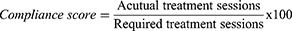Back to Journals » Journal of Pain Research » Volume 16
Fire Needling Therapy versus Manual Acupuncture in Post-Stroke Complex Regional Pain Syndrome of the Upper Limb: Study Protocol for a Pilot Randomised Controlled Trial
Authors Wang M , Yuan F, Xu X, Zhang T, Guo J, Wang G, Wang L, Sun J, Zhang F, Li B
Received 12 April 2023
Accepted for publication 28 June 2023
Published 11 July 2023 Volume 2023:16 Pages 2347—2356
DOI https://doi.org/10.2147/JPR.S416893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Mina Wang,1,2,* Fang Yuan,1,* Xiaobai Xu,1,* Tao Zhang,1 Jing Guo,1 Guiling Wang,1 Linpeng Wang,1 Jingqing Sun,1 Fan Zhang,1 Bin Li1
1Department of Acupuncture and Moxibustion, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, 100010, People’s Republic of China; 2Graduate School, Beijing University of Chinese Medicine, Beijing, 100029, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fan Zhang; Bin Li, Email [email protected]; [email protected]
Introduction: Post-stroke complex regional pain syndrome (CRPS) is a devastating disease that causes severe physical and emotional consequences. Conventional therapies are limited due to the insufficient benefits and side effects, and fire needling therapy is considered an alternative for post-stroke CRPS of the upper limb.
Methods and Analysis: This is a study protocol for a pilot randomised, two-arm, single-centre, clinical trial at Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University. The trial started in March 2023 and is expected to end in December 2024. A total of 60 patients (aged 40– 75 years, male or female) with post-stroke CRPS of the upper limb will be randomly assigned to treatment group (fire needling therapy, 5 sessions per week for 2 weeks) or control group (manual acupuncture, 5 sessions per week for 2 weeks) in a 1:1 ratio using block randomisation and opaque envelopes. Fire needling therapy or manual acupuncture will be performed in ten acupoints. Participants will complete the trial by visiting the research centre at Week 14 for a follow-up assessment. The primary outcome is the response rate. Secondary outcomes include FMA, Barthel Scale/Index (BI), pain threshold (PPT), and muscle elasticity modulus (using shear wave elastography [SWE]). A chi-squared test will be used for response rate. A mixed-effects linear model and a mixed-effects model will be used for FMA, BI, PPT, and SWE, respectively.
Discussion: This is the first standardised protocol to compare the effectiveness of fire needling therapy and manual acupuncture. We will use a rigorous methodology to minimise bias and set up supervising committees to ensure the quality of our study, thus providing trustworthy evidence for better understanding of fire needling therapy in treating post-stroke CRPS of the upper limb.
Keywords: post-stroke complex regional pain syndrome, upper limb, fire needling therapy, manual acupuncture
Introduction
ICD-10 code G90. 5 and G90. 6 for complex regional pain syndrome (CRPS) is a medical classification as listed by the World Health Organization, whereby it is seen as a chronic neurologic condition in response to acute trauma or surgery. It can be further divided into two subtypes: CRPS I, formerly known as reflex sympathetic dystrophy (RSD), which is absent of a major nerve injury, and CRPS II, formerly known as causalgia, where nerve injury is present.1 The prevalence of CRPS reached approximately 5.4–26.2 per 100,000 person-years, and CRPS I occurred more frequently than CRPS II, but with a female predominance in both types.2 A German study reported an incidence disparity between CRPS I and CRPS II as high as 88% and 12%, respectively, and that between women and men as high as 71% and 29%, respectively.3 The study further demonstrated that about 70% of patients developed CRPS in the upper extremity, indicating that the upper limbs were more commonly affected than the lower limbs,3 a finding of other studies too.4
Post-stroke CRPS of the upper limb is also known as post-stroke shoulder-hand syndrome (PSSHS), or reflex sympathetic dystrophy of the upper limb. The clinical manifestations of post-stroke CRPS of the upper limb include severe pain and dysfunction in the shoulder joint, edema and swelling of hand, hyperalgesia, changes in the skin color and temperature, muscle atrophy in upper limbs or even contracture deformity, etc.5 The therapeutic goal is to relieve pain and restore functionality of the affected limb in the early stage of the disease, which is also the best opportunity to recover with positive intervention.
