Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
First-Line Treatment with Tiotropium/Olodaterol Improves Physical Activity in Patients with Treatment-Naïve Chronic Obstructive Pulmonary Disease
Authors Takahashi K , Uchida M , Kato G, Takamori A, Kinoshita T, Yoshida M , Tajiri R, Kojima K, Inoue H, Kobayashi H, Sadamatsu H, Tashiro H, Tanaka M, Hayashi S , Kawaguchi A, Kimura S, Sueoka-Aragane N, Kawayama T
Received 26 June 2020
Accepted for publication 18 August 2020
Published 14 September 2020 Volume 2020:15 Pages 2115—2126
DOI https://doi.org/10.2147/COPD.S268905
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Koichiro Takahashi,1 Masaru Uchida,2 Go Kato,3 Ayako Takamori,4 Takashi Kinoshita,5 Makoto Yoshida,6 Ryo Tajiri,4 Keisuke Kojima,7 Hiroshi Inoue,8 Hiromi Kobayashi,9 Hironori Sadamatsu,1 Hiroki Tashiro,1 Masahide Tanaka,1 Shinichiro Hayashi,10 Atsushi Kawaguchi,4,11 Shinya Kimura,1 Naoko Sueoka-Aragane,1 Tomotaka Kawayama5 Saga-naïve COPD Physical Activity Evaluation (SCOPE) Study Investigator Group
1Division of Hematology, Respiratory Medicine and Oncology, Department of Internal Medicine, Faculty of Medicine, Saga University, Saga, Japan; 2Division of Internal Medicine, Japan Community Health Care Organization Saga Central Hospital, Saga, Japan; 3Division of Respiratory Medicine, Saga Prefectural Medical Center Koseikan, Saga, Japan; 4Clinical Research Center, Saga University Hospital, Faculty of Medicine, Saga University, Saga, Japan; 5Division of Respirology, Neurology, and Rheumatology, Department of Medicine, Kurume University School of Medicine, Fukuoka, Japan; 6Division of Respiratory Medicine, National Hospital Organization Fukuoka Hospital, Fukuoka, Japan; 7Division of Internal Medicine, Imari Arita Kyouritsu Hospital, Saga, Japan; 8Division of Internal Medicine, Karatsu Red Cross Hospital, Saga, Japan; 9Division of Respiratory Medicine, National Hospital Organization East Saga Hospital, Saga, Japan; 10Division of Internal Medicine, Kouhoukai Takagi Hospital, Fukuoka, Japan; 11Education and Research Center for Community Medicine, Faculty of Medicine, Saga University, Saga, Japan
Correspondence: Koichiro Takahashi
Division of Hematology, Respiratory Medicine and Oncology, Department of Internal Medicine, Faculty of Medicine, Saga University, 5-1-1 Nabeshima, Saga 849-8501, Japan
Email [email protected]
Background: Comparative effects on physical activity of mono and dual bronchodilators remain unclear in patients with treatment-naïve chronic obstructive pulmonary disease (COPD). We sought to compare the changes in physical activity before and after tiotropium and tiotropium/olodaterol treatment in treatment-naïve COPD patients.
Methods: A prospective, multicenter, randomized, open-labeled, and parallel interventional study was conducted. Eighty Japanese patients with treatment-naïve COPD were randomized to receive either tiotropium or tiotropium/olodaterol treatment for 12 weeks. Spirometry and dyspnea index were assessed, and COPD assessment test (CAT) and the 6-minute walk distance (6MWD) were conducted before and after treatment. Evaluation of physical activity was assessed by a triaxle accelerometer over a 2-week period before and after treatment.
