Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Frequency of Diabetic Ketoacidosis and Its Determinants Among Pediatric Diabetes Mellitus Patients in Northwest Ethiopia
Authors Kidie AA, Lakew AM, Ayele T
Received 28 August 2021
Accepted for publication 4 December 2021
Published 19 December 2021 Volume 2021:14 Pages 4819—4827
DOI https://doi.org/10.2147/DMSO.S326537
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Atitegeb Abera Kidie,1 Ayenew Molla Lakew,2 Tiruneh Ayele2
1Department of Public Health, College of Medicine and Health Sciences, Woldia University, Woldia, Ethiopia; 2Department of Epidemiology and Biostatistics, Institute of Public Health, University of Gondar, Gondar, Ethiopia
Correspondence: Ayenew Molla Lakew Email [email protected]
Background: Diabetic ketoacidosis (DKA) is one of the most common public health problems and is still a major child killer in sub-Saharan African countries, particularly Ethiopia. There are limited and inconclusive data in Amhara regional state; moreover, predictors for the incidence of DKA were not investigated before. Therefore, this study aimed to assess the frequency of DKA and its determinants among pediatric diabetes mellitus patients in public hospitals in northwest Ethiopia.
Methods: An institutional-based retrospective follow-up study was conducted from September 2015 to February 2018 at selected public hospitals in northwest Ethiopia. A simple random sampling method was used to select 389 study subjects. Statistical analysis was done by R-studio version 1.1.4. Akakia’s information criteria was used for model comparison and the negative binomial regression model was fitted to identify determinants for the frequency of DKA. An adjusted incidence rate ratio with 95% confidence interval was used to declare statistical significance.
Results: The average frequency of DKA was 1.01 per individual. The incidence rate of DKA was increased among diabetes mellitus patients with an infection (adjusted incidence rate ratio (AIRR) = 1.41, 95% CI = 1.05– 2.14), heart diseases (AIRR = 4.1, 95% CI = 1.17– 14.68), treatment discontinuation (AIRR = 2.91, 95% CI = 2.02– 4.22), low level of sodium (AIRR = 1.88, 95% CI = 1.22– 2.89) and low dose of treatment at baseline (AIRR = 0.96, 95% CI = 0.94– 0.97).
Conclusion: Having an infection, heart diseases, taking a low dose of treatment, a low sodium level, and treatment discontinuation were the factors that increase the frequency of DKA.
Keywords: frequency, diabetic ketoacidosis, diabetes mellitus, pediatric, northwest Ethiopia
Introduction
Diabetes mellitus (DM) describes a group of metabolic disorders characterized by increased blood glucose concentration; and the main threat to human health in the 21st century.1
In 2017, there were 425 million DM sufferers globally, and 14.2 million of them were in Africa, 77% were in developing countries, and 5.2% of them were in Ethiopia.2
Diabetic ketoacidosis (DKA) is an acute life-threatening complication of diabetes mellitus characterized by the triad of hyperglycemia, acidosis, and ketosis that occurs in the presence of very low levels of effective insulin action.1,3–7 It is one of the common emergencies among patients with diabetes mellitus and is associated with considerable morbidity and mortality.8
It is still the leading cause of death; and mortality risk is substantially increased in patients with chronically poor glycemic control and frequent DKA.3 Frequent DKA is a serious and common health problem.9
Globally, around 65,000 children aged under 15 years develop type 1 diabetes each year and up to 80% present with DKA.10 In Africa, the mortality rate due to DKA is unacceptably higher.8 In developing countries, it ranges from 6% to 24%. In Ethiopia, the risk of dying from DKA is higher due to poor medical services in the country.4,6,11–13
In addition to the risk of mortality, frequent DKA has a major impact on the quality of life of the individuals and their families, accelerated micro-vascular complications, and costs for families and health care.4,9,14
According to studies conducted in different parts of the world, being female, those diagnosed at the younger age, family history of DM, missing an insulin dose, infection, and psychiatric comorbidities were the common determinants of frequent DKA.9,10,15–20,
The Federal Ministry of Health (FMOH) of Ethiopia developed a National Strategic Action plan (NSAP) for four priority Non-Communicable Diseases (NCD), including DM, by focusing on integration to a three-tier health care system. However, due to limited resources, the country mainly focuses on infectious diseases and pays little attention to non-communicable diseases.2 Besides this little attention, the control of diabetes and the prevention of ketoacidosis were hampered by socioeconomic factors; particularly the cost and unreliability of insulin supplies.21 As a result, DKA remains a common public health issue, and a major child killer in sub-Saharan African countries, including Ethiopia.19 Moreover, there is limited data on the incidence of DKA and its determinants in Amhara Regional State, Ethiopia. Therefore, this study assessed the frequency of DKA and its determinants among pediatric diabetes mellitus patients in public hospitals of Amhara Regional State, northwest Ethiopia.