The conventional treatment has been developed according to the pathogenesis of the disorder, such as inflammation (systemic or neurogenic), autonomic dysfunction, central nervous system changes, and psychological factors. Therefore, the management is categorised into physical/occupational/psychological therapy, pharmacological therapy, and invasive therapy.6 Physical/occupational/psychological therapy, such as mirror therapy5 and psychological consulting,7 is the first-line treatment to reduce pain and improve active mobility, especially in the early stage of the disease. Pharmacological therapy mainly regulates the inflammatory status to alleviate pain, such as non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, bisphosphonates, etc.8 When physical and medical therapies fail to relieve pain, some invasive therapies will be employed, such as sympathetic nerve block, surgical sympathectomy, spinal cord stimulation and even amputation, dorsal root ganglion stimulation, etc.8 However, considering the increased risk of gastrointestinal and cardiovascular diseases, higher cost, and trauma resulting from medical and invasive therapies, complementary and alternative therapy should be applied to post-stroke CRPS patients to relieve pain and restore functionality of the affected limb. Fire needling therapy has been suggested as effective in treating post-stroke CRPS.9
Fire needling therapy is one of the most common forms of acupuncture, which can be dated back thousands of years. Compared with manual acupuncture, fire needling therapy has extra heat stimuli, thus further enhancing the stimulation and initiating a stronger response in the human body. A network meta-analysis has demonstrated that the effect of fire needling therapy is better than manual acupuncture in reducing pain and improving active mobility. However, the methodological quality of included trials is poor, thus restricting reliability and generalisation of fire needling therapy.10 Therefore, our study aims to compare the effectiveness of fire needling therapy (5 sessions per week for 2 weeks) and manual acupuncture (5 sessions per week for 2 weeks) in relieving pain and restoring functionality of the affected upper limb, thus evaluating beneficial effects of fire needling therapy on pain, active functionality as well as quality of life and providing trustworthy evidence of acupuncture in treating post-stroke CRPS of the upper limb.
Methods and Analysis
Study Design and Setting
This study will be a single-centre, two-arm, randomised controlled trial (RCT) conducted at Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University. The trial has been registered with the Chinese Clinical Trial Registry (registered number: ChiCTR2300068841) and approved by the research ethics committee. This study will be conducted in accordance with the Declaration of Helsinki and reported following the Consolidated Standards of Reporting Trials (CONSORT) statement. This protocol has been presented according to the SPIRIT guidelines. Recruitment strategies include online and print advertisements through Wechat (a common social media platform in China), hospital websites, and flyers at outpatient units. The intervention includes 2 weeks of fire needling therapy or manual acupuncture and 12 weeks of follow-up (Figures 1 and 2).
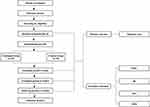 |
Figure 1 Flow diagram. Abbreviations: FMA, Fugl–Meyer Assessment; BI, Barthel Scale/Index; PPT, Pressure Pain Threshold; SWE, Shear-Wave Elastography. |
 |
Figure 2 Schedule for data collection of this trial. Abbreviations: FMA, Fugl–Meyer Assessment; BI, Barthel Scale/Index; PPT, Pressure Pain Threshold; SWE, Shear-Wave Elastography. |
Participants
Participants will be recruited from the outpatient units of acupuncture and moxibustion department by two well-trained researchers who have at least 3 years’ experience in doing clinical trials. The enrollment started in March 2023 and is expected to end in December 2024. Each eligible and consenting patient will be randomly allocated in a 1:1 ratio to receive 2-week fire needling therapy or manual acupuncture.