Results: There were no differences in the mean age (69.8 vs 70.4 years), body mass index (BMI) (22.5 vs 22.6 kg/m2) and mean % forced expiratory volume in 1 second (%FEV1) at baseline (61.5 vs 62.6%) between the two groups. Changes in FEV1 (mean±standard error, 242.8± 28.8 mL) and transient dyspnea index (TDI) (2.4± 0.3 points) before and after tiotropium/olodaterol treatment were greater than with tiotropium treatment (104.1± 31.9 mL, p< 0.01 and 1.5± 0.3, p=0.02, respectively). Changes in the duration of physical activity with 1.0– 1.5 metabolic equivalents (METs) estimated in the sedentary position following tiotropium/olodaterol treatment (− 38.7± 14.7 min) tended to be reduced more than with tiotropium treatment (− 4.6± 10.6 min) (p=0.06), although those with ≥ 2.0 METs numerically increased with both treatments (+10.8± 7.6 min for tiotropium/olodaterol vs +8.3± 7.6 min for tiotropium, p=0.82). Tiotropium/olodaterol treatment reduced the duration of physical activity with 1.0– 1.5 METs (regression coefficient, − 43.6 [95% CI − 84.1, − 3.1], p=0.04) in a multiple regression model adjusted for cofounding factors such as age, FEV1, total CAT scores, 6MWD, and TDI.
Conclusion: This is the first study to report the impact of dual bronchodilator on physical activity in treatment-naïve COPD patients of Japanese with low BMI.
Keywords: chronic obstructive pulmonary disease, physical activity, long-acting muscarinic antagonist, long-acting beta 2 agonist
Introduction
Chronic obstructive pulmonary disease (COPD) ranked third among the global age-standardized death rates in 2015, with approximately 3 million deaths.1 The prevalence of COPD in the population over 40 years old is reported to be around 10%.2 The estimated number of patients with COPD is reported to be more than 5 million in Japan.3
Progressive airflow limitation causes dyspnea, decreasing quality of life (QOL) exercise capacity, and physical activity in patients with COPD.1 Physical inactivity has been reported to be the strongest predictor of all-cause mortality in COPD.4 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has recommended regular physical activity for COPD patients.5 Bronchodilators play an essential role in the treatment of COPD, and inhaled long-acting bronchodilator use is associated with improvement of lung function, reducing symptoms and fewer exacerbations.6,7 It has been reported that combination therapy of tiotropium, a long-acting muscarinic antagonist (LAMA), and olodaterol, a long-acting beta 2 agonist (LABA), provides an improvement in pulmonary function and QOL compared with LAMA monotherapy.8
Although some studies have reported improvements in physical activity with bronchodilators in COPD patients, the effects of dual bronchodilators agent combination on physical activity are still unclear.9–12 Physical activity has been reported to be decreased not only in moderate to severe COPD but also in mild COPD cases.13 Early intervention for milder COPD may make it possible to improve physical activity in patients with COPD.14 It has been reported that not only improvement in high-intensity physical activity but also low-intensity physical activity, such as sedentary time, was important.15 Sedentary behavior is considered an independent predictor of mortality in COPD, even when adjusting for moderate-to-vigorous physical activity. However, there is limited evidence to support whether dual-bronchodilator treatment allows for improvements in physical activity, especially in Japanese patients with treatment-naïve COPD.
The present study aimed to clarify the efficacy of the tiotropium/olodaterol combination in terms of pulmonary function and physical activity when compared to tiotropium monotherapy in patients with treatment-naïve COPD.
Patients and Methods
Study Subjects
Nine public hospitals located in Japan participated in the study. This study included patients with treatment-naïve COPD. Entry period of this study was July 2017 to February 2019. Inclusion criteria were as follows: treatment-naive men or women aged between ≥40 and <85 years old with airway limitation and post-bronchodilator (short-acting beta 2 agonist) forced expiratory volume in 1 second (FEV1) less than 80% of predicted normal and post-bronchodilator FEV1/forced vital capacity (FVC) less than 70% at the point of screening and current or ex-smokers with a smoking history ≥10 pack-years of index. Patients with treatment-naive COPD were defined as those who had not been treated with inhaled corticosteroid (ICS), LABA, and/or LAMA in the last 12 months. Exclusion criteria were the presence or history of asthma, patients who had undergone surgery such as lung lobectomy, patients who impaired mobility, and patients with urination disorder by benign prostatic hyperplasia, glaucoma, and dementia. Patients who had experienced an exacerbation of COPD within the previous 1 month were also excluded. Patients with paroxysmal tachycardia or life-threatening arrhythmias, those with a history of acute myocardial infarction or hospitalization for heart failure in the past year, and those with a history of malignancy within the past 5 years were excluded. Written informed consent was obtained from all patients prior to the study, which was approved by the institutional review board at each participating center (approval number: 2016–12-01, Table S1). The study was conducted according to the principles of the International Conference on Harmonization and Good Clinical Practice, Declaration of Helsinki, Japanese Good Clinical Practice, and all relevant local regulatory, legal, and ethical requirements. This study was also approved by the Japan Registry of Clinical Trials (JRCT; jRCTs071180021). We named this study the Saga-naïve COPD Physical Activity Evaluation (SCOPE) Study.