Materials and Methods
Study Design and Setting
An institutional-based retrospective follow-up study was conducted in northwest Ethiopia from September 2015 to February 2018.
The study was conducted at the University of Gondar Specialized and Referral Hospital (UoGSRH) and Felege Hiwot Specialized and Referral Hospital (FHSRH) of Amhara Regional State, northwest Ethiopia. Both hospitals are public hospitals and are expected to serve five million people. Pediatric emergency, inpatient wards, outpatient follow-up, and an Out Patient Department (OPD) for chronic illness are served at both hospitals. A total of 322 and 301 pediatric DM patients were followed at UoGSRH and FHSRH from 2015 to 2018, respectively.
Study Participants
Pediatric DM patients aged <18 years and who started a follow-up from 2015 to 2018 and attend the follow-up clinic for at least two years were included in the study. The option of Signori normalized Poisson proposed methods were used to determine the sample size.21,22 Based on the signori normalized Poisson proposed approach, using a 95% confidence level and 90% power, the final estimated sample size used for this study was 389. Pediatric DM patients with unknown dates of diagnosis and follow-up were excluded from the study.
Data Collection Procedures
Secondary data were retrieved from patient’s charts using a data extraction form. The extraction form was developed after reviewing the patients’ chart and based on the record of variables in the chart. The data were collected by six well-trained nurses with BScs. Two supervisors who have master’s degrees in public health supervised the data collection processes. The principal investigator also monitored the data collection process.
Statistical Analysis
Epi-data version 4.6 was used for data entry. For nutritional measurements, WHO Anthro version 3.2.2 for children under 5 years old and Anthro plus version 1.0.4 for adolescents were used to categorize nutritional variables. Outliers were identified for continuous variables using a box, scatter, and normal Q-Q plots. For those variables which had outliers, a model was fitted with and without outliers independently, estimates and standard error were estimated and compared, and noted that they did not vary significantly. Therefore, the analysis was made using the original data. Multi-collinearity was checked using variance inflation factor (VIF), that had a global VIF of 1.22 and a maximum of 4.7 which were <10, indicating that multi-collinearity was not a problem.
To determine the association of covariates with the frequency of DKA, the Poisson regression model was fitted. The Poisson regression model assumes that the mean and variance of count variable are equal, known as equidispersion. The other assumption is the skewed distribution of the outcome variable. The distribution of frequency of DKA in this study was right-skewed and the variance (1.96) was greater than the mean (1.01), indicating that the assumption of the Poisson regression model, is violated. The violation of such assumptions helps us to consider methods of dispersion tests. Overdispersion of the data was confirmed by estimating the ratio of residual deviance to the degree of freedom and dispersion test under the “AER” package. The ratio was greater than one (1.58) and the dispersion test was also significant (P-value = 0.00015) which indicated there is overdispersion. Therefore, to account for this overdispersed data, the negative binomial model was considered.
A zero-inflated negative binomial model was also fitted to check whether the data had inflated zeros or not, and a Vuong test was used to determine whether the zero-inflated negative binomial regression model is statistically preferred over the negative binomial regression model. Based on the test, the negative binomial model was the preferred model for the data.
Akaike’s Information Criteria (AIC) was also used to select the best fitted model and a model with smaller AIC was preferred. Variables with a p-value of less than 0.2 in the bi-variable analysis were selected to fit the model in the multivariable analysis. P-values less than 0.05 and (adjusted incidence rate ratio) AIRR with 95% CI was used to declare statistically significant predictors in multivariable analysis.
Results
Socio-Demographic Characteristics of Study Participants
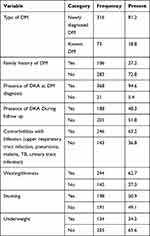
From the total, 389 patients, more than half, 215 (55.3%), of them were males. The average age of the patients was 11 (±4 SD) years. Regarding residence, half, 198 (50.9%), of the patients were from rural areas.