Inclusion Criteria
- Aged 40–75 years, male or female
- Diagnosed with stroke based on National Institute for Health and Clinical Excellence (NICE) Guidelines 2019 Edition11 (within 2 weeks to 3 months after the onset of stroke)
- Total or partial anterior circulation infarction confirmed by computed tomography (CT) or magnetic resonance imaging (MRI)
- Diagnosed with CRSP of the upper limb according to the Budapest criteria,12 and an average pain intensity of ≥4 on an 11-point numerical rating scale (NRS) in the last week
- Muscle strength of affected upper limb of ≤3
- Fugl–Meyer Assessment of ≤54
- Provision of written informed consent
Exclusion Criteria
- Having severe posterior circulation infarction, thalamic infarction, progressive stroke, cerebral embolism, brain tumor
- Having hemorrhagic disease, liver or kidney disease, metabolic disease, hematopoietic system disease, infectious disease or other severe acute or chronic organic diseases that belong to the contraindication of fire needling therapy
- Had two or more strokes
- Shoulder and hand dysfunction existed before the stroke or was caused by other reasons (upper limb fracture, periarthritis of shoulder, peripheral nerve injury, etc.)
- Having sensory abnormalities, especially having an abnormal pain threshold
- Having undergone acupuncture therapy within 3 months
- Having mental disorders or severe cognitive disorders
- Pregnant or breastfeeding women
- Having participated in other clinical trials within 3 months
- Unable to cooperate or unwilling to comply with all study requirements
- Cardiac pacemaker, metal allergy, or fire needle phobia
Randomisation and Allocation Concealment
Participants will be randomly assigned to the treatment group (fire needling therapy) or control group (manual acupuncture) in a ratio of 1:1. The randomisation sequence will be generated with the R software (4.2.1), using block randomisation with a block size of 4 by an independent statistician who is not involved in the later implementation or statistical analysis. The randomisation sequence will be put into an opaque envelope which is marked with an enrollment number on the cover. The generation of randomisation sequence and opaque envelopes will be provided by the Research Center of Clinical Epidemiology (Beijing Institute of Traditional Chinese Medicine). Researchers will be required to open the envelope according to the order of each patient’s enrollment and implement the intervention based on the group information inside the envelope.
Blinding
The acupuncturists and participants will not be blinded because of the nature of the intervention. However, data analysts and outcome assessors will be blinded to group assignment. The allocation of all participants will be concealed until the statistical analysis is completed.
Interventions
Acupuncture prescription was developed from a literature review (Chinese Sinew Theory), as well as the clinical practice. Acupuncturists are required to have a Chinese medicine practitioner licence, and two acupuncturists will be given trial-specific training at the project launching session to understand their role and job in this study. Each participant will receive fire needling therapy or manual acupuncture from one acupuncturist throughout the trial. The acupuncture points in this study are not in classical locations; rather, they are in sinew-knot points that have severe tenderness with stripe or swelling and thickening of local tissue. Ten compulsory points include Jianyuci, Jianliaoci, Bingfengci, Tianzongci, Jianzhenci, Taijianci, Jujianci, Yangxici, Yangchici, and Yangguci (Table 1 and Figure 3).
 |
Table 1 Locations of Acupuncture Points Used in Treatment Group and Control Group |
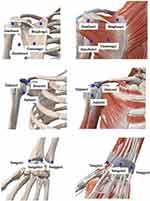 |
Figure 3 Locations of acupuncture points. |
Treatment Group (Fire Needling Therapy)
Fire needling therapy will be performed with a needle made of manganese and tungsten alloy, which will maintain hardness at high temperatures (He’s fire needling instrument, size 0.5mm*25mm). Nail scratch marks will be given at chosen points where the acupuncturists will insert the red-hot needle burnt over spirit cotton and will withdraw the needle swiftly without retention; each chosen point will be pricked twice. The depth of needling should be 5–10mm, depending on the figure of participants. The needle hole will be pressed with a sterilised dry cotton ball for 30 seconds.
Participants in the treatment group will receive fire needling therapy 5 times per week (Monday to Friday) for 2 weeks.
Control Group (Manual Acupuncture)
Manual acupuncture will be performed with sterile disposable needles (Hwato Needles, Sino-foreign Joint Venture Suzhou Hwato Medical Instruments, China, size 0.3mm*40mm). The needles will be inserted to a depth of 15–30mm at the marked place and will be stimulated manually (lifting and thrusting combined with twirling and rotating) for at least 10 seconds to achieve “Deqi” (a compositional sensation of soreness, numbness, distention, and heaviness). The needles will be manually stimulated every 10 minutes and will be retained for 30 minutes.