Study Design
A prospective, multicenter, randomized, open-labeled, paralleled study was conducted (UMIN; UMIN000027190). Patients were randomized 1:1, using an electronic data capture system, according to 3 factors: respiratory function (%FEV1 predicted post-bronchodilator treatment), age, and smoking history (current or past smoking) to receive open-labeled tiotropium or tiotropium/olodaterol for 12 weeks. Oral doses of tiotropium/olodaterol 5 μg/5 μg inhalation solution (2.5 μg/2.5 μg per actuation) and tiotropium 5 μg inhalation solution (2.5 μg per actuation) were administered using the RESPIMAT® (Nippon Boehringer Ingelheim Co., Ltd) soft mist inhaler based on the marketed dose in Japan. The sample size was set based on previous reports and calculated from the change of FEV1 (mean difference=126.0 mL) which was the primary endpoint, with an assumed standard deviation of 190 and 0.8 power to detect a significant difference (two-sided p=0.05). Therefore, we planned to enroll 40 patients in each group after considering dropouts.8
At visit 1, patients were screened by pulmonary function test after bronchodilator administration, and written informed consent was obtained. At visit 2, patients were randomly allocated to either the tiotropium/olodaterol or tiotropium group following factors; predicted %FEV1, age, and smoking status. At visits 3 and 4, patients returned to hospital to verify adherence to bronchodilator treatment and the presence of adverse effects. At visit 5, which occurred after the 12-week bronchodilators treatment, patients were examined for post-bronchodilator pulmonary function. Medications for the comorbidities such as hypertension, diabetes mellitus, dyslipidemia, reflux esophagitis, and osteoporosis were recorded, and there was no difference between the two groups in concomitant medications.
Study Assessments
The primary endpoint was the difference in baseline FEV1 and FEV1 at 60 min post-dose (tiotropium/olodaterol or tiotropium) after 12 weeks between tiotropium/olodaterol and tiotropium treatment groups. The secondary endpoints were the differences in inspiratory capacity (IC), Baseline Dyspnea Index/Transitional Dyspnea Index (BDI/TDI) score, total COPD assessment test (CAT) score, and physical activity between tiotropium/olodaterol and tiotropium treatment groups. Other endpoints were the 6-minute walk distance (6MWD) and Borg’s scales before (visit 2) and after treatments (visit 5).
Analysis of Physical Activity
Physical activity was examined by tri-axis accelerometer (Active style PRO HJA-750C, OMRON, Kyoto, Japan) before (between visits 1 and 2) and after treatment (between visits 4 and 5) for 2 weeks. Each patient was required to wear the accelerometer on the waist from wakeup time to sleeping. The accelerometer screen was set to be not viewable by patients. Physical activity was evaluated as the average number of steps per day, average daily duration (minutes) of ≥3, ≥2, and 1.0–1.5 metabolic equivalents (METs). This study used data collected over 12 hours, from 9 am to 9 pm, to analyze physical activity. Activity data were excluded on rainy days, which have been shown to prevent outdoor activity.14,16 To obtain more reproducible data and assess activity reliably, the data relative to physical activity were measured for at least 3 valid days with 12 hours wearing time of activity,17 and physical activity with 1.0–1.5 METs was adopted as representative for sedentary time.18 One patient in both groups was excluded due to mechanical failure of accelerometer, and 36 patients in both groups were analyzed as the physical activity analysis set (PAAS) (Figure 1).
Subgroup Analyses
Improvement of physical activity was defined as a greater reduction from baseline in the time of 1.0–1.5 METs, or a greater increase from baseline in the time of ≥2.0 METs or ≥3.0 METs. Subgroup analyses were performed for factors associated with improvement in physical activity for each METs category. This study used factors relative to baseline characteristics, including age, body mass index (BMI), IC, FEV1, FVC, CAT score, 6MWD, TDI score, and physical activity (1.0–1.5 METs, ≥2.0 METs, and ≥3.0 METs).