Characteristics of Patients Based on Clinical Variables
Almost all, 382 (98.2%), DM patients had poly symptoms (polyuria, polydipsia, polyphagia) and 109 (28%) had weight loss. About 246 (63%) of the patients had comorbidities with infection (upper respiratory tract infection, pneumonia, malaria, TB, urinary tract infection) and the rest, 18% of them had other comorbidities (trauma, heart diseases, psychiatric illness, asthma, anemia, and skin infections).
One-seventh, 58 (14.9%), of the patients had upper respiratory tract infections, 61 (15.7%) pneumonia, 36 (9.3%) malaria, and the rest of them had other types of infections such as TB, intestinal parasitosis, tinea capitis, scabies, and urinary tract infections. About 26 (6.7%) of the patients had foot ulcers and 41 (10.5%) of them had blurring vision. During the follow up, 79 (20.3%) of the patients had a history of treatment discontinuation. About 111 (28.5%) of the patients had a history of hypoglycemia; and almost all, 368 (94.6%) of them had DKA at diagnosis. Regarding to electrolytes, 124 (31.9%) the patients had normal, while 46 (11.8%) of them had low and 22 (5.7%) had a high level of potassium. Forty-five (11.6%) of the patients had normal, while 125 (32.1%) and 24 (6.2%) of them had low and high levels of sodium, respectively (Table 1).
Pediatric DM Patient Characteristics Based on Measurement Variables
Regarding the baseline measurements, the mean random blood sugar of the patients was 500.8 (±100.9 SD) mg/dl and fasting blood sugar was 272.6 (±80.8 SD) mg/dl. The mean weight was 22.8 (±9.8SD) kg and the creatinine was 0.8 (±0.3 SD) mg/dl (Table 2).
 |
Table 2 Characteristics of Pediatric DM Patients Based on Baseline Measurement Variables at Public Hospitals of Amhara Regional State, Northwest Ethiopia, from 2015 to 2018 |
The Severity of Diabetic Ketoacidosis at DM Diagnosis
Regarding the severity of DKA, 157 (42.7%) of the patients had mild while 101 (27.4%) and 110 (29.9%) of them had moderate and severe DKA at diagnosis, respectively (Figure 1).
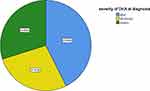 |
Figure 1 Characteristics of pediatric diabetes mellitus patients based on the severity of diabetic ketoacidosis at Public Hospitals of Amhara Regional State, northwest Ethiopia, from 2015 to 2018. |
The glycemic control level meant 132 (33.9%) were well, while 104 (26.7%) and 153 (39.3%) had fairly and poorly controlled glycemic levels.
Precipitating Factors of DKA During Follow-Up
During the follow up, 109 (28%) DKAs were precipitated by infection (5.4% CAP, 10.3% URTI and 2.6% malaria) and 79 (20.3%) of the patients had a history of treatment discontinuation and the rest had other precipitants such as trauma, congenital heart diseases, Down’s syndrome, eating sweet foods/candy and poor injection techniques.
Frequency of DKA
Nearly half, 188 (48.3%) of the DM patients had at least one bout of DKA during the follow up. Among those patients who had DKA, 102 (54.3%) of them were from Felege Hiwot Specialized and Referral Hospital. The minimum and maximum frequencies of DKA were 0 and 6 per individual within the two years of follow-up, respectively. The mean frequency of DKA was 1.01 (Figure 2).
 |
Figure 2 Frequency of diabetic ketoacidosis among pediatric diabetes mellitus patients at Public Hospitals of Amhara Regional State, northwest Ethiopia, from 2015 to 2018. |
Determinants of Frequency of DKA
In the multi-variable analysis, infection, treatment discontinuation, sodium level, heart disease and the dose of treatment were statistically significant variables. The expected mean frequency of DKA was 0.06 when the effect of other covariates were constant. Holding other variables constant, the incidence rate of diabetic acidosis was increased by 41% among DM patients with infection comorbidities (upper respiratory tract infection, pneumonia, malaria, TB, urinary tract infection) compared with those who do not have an infection (AIRR = 1.41, 95% CI = 1.05–2.14). Patients with heart disease as their comorbidity were found to be 4.1 times more likely to have a greater frequency of DKA (AIRR = 4.1, 95% CI = 1.17–14.68) compared to those without heart disease and holding other variables constant.
The incidence rate of DKA was 2.91 times (AIRR = 2.91, 95% CI = 2.02–4.22) greater among DM patients who discontinued their treatment compared to patients who had taken their treatment properly, keeping other variables constant.
