in the initiation of inflammatory reaction. Microglia secrete pro-inflammatory as well as anti-inflammatory cytokines, showing an obvious duality. All three types (neurons, astrocytes, and microglia) are implicated in the inflammatory process. There are complicated mechanisms involved in intercellular interactions. Many details need to be clarified by future study. Clearly, the transplanted stem cells inhibit neuroinflammation and regulate the dynamic remodeling of tissue function, which stimulates neurogenesis and synaptogenesis. The therapeutic effects of BMMSCs are highlighted by the alleviation of neuropathology and the improvement of cognitive deficits in different AD-like models.
Transplanted BMMSCs Recruit Peripheral Blood Monocytes
Certain cytokines secreted by transplanted BMMSCs have positive chemotactic effect and can recruit peripheral leukocytes into the lesion, including CCL5, G-CSF, SCF, and GM-CSF. CCL5 derived from BMMSCs can be activated by Aβ protein to promote microglial migration.49 GM-CSF or CSF2 functions as a cytokine to recruit granulocytes and macrophages. SCF is a costimulatory factor, which combines other cytokines to produce a synergistic effect on the proliferation, differentiation, and survival of stem cells. In particular, its synergy with G-CSF has biological and clinical significance.89 G-CSF is an endogenous neurohematopoietic factor, which has a strong neuroprotection in vivo and in vitro. The therapeutic effect of G-CSF significantly improved the motor coordination and the exploratory behavior of Aβ-induced rats.90 The improvement of memory in Aβ-induced rats and Tg2576 mice was associated with the significant reduction of lipid peroxidation, the inhibition of acetylcholinesterase, and the main increase of antioxidant enzymes.90,91 The long-term effect of G-CSF could improve the cognitive function of Aβ-induced mice and APP/PS1 transgenic mice, which might be through potential mechanisms, such as peripheral blood monocyte recruitment, microglial activation and polarization, neurogenesis, and synaptogenesis.77,91–93 The analysis of cytokine expression revealed there was a high secretion of chemoattractive factor CCL5 after BMMSCs were transplanted into the brains of APP/PS1 mice.49 The levels of leukocyte-chemoattractant factors are affected by stem cell concentration, inoculation position, delivery method, and survival rate. The differential expression profiles of autocrine and paracrine factors remain to be determined when MSCs are transplanted by different methods, such as intrahippocampal, intracerebroventricular, or intravenous. The expression of leukocyte-chemoattractant factors may be regulated by growth factor, cell cycle, and nutrition state.
Aberrant Aβ Plaques and Neurofibrillary Tau Tangles
The neurotoxicity of over-produced Aβ peptides is a critical mechanism in the pathogenesis of AD. The extracellular removal of Aβ deposits is conducted by microglia, astrocytes, and neurons. So far, no evidence demonstrates that transplanted stem cells can directly eliminate aberrant Aβ peptides. However, a multitude of high-profile studies support that the transplanted BMMSCs alter microenvironmental homeostasis by facilitating intercellular communication and participating in molecular transfer among neurons, astrocytes, and microglia, which promote the removal of aberrant Aβ peptides.47,94,95 Moreover, the transplanted BMMSCs can recruit peripheral blood monocytes into the lesion through leucocyte chemoattractant factors, such as GM-CSF and SCF. In neurodegenerative tissue, the functional conversion of monocyte/microglia could accelerate the clearance of Aβ deposits via effective phagocytosis in the Aβ-injected C57BL/6 mice.82 In addition, recruited monocytes might facilitate microglial M1/M2 polarization.96,97 Microglia account for 10–15% of the total brain cells. They act as the main cell type in inflammatory response to phagocytose damaged cells and pathogens.20,84 Adult microglia are a combined population of residents and migrants into the brain by myeloid progenitors. Under normal circumstances, there are a few microglia in the ramified or quiescent state. The quiescent microglia move at a speed of 1.5 µm/min, covering 15–30µm wide territory. Focal brain damage induces a rapid and concerted movement.84 As part of the cellular response, microglia secrete cytokines, chemokines, prostaglandins, NO and reactive oxygen species, which take part in immunoregulation. Additionally, the M2 phenotype of microglia is instrumental in the resolution of the inflammatory response by producing anti-inflammatory cytokines such as IL-4 and IL-10.85,93 The shift of M2 phenotype reduces cerebral amyloid-β load.76,98
The transplantation of BMMSCs could improve cognitive deficits by alleviating neuropathology in animal models of Alzheimer’s disease.26 Potential mechanisms involve (i) the accelerated removal of Aβ protein. Transplanted BMMSCs secret chemoattractant factors, such as G-CSF, SCF, and GM-CSF. GM-CSF takes part in the shift of microglial M1/M2 phenotype to facilitate the removal of Aβ protein;76,99 (ii) the alleviation of Tau pathology. Stem cell therapy decreases intercellular Tau hyperphosphorylated aggregates or Tau tangles, which may be related to the secretion of exosomes and the degradation of p-Tau;57,101,102 (iii) the mitigation of apoptosis due to the clearance of aberrant proteins and the attenuation of oxidative stress; and (iv) the inhibition of neuroinflammation. Transplanted BMMSCs suppress pro-inflammatory IL-1, IL-2, TNF-α and IFN-γ, and enhance anti-inflammatory IL-4 and IL-10.47,52,53 Microglial M1/M2 polarization can be mediated by the recruitment of peripheral blood monocytes, which is an essential mechanism for the functional reconstruction of damaged tissues; (v) synaptogenesis. Transplanted BMMSCs stimulate the production of neurotrophins such as BDNF and NGF.62,103 Their functional activities promote synaptic formation and endogenous neural growth. Furthermore, BMMSCs can shape the crosstalk between T cells and microglia to mediate synaptic plasticity in the brain.25,104,105
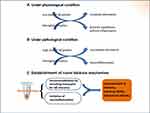
The Establishment of Novel Balance Mechanism
The transplantation of stem cells alters the pathological state in the brain. A series of characteristic changes are induced, which are regulated by autocrine and paracrine cytokines. The BMMSC-mediated functional reconstruction through dynamic remodeling tends to establish a new balance (Figure 4). The new balance mechanism is based on multiple signaling pathways.
- Cytokine signaling. After stem cell therapy, non-specialized BMMSCs have the potential for self-renewal and differentiation, accompanied by the secretion of autocrine and paracrine cytokines. For instance, G-CSF recruits peripheral blood monocytes and exerts neuroprotective effect.89,91,100 The low expression of seladin-1 and nestin in aluminium-induced AD rats is reversed by transplanted BMMSCs via PI3K/Akt and ERK1/2 signaling pathways.86 An increased level of BDNF and total antioxidant capacity are revealed in the hippocampus of Aβ-injected rats following the transplantation of MSCs.62 Of note, the pathophysiological role of autocrine and paracrine factors is a double-edged sword. Some cytokines may be harmful to neurons at certain stages. The therapeutic effects of stem cells are associated with functional construction through new balance mechanism, which involve multi-level signaling crosstalk, including inflammation, peripheral blood monocyte recruitment, and microglial M1/M2 polarization.
- Removal of Aβ peptides and plaques. The transplantation of BMMSCs inhibits neuroinflammation. However, the transplanted BMMSCs can also recruit peripheral blood monocytes into the lesion and further activate them. Activated monocytes exert neuroprotective effect by eliminating Aβ proteins.49 It seems to be a contradictory, but this does happen. Functional cytokines such as CCL5, G-CSF and GM-CSF play a crucial role in the recruitment of peripheral blood monocytes. These monocytes are then activated by extracellular Aβ proteins, which accelerate Aβ clearance as demonstrated in APP/PS1 mice.81,106 The enhanced phagocytosis of aberrant proteins attenuates cortical and hippocampal Aβ deposits, thereby improving memory and cognitive deficits. Similar result has also been observed in the Aβ-injected AD mice.82
- The alleviation of Tau tangles. As compared with the age-matched control brains, AD patients have numerous Aβ plaques and Tau tangles observed in different regions, such as hippocampus, temporal and parietal lobes.107,108 Neurofibrillary tangles are the hyperphosphorylated aggregates of microtubule-associated protein Tau, which are accumulated in neurons of AD. Tau protein can activate caspase-3 activity and cause neuronal apoptosis.12,109 Early studies demonstrated that Tau protein could be abated subsequent to the transplantation of BMMSCs.57,101 However, the underlying mechanism for reducing Tau aggregates still needs to be investigated. It may be associated with oxidative stress and mitochondrial pathway.3,109
- Apoptosis. Transplanted BMMSCs modulate the apoptosis via direct and indirect pathways. Direct pathway includes immediate effects on apoptotic cascade, such as the inhibition of caspase-3 and the enhancement of survivin expression.110,111 Indirect pathway may involve other mediators such as nuclear factor p53 and neuroprotective cytokines BDNF, NGF, IGF-1, and VEGF.112,113 BMMSCs can decrease Aβ-induced apoptotic cell death in the primary culture of hippocampal neurons.114 The transplantation of stem cells suppresses apoptosis and contributes to the functional remodeling of synaptic plasticity.15,22,26,110,115–117 Apoptosis mechanism also regulates the survival of transplanted BMMSCs in the brain, which affects the therapeutic efficiency of stem cells.65
- Oxidative stress. The accumulation of Aβ peptides induces the production of free radicals, oxidative stress, and lipid peroxidation. The transplanted BMMSCs can mitigate Aβ deposits and ameliorate Aβ-induced oxidative stress in Aβ-injected mice, which improve spatial memory impairment in the hippocampus.26,115 Oxidative stress and free radicals in neurons stimulate the release of cytochrome c and the activation of caspase-9, which induces intrinsic apoptosis in the pathogenesis of Alzheimer’s disease. The transfused MSCs alleviate ROS-induced damage and initiate neuroprotective mechanisms via the combined action of neurotrophic factors NGF and BDNF.112
The functional activities of transplanted stem cells involve distinct mechanisms such as inflammation, immunoregulation, autophagy, apoptosis, angiogenesis, and synaptogenesis. Their comprehensive role changes the pathological state in the hippocampus and establishes a new dynamic balance by integrating various signal pathways. The novel balance in the hippocampal microenvironment is a key mechanism by which transplanted BMMSCs alleviate neuropathology and improve cognitive impairment in animal models with Alzheimer’s disease.22,26,62,86
Challenge and Perspective
- Uncertainty. Following the transplantation of BMMSCs, a novel balance is established based on dynamic remodeling, but it is unsure how long the functional state of new balance can be maintained.
- Stem cell parameters. In order to achieve therapeutic effects, it may be necessary to transplant BMMSCs repeatedly. At this moment, relevant parameters on the transplantation of BMMSCs need to be determined, including stem cell concentration, time interval, inoculation position, and delivery method. At present, the most important task is to standardize the protocol for BMMSC administration.
- Biomarkers for surveillance. Currently, monitoring markers (i.e., Aβ42, T-Tau and P-Tau, or exosomes in cerebrospinal fluid and/or peripheral bloodstream) need to be optimized for the evaluation of therapeutic effects.
- The integration of various mechanisms. Transplanted BMMSCs have diverse functions such as immunoregulation, anti-apoptosis, neurogenesis, the activation of autophagy, and angiogenesis. Meantime, immunoregulation can interact with different mechanisms, which is a key regulator in the pathogenesis of AD. However, some details need to be elucidated, including inflammation and synaptic remodeling, the interaction between astrocytes and microglia, and inflammation and autophagy.
- Exosomes. During stem cell therapy, stem cells can produce extracellular vesicles or exosomes to communicate with recipient cells. In transgenic APP/PS1 mice, exosome-mediated immunomodulation and neuroprotection are similar to transplanted stem cells.94,118 However, there are still many influencing variables. The therapeutic advantages of stem cells and exosomes will be determined through parallel comparative studies in the future.
Summary
The transplantation of BMMSCs can improve memory and cognitive deficits by alleviating neuropathology in animal models with Alzheimer’s disease. The underlying mechanisms involve (i) the inhibition of neuroinflammation; (ii) the migration of Aβ and Tau pathology through immunoregulation; (iii) the attenuation of neuronal apoptosis by reducing oxidative stress and ROS generation; (iv) other effects, such as neurogenesis, synaptic plasticity, autophagy, and angiogenesis. The therapeutic effect of stem cells comes from the integral regulation of different mechanisms. The transplantation of BMMSCs acts as a new balance driver and leads to beneficial improvements in AD-like animals. Stem cell therapy may be prospective for the patients with Alzheimer’s disease.
Abbreviation
AD, Alzheimer’s disease; BMMSCs, bone marrow-derived mesenchymal stem cells; Aβ protein, amyloid-beta protein; hBMSCs, human bone marrow stem cells; hMSC, human mesenchymal stem cells; PPARγ, peroxisome proliferator-activated receptor γ; RXRs, retinoid X receptors; FGF, fibroblast growth factor; TIMP-1, tissue inhibitor of metalloproteinases-1; TIMP-2, tissue inhibitor of metalloproteinases-2; CINC-1, cytokine-induced neutrophil chemoattractant-1; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α; G-CSF, granulocyte-colony stimulating factor; GM-CSF granulocyte-macrophage colony-stimulating factor; SCF, stem cell factor; IFN-γ, interferon-γ; Seladin-1, selective Alzheimer’s disease indicator-1; Nrf2, nuclear factor erythroid 2–related factor 2; VEGF-A, vascular endothelial growth factor A; NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; CREB, cAMP response element binding; APP, amyloid precursor protein; GSK-3β, glycogen synthase kinase-3β; SCD-1, stromal cell–derived factor-1α; CXCL-12, C-X-C motif chemokine 12; CXCL-10, C-X-C-motif ligand 10; CCL5, chemokine (C-C motif) ligand 5; Ang-1, angiopoietin-1; Ang-2, angiopoietin-2; AIF, apoptosis-inducing factor; PCNA, proliferating cell nuclear antigen; SDF-1, stromal cell-derived factor 1; HGF, hepatocyte growth factor.
Funding
This work was supported by Beijing Natural Science Foundation (#517100) and National Key Research and Development Project (No. 2017YFA0105200).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–344. doi:10.1056/NEJMra0909142
2. Tang M, Ryman DC, McDade E, et al. Neurological manifestations of autosomal dominant familial Alzheimer’s disease: a comparison of the published literature with the Dominantly Inherited Alzheimer Network observational study (DIAN-OBS). Lancet Neurol. 2016;15(13):1317–1325. doi:10.1016/S1474-4422(16)30229-0
3. Fertan E, Rodrigues GJ, Wheeler RV, et al. Cognitive Decline, Cerebral-Spleen Tryptophan Metabolism, Oxidative Stress, Cytokine Production, and Regulation of the Txnip Gene in a Triple Transgenic Mouse Model of Alzheimer Disease. Am J Pathol. 2019;189(7):1435–1450. doi:10.1016/j.ajpath.2019.03.006
4. Polito VA, Li H, Martini-Stoica H, et al. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol Med. 2014;6(9):1142–1160. doi:10.15252/emmm.201303671
5. Sajjad N, Wani A, Sharma A, et al. Artemisia amygdalina Upregulates Nrf2 and Protects Neurons Against Oxidative Stress in Alzheimer Disease. Cell Mol Neurobiol. 2019;39(3):387–399. doi:10.1007/s10571-019-00656-w
6. Santos RX, Correia SC, Wang X, et al. A synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer’s disease. J Alzheimers Dis. 2010;20(Suppl 2):S401–12. doi:10.3233/JAD-2010-100666
7. Ko YJ, Ko IG. Voluntary Wheel Running Improves Spatial Learning Memory by Suppressing Inflammation and Apoptosis via Inactivation of Nuclear Factor Kappa B in Brain Inflammation Rats. Int Neurourol J. 2020;24(Suppl 2):96–103. doi:10.5213/inj.2040432.216
8. Alexandraki KI, Apostolopoulos NV, Adamopoulos C, et al. Differential Expression of Apoptotic and Low-Grade Inflammatory Markers in Alzheimer Disease Compared to Diabetes Mellitus Type 1 and 2. J Appl Lab Med. 2019;3(6):1003–1013. doi:10.1373/jalm.2018.027623
9. Kim SH, Ko YJ, Kim JY, Sim YJ. Treadmill Running Improves Spatial Learning Memory Through Inactivation of Nuclear Factor Kappa B/Mitogen-Activated Protein Kinase Signaling Pathway in Amyloid-beta-Induced Alzheimer Disease Rats. Int Neurourol J. 2021;25(Suppl 1):S35–43. doi:10.5213/inj.2142164.082
10. Verma S, Kumar A, Tripathi T, Kumar A. Muscarinic and nicotinic acetylcholine receptor agonists: current scenario in Alzheimer’s disease therapy. J Pharm Pharmacol. 2018;70(8):985–993. doi:10.1111/jphp.12919
11. Connelly PJ, Adams F, Tayar ZI, Khan F. Peripheral vascular responses to acetylcholine as a predictive tool for response to cholinesterase inhibitors in Alzheimer’s disease. BMC Neurol. 2019;19(1):88. doi:10.1186/s12883-019-1316-4
12. Huber CM, Yee C, May T, Dhanala A, Mitchell CS. Cognitive Decline in Preclinical Alzheimer’s Disease: amyloid-Beta versus Tauopathy. J Alzheimers Dis. 2018;61(1):265–281. doi:10.3233/JAD-170490
13. White JD, Urbano CM, Taylor JO, et al. Intraventricular murine Abeta infusion elicits hippocampal inflammation and disrupts the consolidation, but not retrieval, of conditioned fear in C57BL6/J mice. Behav Brain Res. 2020;378:112303. doi:10.1016/j.bbr.2019.112303
14. Huang HJ, Chen SL, Huang HY, et al. Chronic low dose of AM404 ameliorates the cognitive impairment and pathological features in hyperglycemic 3xTg-AD mice. Psychopharmacol Berl. 2019;236(2):763–773. doi:10.1007/s00213-018-5108-0
15. Lee M, Ban JJ, Yang S, Im W, Kim M. The exosome of adipose-derived stem cells reduces beta-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res. 2018;1691:87–93. doi:10.1016/j.brainres.2018.03.034
16. Ehrhart J, Darlington D, Kuzmin-Nichols N, et al. Biodistribution of Infused Human Umbilical Cord Blood Cells in Alzheimer’s Disease-Like Murine Model. Cell Transpl. 2016;25(1):195–199. doi:10.3727/096368915X689604
17. Reyes S, Tajiri N, Borlongan CV. Developments in intracerebral stem cell grafts. Expert Rev Neurother. 2015;15(4):381–393. doi:10.1586/14737175.2015.1021787
18. Cha MY, Kwon YW, Ahn HS, et al. Protein-Induced Pluripotent Stem Cells Ameliorate Cognitive Dysfunction and Reduce Abeta Deposition in a Mouse Model of Alzheimer’s Disease. Stem Cells Transl Med. 2017;6(1):293–305. doi:10.5966/sctm.2016-0081
19. Fujiwara N, Shimizu J, Takai K, et al. Cellular and molecular mechanisms of the restoration of human APP transgenic mouse cognitive dysfunction after transplant of human iPS cell-derived neural cells. Exp Neurol. 2015;271:423–431. doi:10.1016/j.expneurol.2015.07.008
20. Shen Z, Li X, Bao X, Wang R. Microglia-targeted stem cell therapies for Alzheimer disease: a preclinical data review. J Neurosci Res. 2017;95(12):2420–2429. doi:10.1002/jnr.24066
21. Naaldijk Y, Jager C, Fabian C, et al. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol. 2017;43(4):299–314. doi:10.1111/nan.12319
22. Bae JS, Jin HK, Lee JK, Richardson JC, Carter JE. Bone marrow-derived mesenchymal stem cells contribute to the reduction of amyloid-beta deposits and the improvement of synaptic transmission in a mouse model of pre-dementia Alzheimer’s disease. Curr Alzheimer Res. 2013;10(5):524–531. doi:10.2174/15672050113109990027
23. Tang X, Chen F, Lin Q, You Y, Ke J, Zhao S. Bone marrow mesenchymal stem cells repair the hippocampal neurons and increase the expression of IGF-1 after cardiac arrest in rats. Exp Ther Med. 2017;14(5):4312–4320. doi:10.3892/etm.2017.5059
24. Hammond TR, Dufort C, Dissing-Olesen L, et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity. 2019;50(1):253–271 e6. doi:10.1016/j.immuni.2018.11.004
25. Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol. 2019;98(5–8):151041. doi:10.1016/j.ejcb.2019.04.002
26. Qin C, Lu Y, Wang K, et al. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer’s disease: a meta-analytic review on potential mechanisms. Transl Neurodegener. 2020;9(1):20. doi:10.1186/s40035-020-00199-x
27. Danielyan L, Beer-Hammer S, Stolzing A, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transpl. 2014;23(Suppl 1):S123–39. doi:10.3727/096368914X684970
28. Wang X, Ma S, Yang B, et al. Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer’s disease. Behav Brain Res. 2018;339:297–304. doi:10.1016/j.bbr.2017.10.032
29. Liu Z, Wang C, Wang X, Xu S. Therapeutic Effects of Transplantation of As-MiR-937-Expressing Mesenchymal Stem Cells in Murine Model of Alzheimer’s Disease. Cell Physiol Biochem. 2015;37(1):321–330. doi:10.1159/000430356
30. Nasiri E, Alizadeh A, Roushandeh AM, Gazor R, Hashemi-Firouzi N, Golipoor Z. Melatonin-pretreated adipose-derived mesenchymal stem cells efficiently improved learning, memory, and cognition in an animal model of Alzheimer’s disease. Metab Brain Dis. 2019;34(4):1131–1143. doi:10.1007/s11011-019-00421-4
31. Kanamaru T, Kamimura N, Yokota T, et al. Intravenous transplantation of bone marrow-derived mononuclear cells prevents memory impairment in transgenic mouse models of Alzheimer’s disease. Brain Res. 2015;1605:49–58. doi:10.1016/j.brainres.2015.02.011
32. Harach T, Jammes F, Muller C, et al. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2017;51:83–96. doi:10.1016/j.neurobiolaging.2016.11.009
33. Hwang JH, Shim SS, Seok OS, et al. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci. 2009;24(4):547–554. doi:10.3346/jkms.2009.24.4.547
34. Fathi E, Farahzadi R, Valipour B, Sanaat Z. Cytokines secreted from bone marrow derived mesenchymal stem cells promote apoptosis and change cell cycle distribution of K562 cell line as clinical agent in cell transplantation. PLoS One. 2019;14(4):e0215678. doi:10.1371/journal.pone.0215678
35. Jin HJ, Bae YK, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–18001. doi:10.3390/ijms140917986
36. Yoon YS, Wecker A, Heyd L, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115(2):326–338. doi:10.1172/JCI22326
37. Chen Q, Liu Y, Ding X, et al. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem. 2020;465(1–2):103–114. doi:10.1007/s11010-019-03671-z
38. Iso Y, Usui S, Toyoda M, Spees JL, Umezawa A, Suzuki H. Bone marrow-derived mesenchymal stem cells inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia after arterial injury in rats. Biochem Biophys Rep. 2018;16:79–87. doi:10.1016/j.bbrep.2018.10.001
39. Cho GW, Noh MY, Kim HY, Koh SH, Kim KS, Kim SH. Bone marrow-derived stromal cells from amyotrophic lateral sclerosis patients have diminished stem cell capacity. Stem Cells Dev. 2010;19(7):1035–1042. doi:10.1089/scd.2009.0453
40. Sun L, Fan X, Zhang L, et al. Bone mesenchymal stem cell transplantation via four routes for the treatment of acute liver failure in rats. Int J Mol Med. 2014;34(4):987–996. doi:10.3892/ijmm.2014.1890
41. Yagi H, Parekkadan B, Suganuma K, et al. Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng Part A. 2009;15(11):3377–3388. doi:10.1089/ten.TEA.2008.0681
42. Wang YH, Wu DB, Chen B, Chen EQ, Tang H. Progress in mesenchymal stem cell-based therapy for acute liver failure. Stem Cell Res Ther. 2018;9(1):227. doi:10.1186/s13287-018-0972-4
43. Li T, Zhu J, Ma K, et al. Autologous bone marrow-derived mesenchymal stem cell transplantation promotes liver regeneration after portal vein embolization in cirrhotic rats. J Surg Res. 2013;184(2):1161–1173. doi:10.1016/j.jss.2013.04.054
44. Wang K, Chen X, Ren J. Autologous bone marrow stem cell transplantation in patients with liver failure: a meta-analytic review. Stem Cells Dev. 2015;24(2):147–159. doi:10.1089/scd.2014.0337
45. Rodriguez-Fuentes DE, Fernandez-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldana HA. Mesenchymal Stem Cells Current Clinical Applications: a Systematic Review. Arch Med Res. 2021;52(1):93–101. doi:10.1016/j.arcmed.2020.08.006
46. Cheleuitte D, Mizuno S, Glowacki J. In vitro secretion of cytokines by human bone marrow: effects of age and estrogen status. J Clin Endocrinol Metab. 1998;83(6):2043–2051. doi:10.1210/jcem.83.6.4848
47. Wei Y, Xie Z, Bi J, Zhu Z. Anti-inflammatory effects of bone marrow mesenchymal stem cells on mice with Alzheimer’s disease. Exp Ther Med. 2018;16(6):5015–5020. doi:10.3892/etm.2018.6857
48. Manshouri T, Estrov Z, Quintas-Cardama A, et al. Bone marrow stroma-secreted cytokines protect JAK2(V617F)-mutated cells from the effects of a JAK2 inhibitor. Cancer Res. 2011;71(11):3831–3840. doi:10.1158/0008-5472.CAN-10-4002
49. Lee JK, Schuchman EH, Jin HK, Bae JS. Soluble CCL5 derived from bone marrow-derived mesenchymal stem cells and activated by amyloid beta ameliorates Alzheimer’s disease in mice by recruiting bone marrow-induced microglia immune responses. Stem Cells. 2012;30(7):1544–1555. doi:10.1002/stem.1125
50. Gordon PM, Dias S, Williams DA. Cytokines secreted by bone marrow stromal cells protect c-KIT mutant AML cells from c-KIT inhibitor-induced apoptosis. Leukemia. 2014;28(11):2257–2260. doi:10.1038/leu.2014.212
51. Guo HD, Wang HJ, Tan YZ, Wu JH. Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng Part A. 2011;17(1–2):45–58. doi:10.1089/ten.TEA.2010.0124
52. Zhou X, Xiu G, Zhu Y, et al. [Bone marrow mesenchymal stem cells modulated the inflammatory response by regulating the expression of IL-4 and RAGE products in the rats with MODS]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29(4):294–299. doi:10.3760/cma.j.issn.2095-4352.2017.04.002. Chinese.
53. Chen S, Yi M, Zhou G, et al. Abdominal Aortic Transplantation of Bone Marrow Mesenchymal Stem Cells Regulates the Expression of Ciliary Neurotrophic Factor and Inflammatory Cytokines in a Rat Model of Spinal Cord Ischemia-Reperfusion Injury. Med Sci Monit. 2019;25:1960–1969. doi:10.12659/MSM.912697
54. Park CW, Kim KS, Bae S, et al. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells. 2009;2(1):59–68. doi:10.15283/ijsc.2009.2.1.59
55. Hwang JH, Lee MJ, Seok OS, et al. Cytokine expression in placenta-derived mesenchymal stem cells in patients with pre-eclampsia and normal pregnancies. Cytokine. 2010;49(1):95–101. doi:10.1016/j.cyto.2009.08.013
56. Madlambayan GJ, Butler JM, Hosaka K, et al. Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood. 2009;114(19):4310–4319. doi:10.1182/blood-2009-03-211342
57. Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28(2):329–343. doi:10.1002/stem.277
58. Norddahl GL, Wahlestedt M, Gisler S, Sigvardsson M, Bryder D. Reduced repression of cytokine signaling ameliorates age-induced decline in hematopoietic stem cell function. Aging Cell. 2012;11(6):1128–1131. doi:10.1111/j.1474-9726.2012.00863.x
59. Masiukiewicz US, Mitnick M, Gulanski BI, Insogna KL. Evidence that the IL-6/IL-6 soluble receptor cytokine system plays a role in the increased skeletal sensitivity to PTH in estrogen-deficient women. J Clin Endocrinol Metab. 2002;87(6):2892–2898. doi:10.1210/jcem.87.6.8577
60. Zhao R, Ying M, Gu S, et al. Cysteinyl Leukotriene Receptor 2 is Involved in Inflammation and Neuronal Damage by Mediating Microglia M1/M2 Polarization through NF-kappaB Pathway. Neuroscience. 2019;422:99–118. doi:10.1016/j.neuroscience.2019.10.048
61. Losurdo M, Pedrazzoli M, D’Agostino C, et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl Med. 2020;9(9):1068–1084. doi:10.1002/sctm.19-0327
62. Esmaeilzade B, Artimani T, Amiri I, et al. Dimethyloxalylglycine preconditioning enhances protective effects of bone marrow-derived mesenchymal stem cells in Abeta- induced Alzheimer disease. Physiol Behav. 2019;199:265–272. doi:10.1016/j.physbeh.2018.11.034
63. Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22(3):1428–1442. doi:10.1111/jcmm.13492
64. Costa MHG, Serra J, McDevitt TC, Cabral JMS, da Silva CL, Ferreira FC. Dimethyloxalylglycine, a small molecule, synergistically increases the homing and angiogenic properties of human mesenchymal stromal cells when cultured as 3-D spheroids. Biotechnol J. 2021;16:e2000389. doi:10.1002/biot.202000389
65. Han L, Zhou Y, Zhang R, et al. MicroRNA Let-7f-5p Promotes Bone Marrow Mesenchymal Stem Cells Survival by Targeting Caspase-3 in Alzheimer Disease Model. Front Neurosci. 2018;12:333. doi:10.3389/fnins.2018.00333
66. Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr Res. 2018;83(1–2):214–222. doi:10.1038/pr.2017.249
67. Rahmat Z, Jose S, Ramasamy R, Vidyadaran S. Reciprocal interactions of mouse bone marrow-derived mesenchymal stem cells and BV2 microglia after lipopolysaccharide stimulation. Stem Cell Res Ther. 2013;4(1):12. doi:10.1186/scrt160
68. Wen T, Meng H, Wang F, Chen Y, Sun T. Early immune response regulated by a bone marrow mesenchymal stem cell model of multiple trauma in rats. Immunotherapy. 2018;10(12):1053–1064. doi:10.2217/imt-2018-0010
69. Guk KD, Kuprash DV. [Interleukin-11, an IL-6 like cytokine]. Mol Biol Mosk. 2011;45(1):44–55.
70. Jiang Y, Gao H, Yuan H, et al. Amelioration of postoperative cognitive dysfunction in mice by mesenchymal stem cell-conditioned medium treatments is associated with reduced inflammation, oxidative stress and increased BDNF expression in brain tissues. Neurosci Lett. 2019;709:134372. doi:10.1016/j.neulet.2019.134372
71. Chen Y, Pan C, Xuan A, et al. Treatment Efficacy of NGF Nanoparticles Combining Neural Stem Cell Transplantation on Alzheimer’s Disease Model Rats. Med Sci Monit. 2015;21:3608–3615. doi:10.12659/msm.894567
72. Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173(4):649–665. doi:10.1111/bph.13139
73. Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19(8):987–991. doi:10.1038/nn.4338
74. Noristani HEH, Perrin FE. Microglia Responses in Acute and Chronic Neurological Diseases: what Microglia-Specific Transcriptomic Studies Taught (and did Not Teach) Us. Front Aging Neurosci. 2017;9:227. doi:10.3389/fnagi.2017.00227
75. von Maydell D, Jorfi M. The interplay between microglial states and major risk factors in Alzheimer’s disease through the eyes of single-cell RNA-sequencing: beyond black and white. J Neurophysiol. 2019;122(4):1291–1296. doi:10.1152/jn.00395.2019
76. Zhu D, Yang N, Liu YY, Zheng J, Ji C, Zuo PP. M2 Macrophage Transplantation Ameliorates Cognitive Dysfunction in Amyloid-beta-Treated Rats Through Regulation of Microglial Polarization. J Alzheimers Dis. 2016;52(2):483–495. doi:10.3233/JAD-151090
77. Terashima T, Nakae Y, Katagi M, Okano J, Suzuki Y, Kojima H. Stem cell factor induces polarization of microglia to the neuroprotective phenotype in vitro. Heliyon. 2018;4(10):e00837. doi:10.1016/j.heliyon.2018.e00837
78. Oh S, Son M, Choi J, Lee S, Byun K. sRAGE prolonged stem cell survival and suppressed RAGE-related inflammatory cell and T lymphocyte accumulations in an Alzheimer’s disease model. Biochem Biophys Res Commun. 2018;495(1):807–813. doi:10.1016/j.bbrc.2017.11.035
79. Meda L, Cassatella MA, Szendrei GI, et al. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374(6523):647–650. doi:10.1038/374647a0
80. von Saucken VE, Jay TR, Landreth GE. The effect of amyloid on microglia-neuron interactions before plaque onset occurs independently of TREM2 in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2020;145:105072. doi:10.1016/j.nbd.2020.105072
81. Casali BT, MacPherson KP, Reed-Geaghan EG, Landreth GE. Microglia depletion rapidly and reversibly alters amyloid pathology by modification of plaque compaction and morphologies. Neurobiol Dis. 2020;142:104956. doi:10.1016/j.nbd.2020.104956
82. Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neurosci Lett. 2009;450(2):136–141. doi:10.1016/j.neulet.2008.11.059
83. Kim M, Kim KH, Song SU, et al. Transplantation of human bone marrow-derived clonal mesenchymal stem cells reduces fibrotic scar formation in a rat spinal cord injury model. J Tissue Eng Regen Med. 2018;12(2):e1034–e1045. doi:10.1002/term.2425
84. Lively S, Schlichter LC. Microglia Responses to Pro-inflammatory Stimuli (LPS, IFNgamma+TNFalpha) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front Cell Neurosci. 2018;12:215. doi:10.3389/fncel.2018.00215
85. Szczepanik AM, Funes S, Petko W, Ringheim GE. IL-4, IL-10 and IL-13 modulate A beta(1–42)-induced cytokine and chemokine production in primary murine microglia and a human monocyte cell line. J Neuroimmunol. 2001;113(1):49–62. doi:10.1016/s0165-5728(00)00404-5
86. Yu S, Hei Y, Liu W. Upregulation of seladin-1 and nestin expression in bone marrow mesenchymal stem cell transplantation via the ERK1/2 and PI3K/Akt signaling pathways in an Alzheimer’s disease model. Oncol Lett. 2018;15(5):7443–7449. doi:10.3892/ol.2017.7543
87. Luciani P, Deledda C, Rosati F, et al. Seladin-1 is a fundamental mediator of the neuroprotective effects of estrogen in human neuroblast long-term cell cultures. Endocrinology. 2008;149(9):4256–4266. doi:10.1210/en.2007-1795
88. Schafer S, Calas AG, Vergouts M, Hermans E. Immunomodulatory influence of bone marrow-derived mesenchymal stem cells on neuroinflammation in astrocyte cultures. J Neuroimmunol. 2012;249(1–2):40–48. doi:10.1016/j.jneuroim.2012.04.018
89. Duarte RF, Frank DA. SCF and G-CSF lead to the synergistic induction of proliferation and gene expression through complementary signaling pathways. Blood. 2000;96(10):3422–3430. doi:10.1182/blood.V96.10.3422
90. Prakash A, Medhi B, Chopra K. Granulocyte colony stimulating factor (GCSF) improves memory and neurobehavior in an amyloid-beta induced experimental model of Alzheimer’s disease. Pharmacol Biochem Behav. 2013;110:46–57. doi:10.1016/j.pbb.2013.05.015
91. Tsai KJ, Tsai YC, Shen CK. G-CSF rescues the memory impairment of animal models of Alzheimer’s disease. J Exp Med. 2007;204(6):1273–1280. doi:10.1084/jem.20062481
92. Cao C, Wang L, Lin X, et al. Caffeine synergizes with another coffee component to increase plasma GCSF: linkage to cognitive benefits in Alzheimer’s mice. J Alzheimers Dis. 2011;25(2):323–335. doi:10.3233/JAD-2011-110110
93. Ji J, Xue TF, Guo XD, et al. Antagonizing peroxisome proliferator-activated receptor gamma facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell. 2018;17(4):e12774. doi:10.1111/acel.12774
94. Cui GH, Wu J, Mou FF, et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32(2):654–668. doi:10.1096/fj.201700600R
95. Kempuraj D, Thangavel R, Natteru PA, et al. Neuroinflammation Induces Neurodegeneration. J Neurol Neurosurg Spine. 2016;1(1):587.
96. Liu W, Rong Y, Wang J, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17(1):47. doi:10.1186/s12974-020-1726-7
97. Zhong Z, Chen A, Fa Z, et al. Adipose-Derived Stem Cells Modulate BV2 Microglial M1/M2 Polarization by Producing GDNF. Stem Cells Dev. 2020;29(11):714–727. doi:10.1089/scd.2019.0235
98. Sackmann V, Ansell A, Sackmann C, et al. Anti-inflammatory (M2) macrophage media reduce transmission of oligomeric amyloid beta in differentiated SH-SY5Y cells. Neurobiol Aging. 2017;60:173–182. doi:10.1016/j.neurobiolaging.2017.08.022
99. Boyette LB, Macedo C, Hadi K, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12(4):e0176460. doi:10.1371/journal.pone.0176460
100. Choudhury ME, Sugimoto K, Kubo M, et al. A cytokine mixture of GM-CSF and IL-3 that induces a neuroprotective phenotype of microglia leading to amelioration of (6-OHDA)-induced Parkinsonism of rats. Brain Behav. 2011;1(1):26–43. doi:10.1002/brb3.11
101. Safar MM, Arab HH, Rizk SM, El-Maraghy SA. Bone Marrow-Derived Endothelial Progenitor Cells Protect Against Scopolamine-Induced Alzheimer-Like Pathological Aberrations. Mol Neurobiol. 2016;53(3):1403–1418. doi:10.1007/s12035-014-9051-8
102. Nakano M, Kubota K, Kobayashi E, et al. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10(1):10772. doi:10.1038/s41598-020-67460-1
103. Li S, Guan H, Zhang Y, et al. Bone marrow mesenchymal stem cells promote remyelination in spinal cord by driving oligodendrocyte progenitor cell differentiation via TNFalpha/RelB-Hes1 pathway: a rat model study of 2,5-hexanedione-induced neurotoxicity. Stem Cell Res Ther. 2021;12(1):436. doi:10.1186/s13287-021-02518-z
104. Monsonego A, Imitola J, Zota V, Oida T, Weiner HL. Microglia-mediated nitric oxide cytotoxicity of T cells following amyloid beta-peptide presentation to Th1 cells. J Immunol. 2003;171(5):2216–2224. doi:10.4049/jimmunol.171.5.2216
105. Zarif H, Hosseiny S, Paquet A, et al. CD4(+) T Cells Have a Permissive Effect on Enriched Environment-Induced Hippocampus Synaptic Plasticity. Front Synaptic Neurosci. 2018;10:14. doi:10.3389/fnsyn.2018.00014
106. Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci. 2012;32(48):17321–17331. doi:10.1523/JNEUROSCI.1569-12.2012
107. Boche D, Nicoll JAR. Invited Review - Understanding cause and effect in Alzheimer’s pathophysiology: implications for clinical trials. Neuropathol Appl Neurobiol. 2020;46(7):623–640. doi:10.1111/nan.12642
108. Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88(4):640–651. doi:10.1016/j.bcp.2013.12.024
109. Kang HJ, Yoon WJ, Moon GJ, et al. Caspase-3-mediated cleavage of PHF-1 tau during apoptosis irrespective of excitotoxicity and oxidative stress: an implication to Alzheimer’s disease. Neurobiol Dis. 2005;18(3):450–458. doi:10.1016/j.nbd.2004.12.004
110. Mo SJ, Zhong Q, Zhou YF, Deng DB, Zhang XQ. Bone marrow-derived mesenchymal stem cells prevent the apoptosis of neuron-like PC12 cells via erythropoietin expression. Neurosci Lett. 2012;522(2):92–97. doi:10.1016/j.neulet.2012.06.002
111. Okazaki T, Magaki T, Takeda M, et al. Intravenous administration of bone marrow stromal cells increases survivin and Bcl-2 protein expression and improves sensorimotor function following ischemia in rats. Neurosci Lett. 2008;430(2):109–114. doi:10.1016/j.neulet.2007.10.046
112. Liu L, Cao JX, Sun B, et al. Mesenchymal stem cells inhibition of chronic ethanol-induced oxidative damage via upregulation of phosphatidylinositol-3-kinase/Akt and modulation of extracellular signal-regulated kinase 1/2 activation in PC12 cells and neurons. Neuroscience. 2010;167(4):1115–1124. doi:10.1016/j.neuroscience.2010.01.057
113. Chen J, Li Y, Zhang R, et al. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005(1–2):21–28. doi:10.1016/j.brainres.2003.11.080
114. Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells attenuate amyloid beta-induced memory impairment and apoptosis by inhibiting neuronal cell death. Curr Alzheimer Res. 2010;7(6):540–548. doi:10.2174/156720510792231739
115. Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells attenuate amyloid beta-induced memory impairment and apoptosis by inhibiting neuronal cell death. Curr Alzheimer Res. 2010;7(6):540–548.
116. Marei HE, Farag A, Althani A, et al. Human olfactory bulb neural stem cells expressing hNGF restore cognitive deficit in Alzheimer’s disease rat model. J Cell Physiol. 2015;230(1):116–130. doi:10.1002/jcp.24688
117. Bae JS, Han HS, Youn DH, et al. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells. 2007;25(5):1307–1316. doi:10.1634/stemcells.2006-0561
118. Harrell CR, Volarevic A, Djonov V, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes as New Remedy for the Treatment of Neurocognitive Disorders. Int J Mol Sci. 2021;22(3):58. doi:10.3390/ijms22031433
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.