Participants in the control group will receive manual acupuncture 5 times per week (Monday to Friday) for 2 weeks.
Outcomes
Primary Outcome Measurement
The primary outcome will be revealed as an effective response rate whereby the proportion of participants whose average pain NRS scores decrease by at least 2 units13 and the FMA scores decrease by at least 7.25 units14 at Week 2 compared with baseline. NRS is an 11-point self-administered instrument, ranging from 0 (no pain at all) to 10 (the worst pain).15 The minimal clinically important difference (MCID) of NRS and FMA is 2 units and 7.25 units, respectively. The effective response rate will be assessed at Week 2 within 1 hour after treatment.
Secondary Outcome Measurement
Motor Function Assessment
The motor function of the upper limb will be assessed using Fugl–Meyer Assessment (FMA), which was designed for post-stroke hemiplegic patients of all ages. The FMA is able to assess motor function, balance, sensation and joint function, and it is applied clinically to evaluate disease severity, motor recovery, and treatment effectiveness. The scale consists of 5 domains and 155 items in total: motor function, sensory function, balance, joint range of motion, joint pain. The motor domain of the upper limb includes 33 items assessing movement, coordination and reflex action of shoulder, elbow, forearm, wrist, and hand. Scoring is based on direct observation of performance and each item is scored using a 3-point ordinal scale: 0=cannot perform, 1=performs partially, and 2=performs fully. The highest score is 66 and the classifications for impairment severity are: <33 – very severe; 33–43 – severe; 44–54 – moderate; 55–64 – mild. Higher scores indicate better motor function.16,17 FMA will be applied at Weeks 0, 1, 2 (within 1 hour after the treatment), and 14 (from 8 a.m. to 9 a.m.).
Activities of Daily Living
The activities of daily living will be assessed using the Barthel Scale/Index (BI). BI includes 10 items: feeding, personal toileting, bathing, dressing and undressing, getting on and off a toilet, controlling bladder, controlling bowel, moving from wheelchair to bed and returning, walking on level surface (or propelling a wheelchair if unable to walk) and ascending and descending stairs. The original BI is a 3-point ordinal scale: 0=unable, 1=needs help, 2=independent. The total score is multiplied by 5 to get a number on a 100-point scale. Most studies apply the 60/61 cutting point: >60 – slight dependency; 60–40 – moderate dependency; 39–20 – severe dependency; <20 – total dependency. A higher score reflects a greater ability to function independently following hospital discharge.18,19 BI will be applied at Weeks 0, 1, 2 (within 1 hour after the treatment), and 14 (from 8 a.m. to 9 a.m.).
Pain Threshold
The most painful point of shoulder, elbow, and wrist joint will be palpated and marked with a brown marker. The pressure pain threshold (PPT) of the three points will be measured using a pressure algometer (Somedic AB, Sweden), which consists of a rod with a circular end (1 cm2). The pressure algometer will be placed perpendicularly to the skin and applied at a gradually increasing rate (0.5 kg/cm2/second) until the participant indicates that the pressure sensation changes to slight pain by raising their right hand. Then the algometer will be removed immediately from the skin. Participants will be familiarised with the test by applying gradual pressure to the palm of their right hand before formal assessment. Each point will be measured three times, and the interval between two points will be two minutes. The lowest value of each point will be recorded by a professional assessor who will implement PPT tests throughout the trial alone. The final value will be the average of three measurements. Lower PPT values reflect greater pain sensitivity.20,21 PPT will be measured at Weeks 0, 1, and 2 (within 1 hour after the treatment).