Regarding electrolytes, patients with low levels of sodium increased the incidence rate of DKA by 88% (AIRR = 1.88, 95% CI = 1.22–2.89). Increasing the dose of treatment by one international unit (IU) decreased the incidence rate of DKA by 4% (AIRR = 0.96, 95% CI = 0.94–0.97) (Table 3).
Discussion
Acute complications of DM are significant contributors to mortality, and frequent DKA has a major impact on the quality of life of the individuals and their families, accelerating micro-vascular complications and costs for families and health care. This study tried to assess the frequency of DKA and identify factors associated with the frequency of DKA among pediatric diabetes mellitus patients.
The present study showed that the average frequency of DKA is 1.01. The minimum and maximum frequencies of DKA were 0 and 6, respectively. This finding is lower than the multicenter study conducted at University Hospital Birmingham, Birmingham Children’s Hospital, City Hospital, Birmingham Heartlands Hospital, and New Cross Hospital, where the average frequency of DKA was 1.1 and 3.2, respectively.23 Another study in Birmingham, Alabama community teaching hospitals showed that the average frequency of DKA was 2.5.22 This variation may be due to the short duration of follow-up used in this study (2 years) compared to the long-duration (15 years) in Birmingham.
This finding revealed a higher average frequency of DKA than the findings in Iraq, where the average was 0.9.24 A possible reason may be that they used a shorter duration of study, which was one year, than this study, which was two years. A relatively long follow up will identify the extent of the frequency of DKA more accurately than a shorter-duration. Another possible reason may be that the country, Ethiopia, is less developed than Iraq, and the patients may be faced with frequent DKA for various socio-economic reasons.
In terms of factors associated with the frequency of DKA, comorbid infection, heart disease, treatment discontinuation, low levels of sodium and dose of treatment were statistically significant factors. In this study, the proportion of patients with infection was 63.2%, and having comorbidity with infection (upper respiratory tract infection, pneumonia, malaria, TB, urinary tract infection) increases the incidence of DKA, which is supported by the studies conducted in Birmingham, Egypt, Tigray, Tikur Anbessa Specialized Hospital, Jimma University Specialized Hospital, and Kovai Medical Centre.4,11,23,25–27 This may be because of DM results in immune dysfunction and makes the patients susceptible to infection, and increasing its virulence in the hyperglycemic environment and finally precipitating complications like DKA.28,29
Based on this study, having a comorbidity with heart disease significantly increased the frequency of DKA. However, there were no comparable results with this finding. This may be to the fact that other studies may not include heart diseases as associated factor of DKA or might not be significantly associated with the frequency of DKA. The possible reason may be having heart diseases result in multiple organ dysfunctions.
This study found that treatment discontinuation results in an increasing for the incidence of DKA by three fold compared to their counterpart. This finding was comparable with the studies conducted in a tertiary care hospital of Dhaka, Bangladesh, and the Soroka University Medical Center of Israel.23,30,31 This might be because when the patients discontinue their treatment, they may be faced insulin deficiency, increased insulin counter-regulatory hormones, and peripheral insulin resistance lead to hyperglycemia, dehydration, ketosis, and electrolyte imbalance, which underlie the pathophysiology of DKA.32 In this study, patients discontinue their treatment for various reasons, including the cost of treatment or discomfort with the daily injection. Patients with a low level of sodium at baseline had a positive significant effect the incidence of DKA. This may be due to the nutritional status of the patient and an insulin-induced low level of sodium.33
The finding of this study showed that a decreasing dose of treatment increased the incidence rate of DKA. In contrast, a study conducted in Denver, Colorado metropolitan area showed that a high dose of treatment increased the rate of DKA.34 The possible reason for the increased rate of DKA as a result of a low dose of treatment may be that lowering the dose of treatment; results in a low production of insulin in the body, and the insulin antagonist hormones will also increase. Finally, the level of glucose will be increased and that leads to an increased hyperglycemic environment and then DKA.
Limitation of the Study
Since the study was based on secondary data, some important risk factors were not available and unable to analyze them.
Conclusion
The findings of this study indicated the average frequency of DKA during follow-up is higher than the expected frequency, which is preferably zero. Comorbidity with an infection, having heart diseases, lowering the dose of treatment, having a low sodium level, and having a history of treatment discontinuation were the factors that increase the frequency of DKA among pediatric DM patients. Parents and health care providers shall emphasize to pediatric diabetic patients to reduce the frequency of DKA, give special attention to infection prevention and the use of treatments for pediatric DM patients. The Federal Ministry of Health has to set priorities and intervention strategies like providing training for health extension workers and awakening them to work with the patients to prevent DKA.
Abbreviations
AIRR, adjusted incidence rate ratio; CAP, community-acquired pneumonia; CI, confidence interval; DKA, diabetic ketoacidosis; DM, diabetes mellitus; FHSRH, Felege Hiwot Specialized and Referral Hospital; FMOH, Federal Ministry of Health; NSAP, National Strategic Action Plan; SD, standard deviation; UoGSRH, University of Gondar Specialized and Referral Hospital; URTI, upper respiratory tract infection; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Informed Consent
Ethical clearance was obtained from the Ethical Committee of the Institute of public health in the University of Gondar. Officials from the University of Gondar Comprehensive Specialized Hospital and Felege Hiwot Specialized and Referral Hospital were communicated through a formal letter which was taken from the institute of public health and permission for data collection was granted from the hospital since consent to collect the data was waived to the hospitals. Confidentiality of information was maintained through not extracting personal identifiers and keeping data in a password secured computer. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We are indebted to the University of Gondar, Institutional Review Board giving an Ethical clearance to conduct this study. Our gratitude extends to University of Gondar and Felege Hiwot Specialized and Referral Hospital health management information system departments and staffs for their contribution by giving the information necessary for this thesis work. We would also like to thank the data collectors and supervisors involved in the data collection process.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There was no funding source for this study; data collection fee was covered by authors.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Yasmin F, Ahmed MAU, Morshed A, Begum T, Mohsin F, Baki M. Precipitating factors, clinical features and outcome of diabetic ketoacidosis in children in a tertiary care hospital of Bangladesh. BIRDEM Medl J. 2019;9(2):121–126. doi:10.3329/birdem.v9i2.41275
2. Non-communicable O. National strategic action plan (nsap) for prevention & control of non-communicable diseases in Ethiopia; 2016.
3. Usher-Smith J, Thompson M, Ercole A, Walter F. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55(11):2878–2894. doi:10.1007/s00125-012-2690-2
4. Hadgu FB, Sibhat GG, Gebretsadik LG. Diabetic ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes in Tigray, Ethiopia: retrospective observational study. Pediatric Health Med Ther. 2019;10:49. doi:10.2147/PHMT.S207165
5. Agarwal A, Yadav A, Gutch M, et al. Prognostic factors in patients hospitalized with Diabetic Ketoacidosis. Endocrinol Metab. 2016;31(3):424–432.
6. Atkilt H. Magnitude and associated risk factors of diabetic ketoacidosis in newly diagnosed diabetes mellitus children in Addis Ababa. Ethiopia: Addis Abeba University; 2015.
7. Balmier A, Dib F, Serret-Larmande A, et al. Initial management of diabetic ketoacidosis and prognosis according to diabetes type: a French multicentre observational retrospective study. Ann Intensive Care. 2019;9(1):91. doi:10.1186/s13613-019-0567-y
8. Iddi S, Francis B, Jaka HM, Mirambo MM, Mushi MF. Clinical presentation and precipitating factors of diabetic ketoacidosis among patients admitted to intensive care unit at a tertiary hospital in Mwanza, Tanzania. Tanzan J Health Res. 2017;19(1). doi:10.4314/thrb.v19i1.6
9. Skinner TC. Recurrent diabetic ketoacidosis: causes, prevention and management. Horm Res Paediatr. 2002;57(Suppl. 1):78–80. doi:10.1159/000053320
10. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19:155–177. doi:10.1111/pedi.12701
11. Bogale H. Precipitating factors and clinical-laboratory features of Diabetic Ketoacidosis (DKA) at Tikur Anbessa Specialized Hospital Emergency Department. Addis Ababa, Ethiopia: Addis Ababa University; 2014.