Safety
Safety endpoints were all adverse events (AEs). If patients showed COPD exacerbation requiring antibiotics or systemic corticosteroids during the study period, they were asked to drop out of the study. If a serious adverse event occurred due to a targeting drug, the secretariat was contacted within 24 hours.
Statistical Analysis
The data are reported as mean and standard deviation (SD) or median and interquartile range (IQR). Differences between two groups for characteristics were tested with Student’s t-test, Fisher’s exact test, or χ2 test. Wilcoxon’s rank sum test was performed as a nonparametric analysis when it was inappropriate to assume a normal distribution from the histogram of the data. As analyses of primary outcomes, the difference of pulmonary function and daily activity duration in the two groups were compared using Welch’s t-test and reported as estimated mean difference, standard error (SE), and 95% confidence intervals (95% CI). The effect of treatment on time spent on activity levels of 1.0–1.5 METs, ≥2.0 METs, and ≥3.0 METs was assessed using univariate linear regression models by subgroups. Thereafter, multivariate analysis was conducted by adjusting for the following potential covariate factors: age, BMI, IC, FEV1, FVC, CAT score, 6MWD, TDI, physical activity 1.0–1.5 METs, physical activity ≥2.0 METs, and physical activity ≥3.0 METs. Statistical significance was indicated as p-value <0.05, and the missing values were excluded. Data were analyzed with SAS 13 (SAS Institute Inc., Cary, NC, USA) and R 3.6.1.
Results
In the present study, 102 patients from 9 public hospitals located in Japan were assessed for eligibility. Twenty-two patients, 18 who declined to participate and 4 satisfying the exclusion criteria, were excluded by the screening test. Of the remaining patients, 80 were randomly allocated to treatments either in combination with tiotropium and olodaterol or tiotropium monotherapy for 12 weeks. Three patients of the tiotropium/olodaterol group and three patients of tiotropium group withdrew consent for participation. Finally, 37 patients in the tiotropium/olodaterol treatment group and 37 patients in the tiotropium monotherapy group completed the study (Figure 1). No treatment was discontinued due to adverse effects.
Baseline characteristics are shown in Table 1. There were no differences in the proportion of male sex (97.3% vs 89.2%), mean age (69.8 vs 70.4 years), BMI (22.5 vs 22.6 kg/m2), and smoking status, or in the population of patients with hypertension, diabetes mellitus, and dyslipidemia in tiotropium/olodaterol compared with tiotropium. Baseline total CAT scores in the tiotropium/olodaterol group were higher than in the tiotropium group, although there was no difference in data relative to pulmonary functions (%predicted normal FEV1, 61.5% vs 62.6%), 6MWD, and duration of different physical activity levels between the two groups (Table 2). Most patients enrolled in this study were characterized by moderate COPD with mild symptoms.
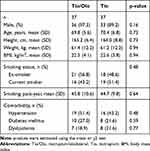 |
Table 1 Patient Demographics and Characteristics |
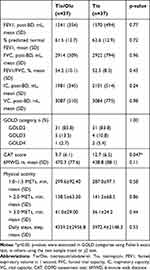 |
Table 2 Pulmonary Function Test and Physical Activity at Baseline |
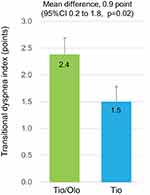
Changes in FEV1 after treatments showed an increase in both tiotropium/olodaterol (mean±SE, +242.8±28.8 mL) and tiotropium (+104.1±31.9 mL) groups (Figure 2A). Difference in FEV1 between the groups, which was the primary endpoint of this study, showed an increase (mean difference, 138.7 mL; 95% CI 52.8 to 224.6, p=0.002). Changes in IC with tiotropium/olodaterol showed a greater difference (mean difference, 115.3; 95% CI −35.9 to 266.6, p=0.13), than the tiotropium (Figure 2B), whereas those of the FVC and VC showed no significant differences between groups (Figure 2C; mean difference, 54.8; 95% CI −92.7 to 202.3, and Figure 2D: mean difference, 7.8; 95% CI −134.0 to 149.6). The TDI score was 2.4±0.3 for the tiotropium/olodaterol vs 1.5±0.3 for tiotropium, which demonstrated tiotropium/olodaterol was more effective in improving dyspnea (Figure 3: mean difference, 0.9; 95% CI 0.2 to 1.8, p=0.02). The 6MWD at the study entry study showed no differences between the two groups (tiotropium/olodaterol, 470.3 vs tiotropium, 438.8 m) (Table S2). Changes in 6MWD after treatment showed improvement (475.7 vs 445.7 m, respectively) though this was also not significantly different between two groups (p=0.10).