Quantification of Muscle Properties (Elasticity Modulus)
Three locations of biceps brachii, triceps brachii and deltoid muscle at 3 cm from the proximal insertion point will be selected. The value of elasticity modulus is expressed in kilopascals (kPa) and these three locations will be measured using AixPlorer real-time shear wave elastography (SWE; L4-15 linear probe [frequency:4–15MHz, range: 180kPa], SuperSonic Imagine). A B-mode image of the three places will be detected first. Then in real time, the elasticity will appear colour coded (colour scaled, ranging from 0 to 40 kPa), and the elasticity modulus value (minimum, maximum, average and standard deviation) at the three locations will be sampled using a round region of interest (ROI) of 10 mm. The distance between the probe and the target locations will be 1 to 2 cm. Each location will be measured three times, and the value of each location will be recorded by a well-trained ultrasound physician who will implement the test throughout the trial alone. The final value will be the average of three measurements.22,23 SWE will be applied at Weeks 0, 1, and 2 (within 1 hour after the treatment).
Compliance of Participants
Participants with scores lower than 80 will be considered poorly compliant at Week 2:
For example, say a participant receives 7 treatment sessions when the total number of required sessions in this study is 10; based on the above equation, their compliance score is 70, which means that that participant is poorly compliant. Participants demonstrating poor compliance will nonetheless still be included for statistical analysis.
Assessment of Safety
All adverse events will be documented by experienced acupuncturists and outcome assessors at Weeks 0 (baseline), 1, 2, 6, 10 and 14. Common acupuncture-related adverse events involve continuous post-needling pain, dizziness, subcutaneous hematoma, infection, etc.
Sample Size
There is no previous study regarding the effective response rate of fire needling therapy on post-stroke CRPS of the upper limb (the proportion of participants whose average pain NRS score decreases by at least 2 units and FMA score decreases by at least 7.25 units at Week 2 compared with baseline). The exploratory nature of the study does not necessarily require a formal sample size calculation. Therefore, a sample size of 60 participants (30 for each group) will be recruited to achieve the practical purpose of the trial based on clinical experience.24
Statistical Analysis
The statistical analyses will be performed by a blinded biostatistician using SAS (SAS Institute, Cary, NC, USA) and R software (4.2.1). Analyses will be conducted as intention-to-treat (ITT) that includes all those participants who have been randomised and given at least one treatment session. Demographic characteristics will be summarised by treatment arm. Continuous variables will be presented as mean (standard deviation) or median (interquartile range) according to the normality of the distribution. Categorical variables will be described in percentages. For primary outcomes, the effective response rate will be compared between treatment group and control group using a chi-squared test. In terms of secondary outcomes, ranked data including FMA and BI will be analysed based on mixed-effects linear models; continuous variables including PPT and SWE will be analysed based on repeated measurement using a mixed-effects model; α will be set at 0.05. The compliance scores and adverse event rates will be described using a chi-squared test. For the dropout analysis, the last observation carried forward method will be used.
Discussion
It is best to treat CRPS during the early acute stages because there exists a small-time window in which treatment is most effective. During the acute phase, patients will show early autonomic signs, including swelling and skin color changes. This is the best time for the patients to receive treatment. However, after these signs disappear, the advanced phase begins where treatment is much less effective. Thus, CRPS causes severe consequences for patients on both physical and emotional sides. Post-stroke CRPS of the upper limb causes pain and dysfunction of the affected limb, and the therapeutic goal is to relieve pain and restore functionality in the early stage of the disease. However, the conventional therapies used in post-stroke CRPS may not completely improve the pain and functionality and may even entail side effects and restrictions of daily life. Therefore, alternative therapies that allow for the reduction of prescribed medication are required to promote therapeutic effects and decrease side effects. In China, fire needling therapy is often used to treat post-stroke CRPS of the upper limb. A network meta-analysis10 included 21 studies that evaluated 1508 patients on the role of fire needling therapy for post-stroke CRPS of the upper limb. The study has suggested that fire needling therapy is effective for post-stroke CRPS of the upper limb and could be the best choice for improving active functionality compared with manual acupuncture, warm acupuncture, and conventional treatment (medication and physical training). However, the study also mentioned that the quality of the original trials is poor, thus high-quality studies using a well-designed methodology are required to assess the effectiveness of fire needling therapy.
