Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Ferroptosis and Traditional Chinese Medicine for Type 2 Diabetes Mellitus
Authors Xie D, Li K, Feng R, Xiao M, Sheng Z, Xie Y
Received 16 March 2023
Accepted for publication 3 June 2023
Published 26 June 2023 Volume 2023:16 Pages 1915—1930
DOI https://doi.org/10.2147/DMSO.S412747
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Dandan Xie,1– 3,* Kai Li,1,* Ruxue Feng,4,* Man Xiao,5 Zhifeng Sheng,2 Yiqiang Xie1
1College of Traditional Chinese Medicine, Hainan Medical University, Haikou, Hainan, People’s Republic of China; 2National Clinical Research Center for Metabolic Diseases, Hunan Provincial Key Laboratory of Metabolic Bone Diseases, Department of Metabolism and Endocrinology, Health Management Center, the Second Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China; 3Department of Clinical Nutrition, the First Affiliated Hospital of Hainan Medical University, Haikou, Hainan, People’s Republic of China; 4Department of Stomatology, Geriatric Hospital of Hainan, Haikou, Hainan, People’s Republic of China; 5Department of Biochemistry and Molecular Biology, Hainan Medical University, Haikou, Hainan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yiqiang Xie, College of Traditional Chinese Medicine, Hainan Medical University, No. 3, Xueyuan Road, Haikou, Hainan, 571199, People’s Republic of China, Tel +86 13036001921, Email [email protected] Zhifeng Sheng, National Clinical Research Center for Metabolic Diseases, Hunan Provincial Key Laboratory of Metabolic Bone Diseases, Department of Metabolism and Endocrinology, Health Management Center, the Second Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China, Tel +86 13574806523, Email [email protected]
Abstract: Ferroptosis, an emerging form of regulated programmed cell death, has garnered significant attention in the past decade. It is characterized by the accumulation of lipid peroxides and subsequent damage to cellular membranes, which is dependent on iron. Ferroptosis has been implicated in the pathogenesis of various diseases, including tumors and diabetes mellitus. Traditional Chinese medicine (TCM) has unique advantages in preventing and treating type 2 diabetes mellitus (T2DM) due to its anti-inflammatory, antioxidant, immunomodulatory, and intestinal flora-regulating functions. Recent studies have determined that TCM may exert therapeutic effects on T2DM and its complications by modulating the ferroptosis-related pathways. Therefore, a comprehensive and systematic understanding of the role of ferroptosis in the pathogenesis and TCM treatment of T2DM is of great significance for developing therapeutic drugs for T2DM and enriching the spectrum of effective T2DM treatment with TCM. In this review, we review the concept, mechanism, and regulatory pathways of ferroptosis and the ferroptosis mechanism of action involved in the development of T2DM. Also, we develop a search strategy, establish strict inclusion and exclusion criteria, and summarize and analyze the application of the ferroptosis mechanism in TCM studies related to T2DM and its complications. Finally, we discuss the shortcomings of current studies and propose a future research focus.
Keywords: ferroptosis, traditional Chinese medicine, type 2 diabetes mellitus, complications
Introduction
With an aging global population and social lifestyle changes, the diabetes mellitus (DM) prevalence rate continues to increase annually. The most recent data released by the International Diabetes Federation (IDF)1 stated that the DM global prevalence in adults aged 20–79 years was expected to be approximately 10.5% (>500 million people) in 2021. Moreover, the largest increase in DM prevalence by 2045 is expected to occur in middle-income countries. In China, the most recent epidemiological survey2–4 revealed that DM prevalence among adults aged ≥18 years increased from 9.7% in 2007–2008 to 11.2% in 2015–2017, among which type 2 DM (T2DM) accounted for >90% of cases. T2DM is a metabolic disease characterized by elevated blood glucose caused by insulin resistance (IR) combined with a relative decrease in insulin secretion. Long-term carbohydrate metabolism disorders and related fat and protein metabolism impairments can cause chronic progressive damage to the kidney, eye, nerves, heart vessels, bone, and other tissues and organs. T2DM and its complications have become an important public health problem that seriously threatens human life and health and represents an important cause of death and disability.5 The etiology and pathogenesis of T2DM are complex, with genetic, environmental, and gut microbiota factors considered the major causes of T2DM, while oxidative stress, inflammation, endothelial cell damage, apoptosis, and autophagy are closely related to its development.
Ferroptosis, a recently identified form of regulated programmed cell death, is characterized by its iron dependence and lipid peroxidation-induced cellular dysfunction. It has been implicated in various diseases, including tumors and neurodegenerative diseases. In the context of T2DM, studies have explored the association between ferroptosis and its occurrence and development.6 Patients with T2DM often exhibit elevated serum iron concentrations and reactive oxygen species (ROS) levels in pancreatic tissues and cells.7,8 Moreover, pancreatic β cells, responsible for insulin secretion, possess weaker antioxidant defenses and are susceptible to ferroptosis compared to other tissues.9–11 Therefore, ferroptosis may contribute to the dysfunction of pancreatic β cells and the development of T2DM. Traditional Chinese medicine (TCM) has been used to treat DM for a long time and was described in ancient Chinese medicinal texts such as Huangdi Neijing (425–221 BC) regarding obesity and overeating. The TCM theory classifies DM into the category of “xiaoke” or “xiaodan”. With the continuous in-depth understanding and practice related to DM among the doctors of previous dynasties, the clinical TCM theory of T2DM has been gradually enriched. TCM is multi-targeted and multi-component, with anti-inflammatory, immunoregulation, antioxidant stress, and intestinal flora regulation effects, which have unique advantages for preventing and treating T2DM. However, its targets and specific mechanisms of action are still not fully elaborated. Increasing evidence suggests that Chinese herbs and their active ingredients may modulate ferroptosis and thereby exert therapeutic effects on T2DM and its complications.12,13 This review paper explores the concept, mechanism, and regulatory pathways of ferroptosis and its involvement in T2DM development of and develops a search strategy with strict inclusion and exclusion criteria, By summarizing and analyzing the mechanisms underlying TCM’s treatment of T2DM and its complications, this study provides a novel theoretical basis and clinical perspective for the utilization of TCM in the management of T2DM.