Reduction in the duration of physical activity with 1.0–1.5 METs and sedentary time, with the tiotropium/olodaterol combination, was greater than the tiotropium group, although the differences were not statistically significant between groups (tiotropium/olodaterol mean±SE, −38.7±14.7 min vs tiotropium, −4.6±10.6 min; mean difference, −34.1, 95% CI −70.4 to 2.2, p=0.06) (Figure 4A). Changes in the duration of physical activity with ≥2.0 METs (tiotropium/olodaterol, +10.8±7.6 min vs tiotropium, +8.3±7.6 min; mean difference, 2.5, 95% CI −19.0 to 24.0, p=0.82) (Figure 4B) and with ≥3.0 METs (tiotropium/olodaterol, +5.2±3.9 min vs tiotropium +2.5±3.3 min; mean difference, 2.7, 95% CI −7.4 to 12.8, p=0.60) (Figure 4C), and daily steps (tiotropium/olodaterol +168.1±392.5 steps vs tiotropium +37.6±192.4 steps, mean difference 131, 95% CI −750 to 1011, p=0.77) (Figure 4D) showed no statistically significant differences between the two groups.
To investigate the effect of tiotropium/olodaterol combination vs tiotropium treatment in time spent on 1.0–1.5 METs, ≥2.0 METs and ≥3.0 METs physical activity levels, subgroup and multivariable analyses were performed. The subgroups were considered by the following baseline factors; age, BMI, IC, FEV1, VC, CAT score, 6MWD, TDI, and the respective time category of physical activity at baseline (1.0–1.5, ≥2.0 and ≥3.0 METs) (Figure 5). In the subgroup analyses, compared with the tiotropium and tiotropium/olodaterol combination treatments, a decrease in the duration of 1.0–1.5 METs was observed in the subgroups with the following baseline characteristics: FEV1, FVC, 6MWD and TDI values greater than or equal to the median. These relationships were not observed in the duration of the other ≥2.0 and ≥3.0 METs activity levels. Multiple regression model analyses were performed in the times for 1.0–1.5 METs, ≥2.0 METs and ≥3.0 METs, considering baseline, age, FEV1, CAT, 6MWD, and TDI values as adjusting factors. The tiotropium/olodaterol combination treatment reduced the duration of physical activity 1.0–1.5 METs (regression coefficient, −43.6 [95% CI −84.1 to −3.1], p=0.04), and then the following baseline factors; age, FEV1, CAT, 6MWD and TDI were not related with the duration of activity 1.0–1.5 METs (Table 3). Concerning the duration of activity ≥2.0 and ≥3.0 METs, any correlations with the considered factors were not observed.
 |
Table 3 Multivariate Analysis of Effect with Tiotropium/Olodaterol versus Tiotropium in Time Spent on 1.0–1.5 METs, ≥2.0 METs and ≥3.0 METs |
In terms of safety, there were no severe AEs in either group during the study period. Mild to moderate AEs are shown in Table S3. There was no exacerbation during the study period.
Discussion
The SCOPE study demonstrated that both dual-bronchodilator treatment with tiotropium/olodaterol and mono-bronchodilator treatment with tiotropium improved pulmonary function, dyspnea levels, and physical activity from baseline to 12 weeks after treatment in patients with treatment-naïve COPD. In addition, dual-bronchodilator treatment resulted in greater improvements in FEV1, TDI score, and reduction of physical activity at 1.0–1.5 METs than tiotropium treatment. This is the first report to show that tiotropium/olodaterol treatment impacts on physical activity in treatment-naïve COPD patients. Dual bronchodilation may represent the first opportunity to change the lifestyle in untreated Japanese patients with COPD.