12. Otieno C, Kayima J, Mbugua P, Amayo A, Mcligeyo S. Prognostic factors in patients hospitalised with diabetic ketoacidosis at Kenyatta National Hospital, Nairobi. East Afr Med J. 2010;87(2):67–74. doi:10.4314/eamj.v87i2.60600
13. Atkilt HS, Turago MG, Tegegne BS. Clinical characteristics of diabetic ketoacidosis in children with newly diagnosed Type 1 diabetes in Addis Ababa, Ethiopia: a cross-sectional Study. PLoS One. 2017;12(1):e0169666. doi:10.1371/journal.pone.0169666
14. World Health Organization. Global report on diabetes; 2016.
15. Hanas R, Lindgren F, Lindblad B. A 2‐yr national population study of pediatric ketoacidosis in Sweden: predisposing conditions and insulin pump use. Pediatr Diabetes. 2009;10(1):33–37. doi:10.1111/j.1399-5448.2008.00441.x
16. Aschner P, Horton E, Leiter L, Munro N, Skyler JS; Management GPfED. Practical steps to improving the management of type 1 diabetes: recommendations from the global partnership for effective diabetes management. Int J Clin Pract. 2010;64(3):305–315. doi:10.1111/j.1742-1241.2009.02296.x
17. Jefferies C, Cutfield SW, Derraik JG, et al. 15-year incidence of diabetic ketoacidosis at onset of type 1 diabetes in children from a regional setting (Auckland, New Zealand). Sci Rep. 2015;5:10358. doi:10.1038/srep10358
18. Maahs DM, Hermann JM, Holman N, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the US, Austria, and Germany. Diabetes Care. 2015;38(10):1876–1882. doi:10.2337/dc15-0780
19. Murunga A, Owira P. Diabetic ketoacidosis: an overlooked child killer in sub‐S aharan A frica? Trop Med Int Health. 2013;18(11):1357–1364. doi:10.1111/tmi.12195
20. Vakharia JD, Agrawal S, Molino J, Topor LS. Family history of diabetes is associated with increased risk of recurrent diabetic ketoacidosis in pediatric patients. Endocr Pract. 2019;26:305–311. doi:10.4158/EP-2019-0351
21. Signorini DF. Sample size for Poisson regression. Biometrika. 1991;78(2):446–450. doi:10.1093/biomet/78.2.446
22. Shieh G. Sample size calculations for logistic and Poisson regression models. Biometrika. 2001;88(4):1193–1199. doi:10.1093/biomet/88.4.1193
23. Wright J, Ruck K, Rabbitts R, et al. Diabetic ketoacidosis (DKA) in Birmingham, UK, 2000—2009: an evaluation of risk factors for recurrence and mortality. Br J Diabetes Vasc Dis. 2009;9(6):278–282. doi:10.1177/1474651409353248
24. Al-Obaidi AH, Alidrisi HA, Mansour AA. Precipitating factors for diabetic ketoacidosis among patients with Type 1 diabetes mellitus: the effect of socioeconomic status. Int J Diabetes Metab. 2019;25(1–2):52–60. doi:10.1159/000499839
25. Desse TA, Eshetie TC, Gudina EK. Predictors and treatment outcome of hyperglycemic emergencies at Jimma University Specialized Hospital, southwest Ethiopia. BMC Res Notes. 2015;8(1):553. doi:10.1186/s13104-015-1495-z
26. Kumar G. Identifying risk factors for development of diabetic ketoacidosis in type 1 diabetes mellitus. Int J Contemp Pediatr. 2019;6(2):769. doi:10.18203/2349-3291.ijcp20185521
27. Hamed ZSS, Gawaly AM, Abbas KM, El Ahwal LM. Epidemiology of infection as a precipitating factor for diabetic ketoacidosis at Tanta University Hospital. Tanta Med J. 2017;45(2):68. doi:10.4103/tmj.tmj_10_17
28. Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl1):S27. doi:10.4103/2230-8210.94253
29. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3–4):259–265. doi:10.1111/j.1574-695X.1999.tb01397.x
30. Brandstaetter E, Bartal C, Sagy I, Jotkowitz A, Barski L. Recurrent diabetic ketoacidosis. Archives of endocrinology and metabolism. Arch Endocrinol Metab. 2019;63:531–535.
31. Islam R, Akhter S, Shelim R, Mohsin F, Begum T, Akhter G. Precipitating factors, clinical features and outcome of diabetic ketoacidosis in children and adolescents admitted in a tertiary care hospital in Dhaka. Bangladesh J Med Sci. 2014;13(1):53–57. doi:10.3329/bjms.v13i1.17429
32. Gosmanov AR, Gosmanova EO, Kitabchi AE. Hyperglycemic crises: diabetic ketoacidosis (DKA), and hyperglycemic hyperosmolar state (HHS). Endotext [Internet]: mDText. com, Inc.; 2018.
33. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. WJCC. 2014;2(10):488. doi:10.12998/wjcc.v2.i10.488
34. Rewers A, Chase HP, Mackenzie T, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287(19):2511–2518. doi:10.1001/jama.287.19.2511
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.