We have cautiously designed the present RCT and the methodological demands such as adequate randomisation, allocation concealment, and blinding of outcome assessors and statisticians are well-established in this trial, aiming to provide trustworthy evidence on the effectiveness of acupuncture in treating post-stroke CRPS of the upper limb. Specifically, we will evaluate the beneficial effects of fire needling therapy on pain, active functionality as well as quality of life. We will use computer-generated randomisation and allocation concealment from a third party to minimise selection bias and apply block randomisation to ensure prognostic balance between intervention groups. Data analysts and outcome assessors will be blinded to group assignment. The allocation of all participants will be concealed until the statistical analysis is completed. All the processes are based on traditional Chinese medicine theory as well as clinical practice. There will be 5 sessions per week in the 2-week treatment phase, giving a total of 10 sessions with a 12-week follow up. The well-designed study may have a profound impact on comparisons between fire needling therapy and manual acupuncture, as well as on the clinical practice of fire needling therapy on post-stroke CRPS of the upper limb.
Our study has several limitations. First, the participants and acupuncturists will not be blinded. Therefore, it’s difficult to exclude the placebo effects from the participants. Moreover, our study is a single-centre RCT and the participants come from the same hospital, which may cause poor representativeness of the participants and poor extrapolation of the findings. Additionally, although acupuncture prescription is based on literature review and clinical practice, the locations are different from the classical ones, which might hinder the clinical application of fire needling therapy for many acupuncturists who are not familiar with sinew theory. Moreover, we only set two groups to compare the effectiveness of fire needling therapy, thus future studies should explore more types of acupuncture treatment to determine whether fire needling therapy is the optimal option for post-stroke CRPS of the upper limb. In addition, fire needling causes a stronger response in the human body, which may lead to poor compliance because of the pain from the stronger response. Finally, the 12-week follow-up phase in our study may cause a high rate of loss after the end of treatment, but we will take several measures throughout the study to maximise participant retention.
In conclusion, we will conduct a single-centre, two-arm RCT to compare the effectiveness of fire needling therapy and manual acupuncture. We will use a rigorous methodology to minimise bias and set up supervising committees to ensure the quality of our study, thus providing trustworthy evidence for better understanding and generalising of fire needling therapy in treating post-stroke CRPS of the upper limb.
Ethics and Dissemination
This protocol has been registered with the Chinese Clinical Trial Registry and approved by Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University Research Ethics Committee (number: 2022BL02-076-02). The Ethics Committee will audit the trial every 3 months to decide on any premature closure of the study. Written informed consent will be obtained from all participants. The CRFs will be preserved in a locked cabinet at the participating hospitals and can be accessed by the research team only. Patient identifiable data will be used to provide clinical care and follow-up only, and the trial database will be anonymised. The aggregated research findings will be submitted for publication in a peer-reviewed clinical journal so as to ensure widespread dissemination. The original paper files and electronic data will be preserved for at least 5 years after publication, which will be available from the corresponding author with appropriate reasons.
Patient Consent for Publication
Obtained.
Funding
This work was supported by Beijing Municipal Administration of Hospitals Incubating Program (code: PZ2022012); Young Elite Scientists Sponsorship Program by BAST (BeiJing Association For Science and Technology) (grant number: BYESS202337); Beijing Traditional Chinese Medicine Science and Technology Development Fund Youth Project (grant number: QN-2020-28); China National Natural Science Foundation (grant number: 82004452; 82074547; 82205246).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Smart KM, Wand BM, O’Connell NE. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst Rev. 2016;2(2):Cd010853. doi:10.1002/14651858.CD010853.pub2
2. Petersen PB, Mikkelsen KL, Lauritzen JB, Krogsgaard MR. Risk factors for post-treatment complex regional pain syndrome (CRPS): an analysis of 647 cases of CRPS from the Danish patient compensation association. Pain Pract. 2018;18(3):341–349. doi:10.1111/papr.12610
3. Ott S, Maihöfner C. Signs and symptoms in 1043 patients with complex regional pain syndrome. J Pain. 2018;19(6):599–611. doi:10.1016/j.jpain.2018.01.004
4. de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129(1–2):12–20. doi:10.1016/j.pain.2006.09.008
5. Saha S, Sur M, Ray Chaudhuri G, Agarwal S. Effects of mirror therapy on oedema, pain and functional activities in patients with poststroke shoulder-hand syndrome: a randomized controlled trial. Physiother Res Int. 2021;26(3):e1902. doi:10.1002/pri.1902
6. Misidou C, Papagoras C. Complex Regional Pain Syndrome: an update. Mediterr J Rheumatol. 2019;30(1):16–25. doi:10.31138/mjr.30.1.16
7. Harden RN, Oaklander AL, Burton AW, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Med. 2013;14(2):180–229. doi:10.1111/pme.12033
8. Shim H, Rose J, Halle S, Shekane P. Complex regional pain syndrome: a narrative review for the practising clinician. Br J Anaesth. 2019;123(2):e424–e433. doi:10.1016/j.bja.2019.03.030
9. Wang S, Zhao W, Qian G, Guo C, Lin G. Professor ZHANG Jiawei’s clinical experience of stage treatment for shoulder-hand syndrome after stroke. Zhongguo Zhen Jiu. 2018;38(8):877–880. doi:10.13703/j.0255-2930.2018.08.020
10. Wang RQ, Wu QZ, Huang CH, Rao WF. [Network Meta-analysis of 4 acupuncture therapies for shoulder hand syndrome after stroke] 4种针刺疗法治疗中风后肩手综合征的网状Meta分析. Zhongguo Zhen Jiu. 2021;41(5):563–569. Chinese. doi:10.13703/j.0255-2930.20200325-k0011
11. National Institute for Health and Clinical Excellence (Great Britain). National Institute for Health and Care Excellence: guidelines. In: Stroke and Transient Ischaemic Attack in Over 16s: Diagnosis and Initial Management. London: National Institute for Health and Care Excellence (NICE) Copyright © NICE 2022; 2022.
12. Harden RN, McCabe CS, Goebel A, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines. Pain Med. 2022;23(Suppl 1):S1–s53. doi:10.1093/pm/pnac046
13. Datta R, Agrawal J, Sharma A, Rathore VS, Datta S. A study of the efficacy of stellate ganglion blocks in complex regional pain syndromes of the upper body. J Anaesthesiol Clin Pharmacol. 2017;33(4):534–540. doi:10.4103/joacp.JOACP_326_16
14. Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791–798. doi:10.2522/ptj.20110009
15. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011;63(11):S240–252. doi:10.1002/acr.20543
16. Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. doi:10.2340/1650197771331
17. Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer Assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240. doi:10.1177/154596802401105171
18. Shah N, Shrivastava M, Kumar S, Nagi RS. Supervised, individualised exercise reduces fatigue and improves strength and quality of life more than unsupervised home exercise in people with chronic Guillain-Barré syndrome: a randomised trial. J Physiother. 2022;68(2):123–129. doi:10.1016/j.jphys.2022.03.007
19. Taghizadeh G, Martinez-Martin P, Meimandi M, et al. Barthel index and modified rankin scale: psychometric properties during medication phases in idiopathic Parkinson disease. Ann Phys Rehabil Med. 2020;63(6):500–504. doi:10.1016/j.rehab.2019.08.006
20. Fernandes GS, Sarmanova A, Warner S, et al. Knee pain and related health in the community study (KPIC): a cohort study protocol. BMC Musculoskelet Disord. 2017;18(1):404. doi:10.1186/s12891-017-1761-4
21. Stefanik JJ, Frey-Law L, Segal NA, et al. The relation of peripheral and central sensitization to muscle co-contraction: the MOST study. Osteoarthritis Cartilage. 2020;28(9):1214–1219. doi:10.1016/j.joca.2020.06.002
22. Nallet C, Pazart L, Cochet C, et al. Prenatal quantification of human foetal lung and liver elasticities between 24 and 39 weeks of gestation using 2D shear wave elastography. Eur Radiol. 2022;32(8):5559–5567. doi:10.1007/s00330-022-08654-1
23. Taljanovic MS, Gimber LH, Becker GW, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. 2017;37(3):855–870. doi:10.1148/rg.2017160116
24. Johanson G, Brooks G. Initial scale development: sample size for pilot studies. Educ Psychol Meas. 2010;70:394–400. doi:10.1177/0013164409355692
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.