In Japan, more than 90% of COPD patients are undiagnosed and untreated by long-acting bronchodilators, although an epidemiologic survey found that the prevalence of COPD was reported to be 8.4% (5.3 million) in the general population.19 A previous study showed that undiagnosed, untreated, and asymptomatic COPD had an increased risk of exacerbations and pneumonia.20 There is limited evidence on the efficacy and safety of dual bronchodilation on the untreated and asymptomatic COPD patients.21 Therefore, our SCOPE study was conducted to investigate the efficacy and safety of dual bronchodilation on patients with undiagnosed and untreated COPD. It is notable that all newly, but not previously, diagnosed COPD patients were selected and covered in the study.
The SCOPE study found that the mean change of FEV1 after 12-week treatment was 243 mL with tiotropium/olodaterol and 104 mL with tiotropium administration, respectively. A previous study compared the difference in peak FEV1 in patients treated with tiotropium/olodaterol and tiotropium was 114 mL (289 mL in tiotropium/olodaterol vs 175 mL in tiotropium, p<0.01) in a treatment-naïve subgroup of patients with COPD. The differences in peak FEV1 in the SCOPE study were comparable to those of previous studies.22,23
Both mono- and dual-bronchodilator treatment improved TDI by more than 1.0 point in the present study. The minimal clinically important difference (MCID) of the TDI score is 1.0 point.24 It was considered that all patients received bronchodilators for the first time, so there was likely an improvement in both groups. Additive bronchodilator effects of adding LABA such as including olodaterol,8 vilanterol,25 and indacaterol26 had already been reported in COPD patients using LAMA monotherapy.27 Since LAMA and LABA have different mechanisms of action than that of bronchodilation,28,29 dual-bronchodilator treatment with a LAMA/LABA combination is more effective than doubling the amount of mono bronchodilator in improving pulmonary function and dyspnea.30
Physical activity is an important predictor of mortality in several chronic diseases including COPD.4,21,31 Physical activity in COPD has been reported as the strongest prognostic factor for survival rather than pulmonary function, exercise tolerance, or dyspnea scale.4 A physical activity of 1.0–1.5 METs means sedentary position, physical activity at 2 METs means standing position or walking less than 55 m/min, and physical activity at 3 METs means walking faster than 55 m/min.32 A recent study demonstrated that not only improvement from high-intensity physical activity but also the positive impact of low-intensity physical activity, which included sedentary behavior improvement, was essential as a COPD treatment strategy.15
Strikingly, the SCOPE study demonstrated that reduction of sedentary time in dual bronchodilator rather than mono-bronchodilator in Japanese patients with treatment-naïve COPD in multivariate analyses with baseline factors including age, FEV1, CAT, 6MWD, and TDI. Sedentary behavior has also been reported to be an independent predictor of mortality in COPD, even adjusting for moderate-to-vigorous physical activity. Mortality was higher in patients with COPD who spend ≥8.5 hours per day in activities requiring <1.5 METs.33 Even in mild-to-moderate COPD, physical activity was lower than that in healthy subjects.13 Increasing physical activity at low intensity rather than that at high intensity contributes to a reduction in the risk of hospitalization in patients with severe COPD.34 Thus, the guidelines on physical activity aim to reduce sedentary time for patients with COPD.35 A previous study reported a slight improvement in physical activity of more than moderate intensity following dual-bronchodilator tiotropium/olodaterol compared to mono-bronchodilator tiotropium.11 COPD patients who participated in the SCOPE study were mostly characterized by moderate COPD with mild symptoms. Interestingly, it was predicted that physical activity with moderate to vigorous intensity would improve due to mild symptoms, and indeed, a reduction of time for physical activity at 1.0–1.5 METs was observed. The reduction in sedentary time with behavior modification and pharmacological intervention is considered to be important in all patients with severe COPD. Since the clinically significant reduction in sedentary time for patients with COPD is unknown, further studies are needed in this regard.
The present study had some limitations. First, it was prospective in nature; furthermore, the study population was small and limited to Japanese patients who were mostly men with COPD. These characteristics limit generalizability to other populations and to female patients. Second, the duration of the study was set for 12 weeks. We consider that adding pulmonary rehabilitation and behavior modification to the protocol or prolonging the treatment period may have even greater effects.36 Third, the CAT score showed differences in baseline levels between the two groups. We considered that the differences in CAT scores were because they were not assessed as a randomization factor for the treatment groups. Although multiple regression model analyses were performed baseline CAT score as adjusting factors, the difference of CAT score in two groups may have some effects improve in physical activity.