Mechanisms of Ferroptosis
Overview of Ferroptosis
In 2012, the Stockwell research team proposed a new form of regulated programmed cell death, termed ferroptosis, which differs from apoptosis, necrosis, and autophagy.14 Under iron-rich and ROS conditions, phospholipids containing polyunsaturated fatty acids (PUFAs) in the cell membranes are prone to peroxidation, resulting in the continuous accumulation of lipid peroxidation products. These products eventually disrupt cell membrane integrity and induce the cell death known as ferroptosis. The main cellular morphological changes associated with ferroptosis are mitochondrial atrophy, which includes the reduction or loss of mitochondrial cristae, outer mitochondrial membrane rupture, and mitochondrial membrane wrinkling. The primary biochemical features include intracellular iron and ROS accumulation, inhibition of the cystine/glutamate antiporter (system Xc−), decreased glutathione peroxidase 4 (GPX4) activity, and reduced glutathione (GSH) production.15,16 The production of oxides of phospholipids containing PUFAs (PLOOHs) enforces ferroptosis, and PLOOH accumulation can lead to rapid and irreparable cell membrane damage, causing cellular iron death.
GSH represents the most abundant reducing agent in mammalian cells and is a cofactor of many enzymes (GPX4 and glutathione-S-transferase). System Xc− is an important intracellular antioxidant system (a transmembrane protein complex composed of the light chain subunit SLC7A11 and the heavy chain subunit SLC3A2) that regulates GSH synthesis by mediating cystine uptake and glutamate release. GPX4 is a selenoprotein that functions as a key enzyme to catalyze the reduction of PLOOHs to the corresponding alcohols to reduce lipid peroxide production.17–19 From this perspective, ferroptosis is involved in several pathophysiological processes and linked to cellular metabolism through iron, selenium, lipid, and redox reactions. Ferroptosis is associated with disease pathogenesis, including tumors, ischemic organ damage, neurodegenerative lesions, pulmonary fibrosis, and endocrine metabolic diseases. Therefore, targeting ferroptosis potentially represents an effective therapeutic modality for ferroptosis-related diseases by regulating the ferroptosis-related mechanisms.20,21
Regulation of Ferroptosis
Mechanisms Governing Ferroptosis
Essentially, ferroptosis occurs when the cellular antioxidant capacity becomes weakened and catalyzed by ferrous ions, intracellular lipid peroxidation metabolites continuously accumulate, intracellular redox homeostasis is imbalanced, and ferroptosis occurs. These factors cause irreparable cell membrane damage and result in cellular dysfunction.20 Therefore, the core molecular mechanism of ferroptosis is an imbalance of cellular metabolism and redox homeostasis, where the key signals include the accumulation of intracellular iron, ROS, and lipid peroxidation products (Figure 1).16,20,21
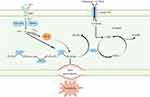 |
Figure 1 Mechanism of ferroptosis occurrence. This figure was created with Figdraw (www.figdraw.com). |
Regulation of Ferroptosis-Suppressing Pathways and Suppressors
It is currently believed that three major systems: cyst(e)ine/GSH/GPX4, FSP1/CoQ (ferroptosis suppressor protein 1, ubiquinone), and GCH1/BH4/DHFR (GTP cyclohydrolase 1, tetrahydrobiopterin, dihydrofolate reductase), effectively inhibit lipid peroxidation and thereby counteract the onset of ferroptosis (Figure 2).20–22
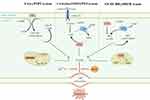 |
Figure 2 Regulation of ferroptosis suppressing pathways and suppressors. This figure was created with Figdraw (www.figdraw.com). Cytoplasmically located GPX4, mitochondrially located GPX4 and DHODH, plasma membrane located FSP1, and GCH1 (“?” indicates that the exact subcellular localization is unknown), together mediating the ferroptosis defense mechanism. |
Cyst(e)ine/GSH/GPX4: The classical ferroptosis-suppressing pathway. Located in the cytoplasm and mitochondria, GPX4 converts reduced GSH into oxidized GSH (glutathione disulfide, GSSG), which is converted to GSH by glutathione reductase (GSR) through the action of electrons donated by nicotinamide adenine dinucleotide phosphate (NADPH), thereby enabling GSSG recycling.20,22,23 CoQ10/FSP1: Located primarily in the plasma membrane, FSP1 inhibits ferroptosis by preventing lipid peroxide accumulation by reducing CoQ to ubiquinol (CoQH2) via NADPH and by acting on α-tocopherol (α-TOH).20–22,24,25 GCH1/BH4/DHFR: GCH1 (exact subcellular localization unknown) is a GPX4-independent ferroptosis suppressor gene identified using the CRISPR/dCas9 screening technique.26,27 GCH1 inhibits ferroptosis through its metabolites BH4 and dihydrobiopterin (BH2).21,26,27
Dihydroorotate dehydrogenase (DHODH) is a recently identified ferroptosis suppressor that is primarily located in the mitochondria.28 DHODH inhibits ferroptosis in the mitochondria by reducing CoQ to CoQH2 in concert with mitochondrial GPX4.21,29
The GPX4 in the cytoplasm, GPX4 and DHODH in the mitochondria, and FSP1 in the plasma membrane form a triad within the cell and together mediate the ferroptosis defense mechanism.29
Ferroptosis and the Pathogenesis of T2DM and Its Related Complications
Ferroptosis and T2DM Pathogenesis
Pancreatic β cell dysfunction and IR are the two main links in T2DM pathogenesis, T2DM occurs when β cells lose compensation to IR. The etiology of pancreatic β cell injury and the pathogenesis of T2DM are closely related to iron overload and ROS accumulation. Pancreatic β-cells are sensitive to ferroptosis. Iron overload7 and increased ROS8 are often present in the pancreatic tissues and cells of patients with T2DM. Compared with other tissues, the pancreas has the weaker antioxidant defense, pancreatic tissues have lower expression and activity of antioxidant enzymes (superoxide dismutase [SOD], catalase [CAT], GPX), and pancreatic β cells are susceptible to ROS-induced oxidative stress damage.9–11 When human pancreatic islet β cells were treated with the ferroptosis inducer erastin in vitro, the glucose-stimulated insulin secretion (GSIS) capacity was significantly reduced, whereas treatment with the ferroptosis inhibitor ferrostatin-1 (Fer-1) or the iron-chelating agent deferoxamine (DFO) rescued GSIS injury.30 These findings suggested that ferroptosis may be involved in T2DM occurrence and development by affecting the insulin secretion capacity of pancreatic β cells.
Environmental factors, such as long-term arsenic exposure and excessive iron intake, are also important factors in T2DM development.31–34 Wei et al35 constructed pancreatic dysfunction models both in vivo and in vitro using NaAsO2-induced Sprague-Dawley rats and MIN6 cells, respectively. They reported that ferroptosis was present in the pancreatic islet β cell injury models both in vivo and in vitro. The NaAsO2-induced mitochondrial damage produced excess mitochondrial ROS (MtROS), increased intracellular free iron levels and MtROS-dependent autophagy, and resulted in imbalanced iron homeostasis. These changes ultimately led to ferroptosis and insulin secretion dysfunction in pancreatic cells, whereas inhibiting the MtROS–autophagy–ferritin pathway improved the insulin secretion capacity of pancreatic β cells. In another study,36 iron stores were associated with the risk of developing DM. Iron regulatory genes, ferritin heavy chain (FTH1), and ferritin light chain (FTL) were highly expressed in islet tissues derived from diabetic patients and high-glucose-cultured INS-1 cells, heme oxygenase-1 (HO-1) and the inhibitor of differentiation proteins (ID1, ID3) may serve as potential endogenous antioxidants for pancreatic β cells against ROS and iron-overload, thereby protecting pancreatic β cells from oxidative stress and ferroptosis in T2DM patients.36
Ferroptosis and the Pathogenesis of T2DM Microangiopathy
Diabetic microvascular complications can affect various tissues and systems throughout the body and are associated with a variety of factors including microcirculatory disorders, inflammatory damage, and oxidative stress, among which nephropathy and retinopathy are the most common. Diabetic kidney disease (DKD) is a common chronic kidney disease and is thought to represent a major cause of end-stage renal disease (ESRD), which is responsible for approximately 30% to 50% of ESRD worldwide.37 Recent studies demonstrated that iron overload, ROS, and lipid peroxidation products accumulated in both mouse models of DKD and human renal tubular epithelial cells (HK-2) cultured under high glucose. The use of DFO or Fer-1 reduced renal iron accumulation and injury.38,39 Kim et al40 reported that renal biopsy samples derived from DKD patients had lower SLC7A11 and GPX4 mRNA expression compared to that from non-diabetic patients. They used streptozotocin (STZ)-induced DKD mice and transforming growth factor-β-1-stimulated proximal tubular epithelial cells in vivo and in vitro experiments, respectively, and found that the concentration of GSH was reduced, total iron levels and malondialdehyde (MDA, a lipid peroxidation product) were increased, SLC7A11 and GPX4 protein and mRNA expression levels were lower than in controls, and lipid peroxidation was enhanced. Fer-1 treatment alleviated these changes and significantly improved kidney damage and proteinuria caused by DM.40 It is suggested that ferroptosis is associated with the development of DKD and that inhibiting or attenuating ferroptosis may improve renal function in DKD.
Diabetic retinopathy (DR) is another common microvascular complication of T2DM. DR is a major cause of blindness in diabetic patients and is closely related to endothelial dysfunction and increased retinal capillary permeability.41 The current main DR treatment modalities include anti-angiogenic drug therapy and laser or surgical treatment.42 However, the benefits of these treatments for patients are also associated with adverse drug reactions or surgical risks. Zhang et al43 reported that human retinal vascular endothelial cell death induced by high-glucose treatment was associated with ferroptosis. Further investigation revealed that the high-glucose treatment upregulated TRIM46 (a member of the E3 ubiquitin ligase family TRIM), facilitated GPX4 ubiquitination, and induced ferroptosis in the cells. It was suggested that inhibiting ferroptosis by targeting TRIM46 and GPX4 represents a potential mechanism for effective DR treatment.
Ferroptosis and the Pathogenesis of T2DM Cardiovascular Complications
Patients with T2DM often have risk factors, such as obesity, abnormal lipid metabolism, and hypertension. Compared with the non-diabetic population, diabetic patients have a substantially increased risk of atherosclerotic vascular disease, which is one of the main causes of death in patients with T2DM.44–46 Diabetic cardiovascular complications can affect the heart, large blood vessels, and myocardial tissue, causing coronary atherosclerotic heart disease, diabetic cardiomyopathy, and other cardiac lesions.
The disorders of lipid and glucose metabolism are closely related to atherosclerosis development.47,48 The pathogenesis of diabetic atherosclerotic vasculopathy may be related to iron accumulation and lipid peroxidation.49–51 Using gene microarray technology (mRNA expression profiling) and bioinformatics analysis, Meng et al identified ferroptosis and HO-1 as important factors in diabetic atherosclerotic vascular disease.52 In vitro and in vivo diabetic atherosclerosis models were constructed using ApoE knockout mice and human umbilical vein endothelial cells (HUVECs), respectively. The results confirmed that Fer-1 reduced ROS production, attenuated high-glucose- and high-fat-induced lipid peroxidation, and reduced diabetic atherosclerosis formation. Similarly, knockout of the HO-1 gene reduced iron content, ROS production, lipid peroxidation, and ferroptosis in the HUVECs under a high-glucose environment. These findings suggested that ferroptosis is involved in diabetic atherosclerosis formation and that HO-1 may be a potential target for the treatment or drug development of diabetic atherosclerotic vascular disease.
Recently, several studies confirmed that diabetic cardiomyopathy development is associated with ferroptosis.53–55 Both DM myocardial ischemia-reperfusion injury model rats and a high-glucose hypoxia-reoxygenation cardiomyocyte model exhibited increased levels of iron ion concentration, ROS, SOD, MDA, and myocardial injury markers (serum creatine kinase MB and lactate dehydrogenase), ferroptosis, endoplasmic reticulum stress (ERS), and myocardial functional impairment. The ferroptosis inducer erastin or the inhibitor Fer-1 aggravated or reduced myocardial cell injury, respectively. Moreover, inhibiting endothelial network stress reduced ferroptosis and cell injury.54 Therefore, these findings indicated that ferroptosis is involved in DM myocardial ischemia–reperfusion-induced cardiomyocyte injury and is associated with ERS.
Endothelial cell injury is another important pathological mechanism in DM and diabetic cardiovascular disease.56 Luo et al57 reported that in HUVECs treated with high glucose and interleukin 1β, cell viability decreased, lipid ROS increased, and GSH and GPX4 concentrations decreased, and after treatment with ferroptosis inhibitors DFO and Fer-1, ROS levels in HUVECs decreased significantly and cell viability and GPX4 concentrations increased compared to pre-treatment. Further, transient transfection of HUVECs using p53 small interfering ribonucleic acid revealed that p53 small interfering ribonucleic acid attenuated the decrease in xCT (the light chain subunit of system Xc−, also known as SLC7A11) and GSH and the increase in ROS induced by HG and IL-1β. In addition, in the aortic endothelium of db/db mice, p53 mRNA was up-regulated, xCT mRNA was down-regulated, and de-endothelialization areas were also observed. These findings suggested that ferroptosis may be involved in the pathogenesis of diabetic vascular endothelial cell dysfunction through the p53-xCT-GSH axis.57
Together, the aforementioned studies suggested that ferroptosis is involved in the pathogenesis of diabetic cardiovascular complications and that inhibiting the ferroptosis-related mechanisms represents a potential therapeutic target for diabetic cardiovascular disease.58
Ferroptosis and the Pathogenesis of Abnormal Bone Metabolism in T2DM
In patients with T2DM, chronic hyperglycemia leads to the accumulation of advanced glycation end-products (AGEs) in the bone matrix, triggering non-enzymatic glycosylation reactions that result in decreased bone quality, increased bone fragility, and a heightened risk of osteoporosis (OP) and fractures.59,60 Recently, Ge et al investigated the role of AGEs in diabetes-related OP and reported that the serum AGEs levels and bone mineral density in patients with OP were positively and negatively correlated with fasting glucose, respectively, and that AGEs and serum from patients with OP and T2DM could promote the development of ferroptosis in hFOB1.19 osteoblast, which was reversed by the ferroptosis inhibitor DFO. The results suggest that AGEs may promote OP by disrupting osteoblast function.61 The loss of osteocyte viability is another important factor in the development of diabetic osteoporosis. Another recent study62 reported that osteocytes cultured in a diabetic microenvironment had increased lipid peroxidation, iron overload, ferroptosis pathway activation, and significant upregulation of HO-1 expression. Moreover, targeting ferroptosis or HO-1 rescued osteocyte death and improved bone structural degeneration in the diabetic OP. These studies suggested that ferroptosis is involved in the development of diabetic OP and that targeting ferroptosis may represent an effective mechanism-based strategy for OP treatment.
Application of Ferroptosis in TCM Treatment of T2DM and Its Related Complications
Search Strategy
We searched PubMed, Web of Science, the Cochrane Library, the Chinese National Knowledge Infrastructure database (CNKI), the Chinese Biomedical Literature database (CBM), the Chinese Scientific Journal database (VIP), and the Wan Fang database for articles published from January 1, 2012, to March 27, 2022. No language restrictions were imposed. The medical subject headings and main keywords used for the search were (“Diabetes Mellitus” OR diabet* OR glucose) AND (“Ferroptosis” OR ferropto* OR “iron death” OR (iron AND “cell death”)). The full search strategy used is shown in the Supplemental Appendix. The supplemental literature was searched manually.
Selection Criteria
Inclusion criteria: (1) study type: clinical trials or basic experimental studies; (2) study object: patients with T2DM or its related complications, animal or cell models; (3) interventions: active ingredients, monomers, or compound preparation of TCM; (4) mechanism of action: ferroptosis. Correspondence, comments, editorials, reviews, meta-analyses, and conference abstracts were excluded.
Data Extraction
Two investigators independently reviewed the full text of the studies that met the selection criteria and extracted the following data: first author’s name, year of publication, disease type, study object, ferroptosis regulation mechanism, and characteristics of action (Table 1). When there was disagreement between the two investigators, a third researcher was consulted to make the final decision.
 |
Table 1 The Role of Ferroptosis in the Therapeutic Use of TCM for T2DM and Its Related Complications |
Results
Figure 3 illustrates the inclusion screening process employed in this study. A comprehensive database search yielded a total of 726 potentially relevant studies. Additionally, two articles were manually searched, bringing the cumulative number of potentially relevant studies to 728. Subsequently, eliminating 205 duplicate articles and 502 articles that were deemed not relevant after reading the titles and abstracts, 21 articles were entered in the full-text review. After a critical evaluation of the complete texts according to the predetermined inclusion and exclusion criteria, eight articles were excluded. Ultimately, a total of 13 eligible studies were included in the final analysis.
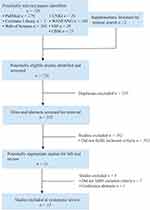 |
Figure 3 Flow chart of the literature retrieval and screening process. |
These 13 studies primarily consisted of basic experimental investigations conducted on animal or cell models. Among them, five studies were relevant to T2DM, four to DKD, three to diabetic cardiomyopathy, and one addressed DR. Notably, no study exploring the treatment of diabetes-related OP through TCM via the modulation of ferroptosis was identified. Table 1 provides an overview of the main characteristics of the studies included in this review.
Analysis
Application of Ferroptosis in TCM Treatment of T2DM
Diabetic patients have elevated ROS levels8 and dietary iron intake is associated with the risk of developing T2DM.76,77 Iron overload tends to lead to cellular oxidative damage, promoting the occurrence of ferroptosis, causing pancreatic β cell dysfunction, and thereby participating in T2DM occurrence and development.78 Recent studies64,79–81 indicate that natural polyphenolic compounds possess iron-chelating properties in addition to their well-known antioxidant, anti-inflammatory, and anti-tumor effects, enabling them to regulate ferroptosis and reduce blood glucose levels.
Curcumin, derived from the rhizomes of turmeric (Curcuma longa L.) and other ginger family plants, (-)-Epigallocatechin-3-gallate (EGCG) found in tea, especially green tea, and grapeseed procyanidin extract (GSPE) abundant in various plants, particularly grape seeds, all containing polyphenols, have been studied. Treatment with these polyphenolic compounds, such as curcumin, EGCG, and GSPE, has shown an increase in cell viability and a decrease in iron accumulation, depletion of GSH, inactivation of GPX4, levels of acyl-CoA synthetase long-chain family member 4 (ACSL4) and lipid peroxidation in mouse pancreatic MIN6 cells when compared to control cells exposed to erastin alone.63,64 Consistent with these findings, diabetic rats exhibited decreased iron content, increased GSH activity in pancreatic tissue, alleviation of ferroptosis and pancreatic damage, increased insulin levels, and reduced blood glucose levels.65 These effects may be associated with the activation of Nrf2-related signaling pathways.63,64
Quercetin, a flavonoid found widely in various plants,82–84 has been found to potentially regulate ferroptosis.66 Compared to the control group, quercetin reduced the iron content in the T2DM mice pancreas, increased the expression of GSH and GPX4, and reduced oxidative stress in pancreatic tissues. Furthermore, quercetin demonstrated the viability to restore the viability of pancreatic β cells under high-glucose stimulation, suggesting its potential beneficial effects on T2DM by inhibiting pancreatic iron accumulation and ferroptosis in pancreatic β cells.
Additionally, studies focused on mulberry (Morus alba L.) leaf extract, a Chinese herbal medicine, revealed its potential mechanism to regulate abnormalities in glycolipid metabolism.85–87 Cryptochlorogenic acid,67 the primary active substance in mulberry leaves, ameliorated islet damage in diabetic rats by inhibiting ferroptosis, reducing iron overload and accumulation of lipid peroxides, and lowering blood glucose levels. The mechanism underlying these effects involves the activation of the cystine/Xc−/GPX4/Nrf2 pathway and the inhibition of nuclear receptor coactivator 4 (NCOA4).67
Taken together, these findings underscore the potential of TCM Taken together, these findings underscore the potential of Chinese herbal medicine to elevate GSH and GPX4 levels in mice with T2DM by modulating ferroptosis in pancreatic tissues or cells, possibly involving the cystine/ Xc−/GPX4/Nrf2 pathway.
Application of Ferroptosis in TCM Treatment of T2DM Microangiopathy
TCM has demonstrated significant efficacy in ameliorating kidney damage and preserving kidney function and is widely used in DKD treatment in some countries, including China. Numerous studies have elucidated that the therapeutic protective effects of TCM on DKD are closely intertwined with its regulation of glucose/lipid metabolism, antioxidant activity, anti-inflammatory response, anti-fibrotic properties, and protection of podocytes.88 Recent reports have highlighted the pivotal role of ferroptosis in the TCM treatment of DKD. Notably, TCM rhubarb (Rheum palmatum L.) has shown remarkable efficacy in improving lipid metabolism in DKD patients.89 Ding et al68 reported that sennoside A, an active compound found in rhubarb, reduced MDA levels, downregulated the expression of HO-1 and PTGS2, and increased GSH concentration to inhibit ferroptosis in DKD mice, thereby improving oxidative stress and renal injury in DKD. Another active compound, berberine, extracted from the rhizome of Coptis chinensis Franch (known as Huanglian in Chinese), has been found to effectively reduce ROS, PTGS2, and ACSL4 levels while upregulating the expression of Nrf2, HO-1, and GPX4 to improve ferroptosis in high-glucose-induced podocytes.69 These effects were achieved through the regulation of the Nrf2/HO-1/GPX4 pathway, thus providing a new theoretical foundation for the application of berberine in DKD treatment. Additionally, a study70 investigating the mechanism of action of umbelliferone, a coumarin derivative found in traditional herbal components such as Cnidium monnieri (L.) Cuss, Angelica dahurica (Fisch. ex Hoffm) Benth. et Hook. f., and Peucedanum praeruptorum Dunn, revealed that it protected against DKD. Umbelliferone treatment decreased ROS accumulation, downregulated ACSL4, and upregulated GPX4, Nrf2, and HO-1 expression, resulting in the alleviation of ferroptosis and renal pathological damage in db/db DKD mice.70 Knockdown of Nrf2 blocked the inhibitory effect of umbelliferone on ferroptosis in DKD model cells. Moreover, platycodin D, a triterpene saponin derived from the dried root of Platycodon grandiflorum (Jacq.) A. DC., exhibited various pharmacological effects, including anti-tumor, anti-inflammatory, and neuroprotective properties.90–92 In a recent study utilizing high-glucose-induced HK-2 cells as an in vitro DKD model, platycodin D treatment inhibited high-glucose-induced ferroptosis, upregulated GPX4, FTH-1, and SLC7A11 expression, and downregulated ACSL4 and TFR1 expression. These effects led to increased cell viability and reduced cellular damage.71 Collectively, these studies indicate that TCM may exert therapeutic effects on DKD by inhibiting ferroptosis through the regulation of Nrf2/HO-1-related pathways.
Astragaloside IV, an active ingredient of the TCM Astragalus membranaceus (Fisch.) Bge., exhibits anti-inflammatory, antioxidative stress, and immunomodulatory effects. Astragaloside IV has been used in the treatment of various diseases, including tumors, DM, and autoimmune diseases.93–95 Recent findings have also demonstrated its effective inhibition of retinal endothelial cell death and amelioration of pathological damage associated with DR.96,97 In an in vitro model of DR utilizing a high-glucose culture of ARPE-19 cells, Tang et al reported that astragaloside IV attenuated the decrease of Sirt1 and Nrf2 levels induced by high glucose in retinal pigment epithelial cells. It increased the levels of GPX4, glutamate cysteine ligase (GCLM), and glutamate cysteine ligase catalytic subunit (GCLC), leading to the reduction of ferroptosis, increased cell viability, and enhanced antioxidant capacity. These effects may be associated with the inhibition of miR-138-5p expression and activation of the Sirt1/Nrf2 pathway.75
In conclusion, TCM demonstrates a beneficial role in the management of T2DM microangiopathy, including DKD and DR, through the regulation of ferroptosis. The underlying mechanism is likely associated with the modulation of Nrf2-related pathways.
Application of Ferroptosis in TCM Treatment of T2DM Cardiovascular Complications
Resveratrol, a natural polyphenolic compound found in various Chinese herbal medicine plants such as Veratrum nigrum L. and Polygonum cuspidatum Sieb. et Zucc., exhibits varied pharmacological effects including anti-inflammatory and antioxidative stress properties. It is commonly used in the prevention and treatment of tumors, cardiovascular diseases, and DM.98–100 In an in vitro model of diabetic myocardial injury utilizing H9c2 cells cultured in a high-glucose environment, resveratrol demonstrated significant effects. It notably increased cell viability, SOD activity, and protein levels of HSF1, GPX4, and SLC7A11, while decreasing MDA levels and iron ion content in H9c2 cells. These findings indicate that resveratrol may improve high-glucose-induced cardiomyocyte injury by inhibiting ferroptosis through the upregulation of HSF1 expression.72
Previous studies have confirmed the positive effects of puerarin, the main active flavonoid in Pueraria lobata (Willd.) Ohwi, on improving cardiac function in rats with heart failure by reducing lipid peroxidation and ferroptosis.101 Similarly, baicalein, the active ingredient of Scutellaria baicalensis Georgi, has shown a neuroprotective role as a natural ferroptosis inhibitor.102 Building upon this knowledge, Yu et al observed the effects of Gegen Qinlian decoction, a Chinese herbal compound preparation composed mainly of P. lobata (Willd.) Ohwi and S. baicalensis Georgi, on the cardiac diastolic function of diabetic mice with the damp-heat syndrome.73 The study revealed that Gegen Qinlian decoction upregulated GPX4 and SLC7A11 levels, while downregulating ACSL4 and PTGS2 levels. It also reduced MDA content and alleviated damp-heat symptoms, such as elevated blood glucose, reduced diet, and urination. Furthermore, Gegen Qinlian decoction mitigated lipid peroxidation in myocardial tissue, improved cardiac diastolic function, and reversed myocardial remodeling in the mice. These findings demonstrated that Gegen Qinlian decoction’s beneficial effects on cardiac remodeling and diastolic function in diabetic mice with the damp-heat syndrome may be associated with the inhibition of ferroptosis in cardiomyocytes.
Prolonged elevated blood glucose levels in T2DM contribute to significant production and accumulation of AGEs in the body, particularly in the extracellular matrix of the heart., AGEs are an important feature of diabetic cardiomyopathy (DCM) pathogenesis. Sulforaphane, an isothiocyanate widely found in plants like broccoli, possesses anti-tumor and antioxidant effects. It has been shown to alleviate diabetes-induced oxidative stress and cardiac functional impairment.103,104 Further studies74 have revealed that sulforaphane alleviated ferroptosis and lipid peroxidation through AMPK-mediated activation of Nrf2, leading to amelioration of cardiac injury in mice with AGE-induced DCM and enhancing the cardioprotective effect.
Overall, these findings suggest that TCM may improve AGE accumulation and reduce lipid peroxidation in T2DM cardiovascular complications by regulating ferroptosis, thereby enhancing the cardioprotective effect on the heart.
Summary
Through the above-detailed analysis of the therapeutic effects of TCM and its active ingredients on T2DM,63–67 as well as related complications such as DKD,68–71 DR75 and DCM,72–74 we found that Chinese herbs and their main active ingredients exerted therapeutic or protective effects by inhibiting ferroptosis, and the specific mechanisms are summarized in Figure 4.
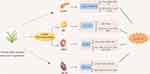 |
Figure 4 Mechanism in the treatment of T2DM and its complications with TCM by inhibiting ferroptosis. This figure was created with Figdraw (www.figdraw.com). |
Discussion
Ferroptosis is a recently proposed new cell death model and is closely related to the occurrence and development of various diseases (tumors, ischemia-reperfusion injury, neurological diseases, and metabolic diseases).6,20 Recent studies have confirmed that, in addition to oxidative stress, the inflammatory response, endothelial cell damage, apoptosis, and autophagy, iron-overload, ROS, and lipid peroxide accumulation are also important pathogenic mechanisms of T2DM and the related complications, and blocking the iron-dependent death pathways with ferroptosis inhibitors or iron-chelating agents can treat or delay the progression of T2DM and its related complications.105
TCM has a long history of use for treating T2DM, being multi-component, multi-target, systematic, and basing treatment on syndrome differentiation (different conditions of each patient), and is highly effective in the clinical treatment and early prevention of disease progression of T2DM. With progressive research on the relationship between the mechanism of TCM and ferroptosis, several studies have confirmed that TCM exerts therapeutic effects on T2DM and its complications by regulating the ferroptosis-related pathways.102,106–108 In this paper, the concept, mechanism of occurrence, regulatory pathways of ferroptosis, and its correlation with T2DM and its related complications were described. The application of ferroptosis to related studies of TCM for treating T2DM and its complications was summarized and analyzed for the first time. In this review, we identified the existence of ferroptosis in T2DM and its related complications, Chinese herbs or their active ingredients (quercetin, curcumin, cryptochlorogenic acid, resveratrol, platycodin D, astragaloside IV) exert beneficial effects on T2DM and its complications by inhibiting ferroptosis. These TCMs all exert their therapeutic effects by inhibiting ferroptosis, with different regulatory mechanisms. However, the number of related studies is relatively small, and all of them are basic experiments. Therefore, future studies should continue to target ferroptosis, explore the mechanism of T2DM occurrence and progression, further clarify the exact mechanism by which different TCMs and their active ingredients mediate their therapeutic effects, and actively explore the relevant regulatory signaling pathways and specific molecular markers. The elucidation of these mechanisms will provide a new theoretical basis for TCM treatment of T2DM and will be crucial in leveraging the knowledge of ferroptosis for the clinical therapeutic benefit.21
Despite the comprehensive and systematic literature search, our review has several limitations. First, the studies included in this review were all basic experimental studies and no published clinical trials were retrieved. Moreover, given the wide variation in animal and in vitro conditions, the generalization of the experimental results to humans requires careful evaluation in rigorous clinical trials. Second, as all experiments included studies that were conducted in China, there may be geographical bias. Third, most of the Chinese herbal medicines used in the included studies were active ingredients of TCM or herbal monomers, and there is a need to increase the study of ferroptosis-related mechanisms of single herbs or TCM compound preparations. Finally, there were only a small number of studies on ferroptosis for TCM treatment of T2DM and its related complications, and TCM treatments for the common complications of T2DM (diabetic neuropathy and abnormal bone metabolism) were not retrieved.
Conclusion and Prospects
TCM treatment of T2DM and its related complications is an effective treatment modality. T2DM is closely associated with ferroptosis and lipid peroxidation, and TCM interventions may play a therapeutic or beneficial role by inhibiting ferroptosis, and the specific mechanism may be relevant to Nrf2-related pathways. Currently, research efforts focusing on the mechanism of ferroptosis in TCM treatment of T2DM primarily concentrate on TCM extracts. However, future studies should delve into the mechanism of ferroptosis in the treatment of T2DM using single herbs and Chinese herbal compounds. This will contribute to the development of new theoretical foundations and potential therapeutic strategies for T2DM and its complications using TCM.
Abbreviations
ACSL4, acyl-CoA synthetase long-chain family member 4; AMPK, AMP-activated protein kinase; BH2, dihydrobiopterin; BH4, tetrahydrobiopterin; CoQ10, Coenzyme Q10; CoQ10H2, ubiquinol; DCM, diabetic cardiomyopathy; DHFR, dihydrofolate reductase; DHODH, dihydroorotate dehydrogenase; DKD, diabetic kidney disease; DR, diabetic retinopathy; FSP1, ferroptosis suppressor protein 1; FTH-1, ferritin heavy chain 1; GCH1, GTP cyclohydrolase 1; GCLC, glutamate cysteine ligase catalytic subunit; GCLM, glutamate cysteine ligase; GPX4, glutathione peroxidase 4; GSH, glutathione; HO-1, heme oxygenase-1; HSF1, heat shock factor 1; MDA, malondialdehyde; NADPH, nicotinamide adenine dinucleotide phosphate; NCOA4, nuclear receptor coactivator 4; Nrf2, nuclear factor erythroid 2-related factor 2; PLOOHs, phospholipid hydroperoxides; PTGS2, prostaglandin-endoperoxide synthase 2; PUFA, polyunsaturated fatty acid; PUFA-PL, phospholipid containing PUFA chain; ROS, reactive oxygen species; SLC7A11, solute carrier family 7 member 11; TCM, traditional Chinese medicine; T2DM, type 2 diabetes mellitus; TFR1, transferrin receptor 1.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China [grant numbers 82174334 and 81870622], the Changsha Municipal Natural Science Foundation [grant number kq2014251], Hunan Provincial Innovation Foundation for Postgraduate [grant number CX20210372], Scientific Research Project of Hunan Provincial Health Commission [grant number 202112070631], and the Research Projects in the Health Industry of Hainan Province [grant number 22A200053].
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
2. Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997.
3. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959.
4. Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Diabetes Mellitus. 2021;13(4):315–409.
5. Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal Associations Between Neighborhood Physical and Social Environments and Incident Type 2 Diabetes Mellitus: the Multi-Ethnic Study of Atherosclerosis (Mesa). JAMA Intern Med. 2015;175(8):1311–1320.
6. Yang WS, Stockwell BR. Ferroptosis: death by Lipid Peroxidation. Trends Cell Biol. 2016;26(3):165–176.
7. Coffey R, Knutson MD. The plasma membrane metal-ion transporter ZIP14 contributes to nontransferrin-bound iron uptake by human β-cells. Am J Physiol Cell Physiol. 2017;312(2):C169–C175.
8. Newsholme P, Cruzat VF, Keane KN, et al. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. 2016;473(24):4527–4550.
9. Tiedge M, Lortz S, Drinkgern J, et al. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46(11):1733–1742.
10. Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20(3):463–466. doi:10.1016/0891-5849(96)02051-5
11. Li N, Frigerio F, Maechler P. The sensitivity of pancreatic beta-cells to mitochondrial injuries triggered by lipotoxicity and oxidative stress. Biochem Soc Trans. 2008;36(Pt 5):930–934.
12. Wu L, Liu M, Liang J, et al. Ferroptosis as a New Mechanism in Parkinson’s Disease Therapy Using Traditional Chinese Medicine. Front Pharmacol. 2021;12:659584.
13. Du J, Wang L, Huang X, et al. Shuganning injection, a traditional Chinese patent medicine, induces ferroptosis and suppresses tumor growth in triple-negative breast cancer cells. Phytomedicine. 2021;85:153551.
14. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072.
15. Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861(8):1893–1900.
16. Mou Y, Wang J, Wu J, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34.
17. Forcina GC, Dixon SJ. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics. 2019;19(18):e1800311.
18. Cardoso BR, Hare DJ, Bush AI, et al. Glutathione peroxidase 4: a new player in neurodegeneration? Mol Psychiatry. 2017;22(3):328–335.
19. Dixon SJ, Patel DN, Welsch M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523.
20. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–282.
21. Stockwell BR. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401–2421.
22. Zheng J, Conrad M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020;32(6):920–937.
23. Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144–152.
24. Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692.
25. Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698.
26. Kraft VAN, Bezjian CT, Pfeiffer S, et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci. 2020;6(1):41–53.
27. Soula M, Weber RA, Zilka O, et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol. 2020;16(12):1351–1360.
28. Vasan K, Werner M, Chandel NS. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020;32(3):341–352.
29. Mao C, Liu X, Zhang Y, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593(7860):586–590.
30. Bruni A, Pepper AR, Pawlick RL, et al. Ferroptosis-inducing agents compromise in vitro human islet viability and function. Cell Death Dis. 2018;9(6):595.
31. Islam R, Khan I, Hassan SN, et al. Association between type 2 diabetes and chronic arsenic exposure in drinking water: a cross sectional study in Bangladesh. Environ Health. 2012;11:38.
32. Lucio M, Barbir R, Vučić Lovrenčić M, et al. Association between arsenic exposure and biomarkers of type 2 diabetes mellitus in a Croatian population: a comparative observational pilot study. Sci Total Environ. 2020;720:137575.
33. Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51(8):2348–2354.
34. Fernández-Real JM, López-Bermejo A, Ricart W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin Chem. 2005;51(7):1201–1205.
35. Wei S, Qiu T, Yao X, et al. Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J Hazard Mater. 2020;384:121390.
36. Hamad M, Mohammed AK, Hachim MY, et al. Heme Oxygenase-1 (HMOX-1) and inhibitor of differentiation proteins (ID1, ID3) are key response mechanisms against iron-overload in pancreatic β-cells. Mol Cell Endocrinol. 2021;538:111462.
37. Ruiz-Ortega M, Rodrigues-Diez RR, Lavoz C, et al. Special Issue “Diabetic Nephropathy: diagnosis, Prevention and Treatment”. J Clin Med. 2020;9(3):545.
38. Chaudhary K, Chilakala A, Ananth S, et al. Renal iron accelerates the progression of diabetic nephropathy in the HFE gene knockout mouse model of iron overload. