Back to Journals » OncoTargets and Therapy » Volume 13
FTY720 Suppresses Liver Tumor Growth and Metastasis by Reducing Circulating Regulating T Cells and Enhancing the Anti-Tumor Effect of Rapamycin
Authors Li CX, Yang XX, Wang HW, Li XC, Ng KTP , Lo CM, Man K
Received 12 October 2019
Accepted for publication 25 February 2020
Published 26 May 2020 Volume 2020:13 Pages 4743—4754
DOI https://doi.org/10.2147/OTT.S234394
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Chang Xian Li,1 Xin Xiang Yang,2 Hong Wei Wang,1 Xiang Cheng Li,1 Kevin Tak-Pan Ng,2 Chung Mau Lo,2 Kwan Man2
1Hepatobiliary Center, The First Affiliated Hospital of Nanjing Medical University, Key Laboratory of Living Donor Liver Transplantation, Nanjing, Jiangsu Province, People’s Republic of China; 2Department of Surgery, The University of Hong Kong, Hong Kong, People’s Republic of China
Correspondence: Kwan Man
Department of Surgery, The University of Hong Kong, L9-55, Faculty of Medicine Building, 21 Sassoon Road, Hong Kong, People’s Republic of China
Tel +852 39179646
Fax +852 39179634
Email [email protected]
Background: In this study, we aimed to study the effect of FTY720 treatment in reducing circulating Tregs level and then suppressing liver tumor metastasis after hepatectomy and I/R injury in animal models. Furthermore, we also investigated the synergistic anti-tumor effect of FTY720 combined with rapamycin on hepatocellular carcinoma.
Methods: The effect of FTY720 on suppressing Tregs mobilization and tumor metastasis after hepatectomy was investigated in an orthotopic liver tumor rat model with hepatectomy and hepatic ischemia/reperfusion (I/R) injury. The synergistic anti-tumor effect of FTY720 combined with rapamycin was further explored both in in vitro functional study and in orthotopic liver tumor mouse model.
Results: In rat model, hepatic I/R promoted tumor metastasis and increased circulating Tregs after hepatectomy. The treatment of FTY720 reduced liver tumor metastasis and the number of circulating Tregs. Furthermore, FTY720 enhanced the anti-tumor capacity of rapamycin by inhibiting tumor cell proliferation and migration in vitro and reducing tumor growth in vivo through suppressing hepatic stellate cell activation and tumor angiogenesis.
Conclusion: FTY720 suppressed liver tumor growth and metastasis by reducing the population of circulating Tregs and enhancing the anti-tumor effect of rapamycin. It was suggested that FTY720 single or combined with rapamycin might provide novel insight for suppressing tumor growth and metastasis for HCC patients.
Keywords: FTY720, rapamycin, regulatory T cells, hepatic ischemia/reperfusion, tumor recurrence
Background
Hepatocellular carcinoma (HCC) is one of the most common cancers and the third-leading cause of cancer-related death worldwide. More than 50% of new cases occur in China.1 Liver surgery such as hepatectomy and liver transplantation are curative treatment options for selected HCC patients.2 However, tumor recurrence and metastasis after surgical treatment is quite common, with 5-year recurrence rates reaching 70% after liver resection and 35% post-liver transplantation.3 Hepatic ischemia reperfusion (I/R) injury is an inevitable consequence during liver surgery.4 Increasing clinical and experimental evidences have shown that hepatic I/R injury promoted tumor recurrence after liver transplantation or liver resection.5–7 Hepatic I/R injury can trigger a series of inflammatory cascades, which may not only activate the cell signaling pathways regulating tumor cell invasion and migration, but also mobilize the circulating progenitor and immune cells including regulatory T cells (Tregs) to facilitate tumor recurrence and metastasis.8,9 Targeting at hepatic I/R injury and reducing Tregs mobilization may be potential therapy for suppressing tumor growth and metastasis after liver surgery for HCC patients.
FTY720 is an immunomodulatory drug and has been approved by the Food and Drug Administration for treating multiple sclerosis through down regulating sphingosine-1 phosphate receptor inhibited lymphocyte egress from lymphoid tissues.10 Baer et al reported that FTY720 inhibited T cell activation through inhibition of distal TCR signaling.11 FTY720 was also demonstrated to prolong allograft survival in several solid organ transplantation by regulating lymphocytes migration and apoptosis.12 Several groups have shown that FTY720 can attenuate hepatic I/R injury by ameliorating acute-phase inflammatory response and up-regulating several protective genes including heat shock proteins and anti-apoptotic genes.13 Furthermore, FTY720 has been demonstrated a strong anti-tumor effect on liver cancer, breast cancer, bladder cancer and so on.14–17 FTY720 enhanced the anti-tumor activity of carboplatin and tamoxifen in a patient-derived xenograft model of ovarian cancer.18 Our previous data also showed that FTY720 suppressed liver tumor metastasis after hepatectomy and I/R injury through reducing the numbers of circulating endothelial progenitor cells.19
In this study, we aimed to study the effect of FTY720 treatment in reducing circulating Tregs and then suppressing liver tumor metastasis in an orthotopic rat liver tumor model. Furthermore, we also investigated whether FTY720 treatment can enhance the anti-tumor effect of rapamycin. The significance of this study will hopefully open a novel therapy to inhibit liver tumor growth and metastasis after liver surgery for HCC patients.
Methods
Rat Liver Tumor Model
The rat liver tumor cell line McA-RH7777 purchased from the American Type Culture Collection (number CRL1601, ATCC, Manassas, VA, USA, 5×105/100ul) was applied for establishing the orthotropic Rat liver tumor model described in our previous paper.19 In order to investigate the role of hepatic I/R injury in liver tumor metastasis, major hepatectomy (the left lobe with tumor) was performed with or without hepatic I/R injury (The right portal vein and hepatic artery were clamped for 30 minutes) after two weeks orthotopic liver tumor implantation. Rats were housed in a standard animal laboratory with free activity and access to water and food. The animals were obtained from Animal Lab of The University of Hong Kong and had been licensed according to Animal Ordinance Chapter 340 by the Department of Health, Hong Kong Special Administrative Region (4325–17). The welfare of the laboratory animals was followed by guidelines of The University of Hong Kong committee on the use of animal livers in teaching & research (M.292/108).
In order to explore the role and mechanism of FTY720 in tumor growth and metastasis after major hepatectomy and I/R injury. The treatment of FTY720 (2 mg/kg) or saline water (control group) were administrated through the portal vein before and after I/R injury. Circulating Tregs were detected at days 0, 1, 3, 7, 14, 21 and 28. The Buffalo rats were sacrificed under anesthesia with overdose pentobarbitone (100mg/kg) at day 28 for investigating tumor metastasis and detecting rat bone-marrow Tregs.
Nude Mice Orthotopic Liver Tumor Model
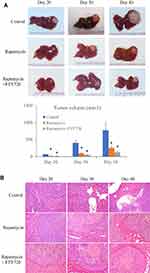
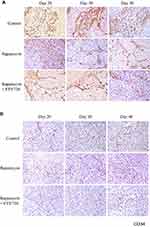
In order to investigate the synergistic anti-tumor effect of FTY720 combined with rapamycin, the nude mice orthotopic liver tumor model was established, which was the same as the rat orthotopic tumor model. One week after tumor implantation, rapamycin alone or combine with FTY720 were used for the treatment of liver cancer. In the single treatment group, rapamycin was given by intraperitoneal injection at 0.8mg/kg/3 days. In the combination treatment group, rapamycin (0.5mg/kg) and FTY720 (2mg/kg) were administrated by intraperitoneal injection every 3 days. The mice were sacrificed at day 20, 30 and 40 after treatment, respectively. The tumor growth, proliferation (Ki67), and apoptosis (TUNEL) were compared among control group, rapamycin alone treatment group and rapamycin combined FTY720 treatment group. Hepatic stellate cell activation and angiogenesis (CD34) in the tumor tissue were compared.
Detection of Circulating and Bone-Marrow Tregs by Flow Cytometry
The numbers of circulating and bone-marrow Tregs were detected by flow cytometry. The details of flow cytometry were described in our previous paper.8 Cells were stained with PE-Cy5-conjugated anti CD4 (eBioscience, San Diego, CA) and PE-conjugated anti CD25 (BD Pharmingen, San Diego, CA), and then permeabilized with fixation/permeabilization working solution and incubated with FITC-conjugated anti-Foxp3 (eBioscience, San Diego, CA). The matched isotype controls were also prepared at the same time.
Hematoxylin and Eosin (H&E), TUNEL and Immunohistochemical Staining
The histological changes and cell TUNEL were detected by H&E staining and TUNEL staining, respectively. The expressions of Foxp3, CD34, ki-67 and α-smooth muscle actin (α-SMA) were detected by immunohistochemical staining (IHC). The details of H & E, TUNEL and IHC staining were described in our previous paper.20
Cell Culture, Colony Formation Assay and Wound Healing Assay
The human liver cancer cell line MHCC97L was obtained from the Liver Cancer institute of Fudan University, Shanghai, the People’s Republic of China. The use of this cell line was approved by The University of Hong Kong institutional research ethics committee. In order to explore the effect of FTY720 combined with rapamycin in proliferation and migration of HCC cells, colony formation assay and wound healing assay were performed by treating the cells with 4 different conditions: (1) control group without any treatment; (2) 1ug/mL of rapamycin; (3) 1uM of FTY720 and (4) 1ug/mL of rapamycin plus 1uM of FTY720.
Statistics and Data Analyses
Continuous variables were expressed as median with range. Mann–Whitney U-test was used for statistical comparison. χ2 test was used to compare incidence of intrahepatic and lung metastasis after hepatectomy and I/R injury. P < 0.05 was considered statistically significant. Calculations were performed by using the SPSS computer software version 15 (SPSS, Chicago, IL).
Results
Hepatic Ischemia-Reperfusion Promoted Liver Tumor Local and Distant Metastasis After Hepatectomy
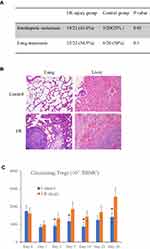
In order to investigate the effect of ischemia-reperfusion on the liver tumor metastasis after hepatectomy, a rat orthotopic liver tumor model with local and distant metastatic potentials was established. Tumor metastasis after hepatectomy is presented in Figure 1A. Compared to the control group, hepatic I/R injury promoted liver tumor intrahepatic and lung metastasis after major hepatectomy. The incidence of intrahepatic metastasis was much higher in the I/R injury group [14/22 (63.6%)] compared to that in the control group [5/20 (25%)]. Furthermore, the incidence of lung metastasis increased from 6/20 (30%) to 12/22 (54.5%) after hepatic I/R injury (Figure 1A). Compared to the control group, the infiltrative growth pattern of the tumor and venous invasion was mainly found in I/R injury group (Figure 1B).
Hepatic Ischemia-Reperfusion Increased the Circulating Tregs After Hepatectomy
Tregs can suppress the immune responses against tumors and thereby promote tumor growth. In order to investigate the role of hepatic I/R injury in Tregs mobilization after hepatectomy, we analyzed the number of Tregs at different time points after hepatectomy with or without hepatic I/R injury. Before hepatectomy, there was no difference of circulating Tregs between the I/R group and control group. The results showed that the number of circulating Tregs was reduced at day 1 after the major hepatectomy in both groups. More circulating Tregs were found in the I/R injury group at day 3, 7, 14 and 28 after hepatectomy than those without I/R injury (Figure 1C).
The Treatment of FTY720 Repressed Liver Tumor Metastasis After Hepatectomy and Hepatic I/R Injury
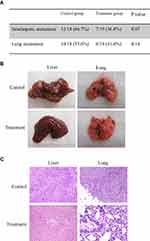
The result showed that compared with control group, the treatment of FTY720 decreased the incidence of intrahepatic metastasis (12/18 (66.7%) vs to 7/19 (36.8%); P =0.07). Similarly, the incidence of lung metastasis was reduced from 10/18 (55.6%) to 6/19 (31.5%) after FTY720 treatment (Figure 2A, B). Intrahepatic and lung metastasis were confirmed by histology examination (Figure 2C).
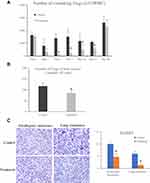
The Treatment of FTY720 Reduced the Number of Circulating and Bone-Marrow Tregs
Before the hepatectomy and I/R injury, there was no difference in circulating Tregs between the treatment group and control group (Figure 3A). The number of circulating Tregs was significantly decreased after FTY720 treatment at day 3, 7 14 and 21 after hepatectomy and I/R injury (Figure 3A). Furthermore, the treatment of FTY720 also decreased the number of Tregs in bone-marrow at day 28 after hepatectomy and I/R injury compared to the control group (116.5 vs 85/105 Cells, P<0.05) (Figure 3B). In current study, we also found that there were less Foxp3 positive cells both in recurrent liver and lung tumors after FTY720 treatment (Figure 3C).
FTY720 Enhanced Anti-Tumor Capacity of Rapamycin by Inhibiting Proliferation and Migration
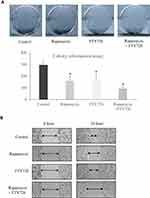
In order to explore whether FTY720 treatment can enhance the anti-tumor effect of rapamycin, colony formation assay and wound healing assay were performed in vitro functional study. In colony formation assay, the result showed that the treatment of FTY720 or rapamycin alone reduced the colony number and size (Figure 4A). More importantly, the colony number and size were significantly decreased in FTY720 combined with rapamycin group. Furthermore, the combination treatment significantly suppressed migration of HCC cells in wound healing assay (Figure 4B).
FTY720 Combined with Rapamycin Suppressed Tumor Growth and Invasiveness in Mice Liver Cancer Model
In nude mice liver cancer model, the treatment of rapamycin suppressed tumor growth at day 20, 30 and 40 after mice orthotopic liver tumor implantation compared with control group (Figure 5A). More importantly, the treatment of FTY720 combined with rapamycin further suppressed tumor growth compared to rapamycin group. A relatively clear margin between tumor and adjacent non-tumor tissues was found in the FTY720 combined with rapamycin group. On the contrary, invasive patterns of tumor growth were present in control group (Figure 5B). Furthermore, compared to control group, there were more apoptotic cells and less ki-67 positive cells in FTY720 combined with rapamycin group (Supplementary Figure 1). The results suggested that the treatment of FTY720 may enhance the anti-tumor growth and invasiveness capacity of rapamycin.
FTY720 Inhibited Hepatic Stellate Cells Activation and Tumor Angiogenesis
The ability to manipulate the microenvironment is one of the hallmarks of promoting tumor progression, invasion and metastasis.21 The effect of FTY720 treatment on tumor-microenvironment was therefore studied from different aspects. Compared to the control group, the number of activated hepatic stellate cells, revealed by immunohistochemical staining of α-SMA protein, was significantly suppressed after FTY720 combined with rapamycin treatment (Figure 6A). In order to study the effect of FTY720 on angiogenesis of HCC, the intra-tumoral CD34 expression which indicated the formation of new vessels was determined. The result showed that FTY720 combined with rapamycin treatment significantly decreased the CD34 expression and microvessel density (MVD) in tumor compared to control group (Figure 6B).
Discussion
Accumulating evidences showed that hepatic I/R injury promoted tumor recurrence and metastasis after liver transplantation or liver resection.5–7 In this study, we confirmed that hepatic I/R injury promoted tumor recurrence and metastasis after hepatectomy in orthotopic rat liver tumor model. Our results also showed that hepatic I/R injury increased the levels of circulating Tregs. Hepatic I/R injury was a typical inflammatory response involving a complex web of interactions between various cellular and molecular signals, which trigger a series of inflammatory cascades such as CXCL10/CXCR3 signaling.21–23 CXCL10 can recruit more Tregs trafficking to the liver through their surface receptor CXCR3.24,25 Our previous study also demonstrated that CXCL10/CXCR3 signaling induced the mobilization and recruitment of Tregs and endothelial progenitor cells, which further promoted liver tumor growth and recurrence after transplantation.8,9,26 Tregs plays important roles in different cancers, which were associated with the mortality.27–29 Tregs can help tumor to escape from the host immunosurveillance by their immunosuppressive activity against effector immune cell response.30 Therefore, it is worthwhile to study whether attenuating hepatic I/R injury and reducing Tregs mobilization and recruitment could suppress liver tumor metastasis after surgical resection.
In the present study, the effect of FTY720 on inhibition of liver tumor metastasis after liver resection and I/R injury was confirmed in an orthotopic rat liver tumor model. FTY720, is synthetically derived from myriocin (ISP-1), a metabolite isolated from ascomycete, Isaria sinclarii. Several groups have shown that FTY720 can attenuate hepatic I/R injury by ameliorating acute phase inflammatory response and up-regulating several protective genes including heat shock proteins and anti-apoptotic genes.13 Our previous study indicated that FTY720 attenuated hepatic I/R injury and down-regulated intracellular mRNA and protein levels of CXCL10 and CXCR3.19 CXCL10/CXCR3 signaling plays an important role in the mobilization of circulating Tregs. Our previous results showed that the knockout of CXCL10 and CXCR3 decreased circulating and hepatic Tregs after hepatectomy and hepatic I/R injury.8 Furthermore, the number of CXCR3+ Tregs recruited into the liver was decreased in CXCL10-/- mice. In the present study, our data showed that FTY720 significantly reduced circulating and bone marrow Tregs after liver resection and hepatic I/R injury. These results suggested that FTY720 inhibited liver tumor metastasis after liver resection and I/R injury maybe through attenuating CXCL10/CXCR3 signaling and decreasing circulatory Tregs. Targeting at hepatic I/R injury and circulating Tregs by FTY720 treatment may effectively decrease tumor recurrence and metastasis after liver surgery. However, the specific mechanisms of these molecules on FTY720-mediated suppression of circulating Tregs need to be further clarified.
In addition to the inhibition of tumor recurrence and Tregs mobilization, we also demonstrated that FTY720 may enhance the anti-tumor effect of rapamycin both in vivo and vitro. Rapamycin is the targeting inhibitor of mammalian target of rapamycin (mTOR), which is a serine/threonine-protein kinase downstream of the phosphoinositide-3-kinase (PI3K)-related kinase family. There is growing evidence to suggest that mTOR is a central regulator of various oncogenic processes including cell growth, proliferation, metabolism, and angiogenesis.31 Blockade of mTOR pathways can promote cell autophagy, induce apoptosis and reduce proliferation in HCC cell lines.32,33 However, clinical trials that have targeted mTOR with rapamycin or rapamycin analogs lonely have had minimal antitumor impact and its efficacy varies by dose in several contexts.34,35 Furthermore, mTOR inhibitors were considered to be attractive immunosuppressants for post-liver transplantation patients with HCC because of its capabilities of effective immunosuppression and concomitant prevention of hepatocellular tumorigenesis.36 Our results showed that FTY720 combined with rapamycin further suppressed tumor growth and invasion compared to rapamycin single treatment. Furthermore, the combination therapy inhibited tumor angiogenesis and hepatic stellate cells activation. These results suggested that FTY720 may enhance the anti-tumor growth and invasiveness capacity of rapamycin.
Conclusions
FTY720 suppressed liver tumor growth and metastasis by reducing the population of circulating Tregs and enhancing the anti-tumor effect of rapamycin (Supplementary figure 2). It was suggested that FTY720 single or combined with rapamycin might provide novel insight for suppressing tumor growth and metastasis for HCC patients.
Abbreviations
I/R, hepatic ischemia/reperfusion; HCC, hepatocellular carcinoma; Tregs, regulatory T cells; mTOR, mammalian target of rapamycin.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article.
Ethics Approval and Consent to Participate
All animal studies were approved by the Department of Health, Hong Kong Special Administrative Region.
Consent for Publication
All authors read and approved the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi:10.1634/theoncologist.2010-S4-05
2. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi:10.1016/S0140-6736(11)61347-0
3. Grazi GL, Ercolani G, Pierangeli F, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234(1):71–78. doi:10.1097/00000658-200107000-00011
4. Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005;37(4):1653–1656. doi:10.1016/j.transproceed.2005.03.134
5. Man K, Ng KT, Lo CM, et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases–activation of cell invasion and migration pathways. Liver Transpl. 2007;13(12):1669–1677. doi:10.1002/lt.21193
6. Nagai S, Yoshida A, Facciuto M, et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology. 2015;61(3):895–904. doi:10.1002/hep.27358
7. Li CX, Man K, Lo CM. The impact of liver graft injury on cancer recurrence posttransplantation. Transplantation. 2017;101(11):2665–2670. doi:10.1097/TP.0000000000001844
8. Li CX, Ling CC, Shao Y, et al. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J Hepatol. 2016;65(5):944–952. doi:10.1016/j.jhep.2016.05.032
9. Ling CC, Ng KT, Shao Y, et al. Post-transplant endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling promotes liver tumor growth. J Hepatol. 2014;60(1):103–109. doi:10.1016/j.jhep.2013.08.017
10. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–415. doi:10.1056/NEJMoa0907839
11. Baer A, Colon-Moran W, Bhattarai N. Characterization of the effects of immunomodulatory drug fingolimod (FTY720) on human T cell receptor signaling pathways. Sci Rep. 2018;8(1):10910. doi:10.1038/s41598-018-29355-0
12. Kimura T, Hasegawa T, Nakai H, et al. FTY720 reduces T-cell recruitment into murine intestinal allograft and prevents activation of graft-infiltrating cells. Transplantation. 2003;75(9):1469–1474. doi:10.1097/01.TP.0000058816.13525.92
13. Man K, Ng KT, Lee TK, et al. FTY720 attenuates hepatic ischemia-reperfusion injury in normal and cirrhotic livers. Am J Transplant. 2005;5(1):40–49. doi:10.1111/j.1600-6143.2004.00642.x
14. Lee TK, Man K, Ho JW, et al. FTY720: a promising agent for treatment of metastatic hepatocellular carcinoma. Clin Cancer Res. 2005;11(23):8458–8466. doi:10.1158/1078-0432.CCR-05-0447
15. Azuma H, Takahara S, Ichimaru N, et al. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62(5):1410–1419.
16. Azuma H, Takahara S, Horie S, Muto S, Otsuki Y, Katsuoka Y. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J Urol. 2003;169(6):2372–2377. doi:10.1097/01.ju.0000064938.32318.91
17. Zhou C, Ling MT, Kin-Wah Lee T, Man K, Wang X, Wong YC. FTY720, a fungus metabolite, inhibits invasion ability of androgen-independent prostate cancer cells through inactivation of RhoA-GTPase. Cancer Lett. 2006;233(1):36–47. doi:10.1016/j.canlet.2005.02.039
18. Kreitzburg KM, Fehling SC, Landen CN, et al. FTY720 enhances the anti-tumor activity of carboplatin and tamoxifen in a patient-derived xenograft model of ovarian cancer. Cancer Lett. 2018;436:75–86. doi:10.1016/j.canlet.2018.08.015
19. Li CX, Shao Y, Ng KT, et al. FTY720 suppresses liver tumor metastasis by reducing the population of circulating endothelial progenitor cells. PLoS One. 2012;7(2):e32380. doi:10.1371/journal.pone.0032380
20. Li CX, Ng KT, Shao Y, et al. The inhibition of aldose reductase attenuates hepatic ischemia-reperfusion injury through reducing inflammatory response. Ann Surg. 2014;260(2):317–328. doi:10.1097/SLA.0000000000000429
21. Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11(8):1563–1569. doi:10.1111/j.1600-6143.2011.03579.x
22. Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6(4):652–658. doi:10.1111/j.1600-6143.2005.01228.x
23. Zhai Y, Shen XD, Gao F, et al. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology. 2008;47(1):207–214. doi:10.1002/hep.21986
24. Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology. 2009;49(4):1267–1276. doi:10.1002/hep.22761
25. Hoerning A, Koss K, Datta D, et al. Subsets of human CD4(+) regulatory T cells express the peripheral homing receptor CXCR3. Eur J Immunol. 2011;41(8):2291–2302. doi:10.1002/eji.201041095
26. Man K, Shih KC, Ng KT, et al. Molecular signature linked to acute phase injury and tumor invasiveness in small-for-size liver grafts. Ann Surg. 2010;251(6):1154–1161. doi:10.1097/SLA.0b013e3181d96e3d
27. Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606–612.
28. Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–4772.
29. Gallimore A, Sakaguchi S. Regulation of tumour immunity by CD25+ T cells. Immunology. 2002;107(1):5–9. doi:10.1046/j.1365-2567.2002.01471.x
30. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi:10.1038/nrc1586
31. Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60(4):855–865. doi:10.1016/j.jhep.2013.11.031
32. Newell P, Toffanin S, Villanueva A, et al. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol. 2009;51(4):725–733. doi:10.1016/j.jhep.2009.03.028
33. Wang SS, Chen YH, Chen N, et al. Hydrogen sulfide promotes autophagy of hepatocellular carcinoma cells through the PI3K/Akt/mTOR signaling pathway. Cell Death Dis. 2017;8(3):e2688. doi:10.1038/cddis.2017.18
34. Mukhopadhyay S, Frias MA, Chatterjee A, Yellen P, Foster DA. The enigma of rapamycin dosage. Mol Cancer Ther. 2016;15(3):347–353. doi:10.1158/1535-7163.MCT-15-0720
35. Mukhopadhyay S, Chatterjee A, Kogan D, Patel D, Foster DA. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) enhances the efficacy of rapamycin in human cancer cells. Cell Cycle. 2015;14(20):3331–3339. doi:10.1080/15384101.2015.1087623
36. Monaco AP. The role of mTOR inhibitors in the management of posttransplant malignancy. Transplantation. 2009;87(2):157–163. doi:10.1097/TP.0b013e318193886e
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.