Conclusions
Dual bronchodilator with tiotropium/olodaterol treatment improved FEV1 and TDI compared with tiotropium in patients with treatment-naïve COPD. Tiotropium/olodaterol also decreased the amount of time spent in the sedentary position. These data indicate that dual bronchodilator may improve not only lung function and respiratory symptoms but also the lifestyle of Japanese patients with treatment-naïve COPD. Dual-bronchodilator treatment may be recommended more than mono bronchodilators as the first-line approach in patients with treatment-naïve COPD.
Abbreviations
METs, metabolic equivalents; 6MWD, 6-minute walk distance; AE, adverse events; BDI/TDI, Baseline Dyspnea Index/Transitional Dyspnea Index score; CAT, chronic obstructive pulmonary disease assessment tests; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IC, inspiratory capacity; LABA, long-acting beta 2 agonist; LAMA, long-acting muscarinic antagonist; QOL, quality of life; TDI, transient dyspnea index.
Data Sharing Statement
The data of this study are stored and managed at the Saga University Clinical Research Center. The reader needs to contact the corresponding author regarding these requests.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
This study was financially supported by Nippon Boehringer Ingelheim Co., Ltd. as an independent investigator study. Dr. Koichiro Takahashi received lecture fees from Nippon Boehringer Ingelheim and AstraZeneca. Dr. Takashi Kinoshita received grants from Daiichi Sankyo. Dr. Makoto Yoshida received lecture fees from AstraZeneca, GlaxoSmithKline and Novartis pharma. Dr. Tomotaka Kawayama received grants from Novartis Pharma, and lecture fees from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Novartis Pharma, Teijin Pharma and Home Healthcare, Sanofi, Kyorin Pharmaceutical, and Meiji Seika Pharma. Dr. Masaru Uchida, Dr. Go Kato, Dr. Ayako Takamori, Dr. Makoto Yoshida, Dr. Ryo Tajiri, Dr. Keisuke Kojima, Dr. Hiroshi Inoue, Dr. Hiromi Kobayashi, Dr. Hironori Sadamatsu, Dr. Hiroki Tashiro, Dr. Masahide Tanaka, Dr. Shinichiro Hayashi, Dr. Atsushi Kawaguchi, Dr. Shinya Kimura and Dr. Naoko Sueoka-Aragane did not have any conflicts of interests.
References
1. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/S0140-6736(17)31222-9
2. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi:10.1016/S0140-6736(07)61377-4
3. Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the nippon COPD epidemiology study. Respirology. 2004;9(4):458–465. doi:10.1111/j.1440-1843.2004.00637.x
4. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:10.1378/chest.10-2521
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
6. Calzetta L, Rogliani P, Matera MG, Cazzola M. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest. 2016;149(5):1181–1196. doi:10.1016/j.chest.2016.02.646
7. Zider AD, Wang X, Buhr RG, Sirichana W, Barjaktarevic IZ, Cooper CB. Reduced COPD exacerbation risk correlates with improved FEV1: a meta-regression analysis. Chest. 2017;152(3):494–501. doi:10.1016/j.chest.2017.04.174
8. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015;45(4):969–979. doi:10.1183/09031936.00136014
9. Watz H, Troosters T, Beeh KM, et al. ACTIVATE: the effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2545–2558. doi:10.2147/COPD.S143488
10. Troosters T, Maltais F, Leidy N, et al. Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(8):1021–1032. doi:10.1164/rccm.201706-1288OC
11. Ichinose M, Minakata Y, Motegi T, et al. Efficacy of tiotropium/olodaterol on lung volume, exercise capacity, and physical activity. Int J Chron Obstruct Pulmon Dis. 2018;13:1407–1419. doi:10.2147/COPD.S166023
12. Minakata Y, Motegi T, Ueki J, et al. Effect of tiotropium/olodaterol on sedentary and active time in patients with COPD: post hoc analysis of the VESUTO ((R)) study. Int J Chron Obstruct Pulmon Dis. 2019;14:1789–1801. doi:10.2147/COPD.S208081
13. Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi:10.1164/rccm.200407-855OC
14. Watz H, Pitta F, Rochester CL, et al. An official European respiratory society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi:10.1183/09031936.00046814
15. Tremblay MS, Aubert S, Barnes JD, et al. Sedentary Behavior Research Network (SBRN) terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. doi:10.1186/s12966-017-0525-8
16. Byrom B, Rowe DA. Measuring free-living physical activity in COPD patients: deriving methodology standards for clinical trials through a review of research studies. Contemp Clin Trials. 2016;47:172–184. doi:10.1016/j.cct.2016.01.006
17. Sugino A, Minakata Y, Kanda M, et al. Validation of a compact motion sensor for the measurement of physical activity in patients with chronic obstructive pulmonary disease. Respiration. 2012;83(4):300–307. doi:10.1159/000330046
18. Sedentary Behaviour Research N. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37(3):540–542. doi:10.1139/h2012-024
19. Matsumoto K, Seki N, Fukuyama S, et al. Prevalence of asthma with airflow limitation, COPD, and COPD with variable airflow limitation in older subjects in a general Japanese population: the Hisayama Study. Respir Investig. 2015;53(1):22–29. doi:10.1016/j.resinv.2014.08.002
20. Colak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5(5):426–434. doi:10.1016/S2213-2600(17)30119-4
21. Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107(19):2435–2439. doi:10.1161/01.CIR.0000066906.11109.1F
22. Ferguson GT, Flezar M, Korn S, et al. Efficacy of tiotropium + olodaterol in patients with chronic obstructive pulmonary disease by initial disease severity and treatment intensity: a post hoc analysis. Adv Ther. 2015;32(6):523–536. doi:10.1007/s12325-015-0218-0
23. Singh D, Gaga M, Schmidt O, et al. Effects of tiotropium + olodaterol versus tiotropium or placebo by COPD disease severity and previous treatment history in the OTEMTO (R) studies. Respir Res. 2016;17(1):73. doi:10.1186/s12931-016-0387-7
24. Witek TJ
25. Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2(6):472–486. doi:10.1016/S2213-2600(14)70065-7
26. Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. doi:10.1183/09031936.00200212
27. Derom E, Brusselle GG, Joos GF. Efficacy of tiotropium-olodaterol fixed-dose combination in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:3163–3177. doi:10.2147/COPD.S92840
28. Calzetta L, Matera MG, Cazzola M. Pharmacological interaction between LABAs and LAMAs in the airways: optimizing synergy. Eur J Pharmacol. 2015;761:168–173. doi:10.1016/j.ejphar.2015.05.020
29. Ikeda T, Anisuzzaman AS, Yoshiki H, et al. Regional quantification of muscarinic acetylcholine receptors and beta-adrenoceptors in human airways. Br J Pharmacol. 2012;166(6):1804–1814. doi:10.1111/j.1476-5381.2012.01881.x
30. Calzetta L, Matera MG, Cazzola M. Pharmacological mechanisms leading to synergy in fixed-dose dual bronchodilator therapy. Curr Opin Pharmacol. 2018;40:95–103. doi:10.1016/j.coph.2018.03.011
31. Hamer M, Stamatakis E. Physical activity and mortality in men and women with diagnosed cardiovascular disease. Eur J Cardiovasc Prev Rehabil. 2009;16(2):156–160. doi:10.1097/HJR.0b013e32831f1b77
32. Park J, Ishikawa-Takata K, Tanaka S, Mekata Y, Tabata I. Effects of walking speed and step frequency on estimation of physical activity using accelerometers. J Physiol Anthropol. 2011;30(3):119–127. doi:10.2114/jpa2.30.119
33. Furlanetto KC, Donaria L, Schneider LP, et al. Sedentary behavior is an independent predictor of mortality in subjects with COPD. Respir Care. 2017;62(5):579–587. doi:10.4187/respcare.05306
34. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al. Benefits of physical activity on COPD hospitalisation depend on intensity. Eur Respir J. 2015;46(5):1281–1289. doi:10.1183/13993003.01699-2014
35. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–2028. doi:10.1001/jama.2018.14854
36. Shioya T, Sato S, Iwakura M, et al. Improvement of physical activity in chronic obstructive pulmonary disease by pulmonary rehabilitation and pharmacological treatment. Respir Investig. 2018;56(4):292–306. doi:10.1016/j.resinv.2018.05.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.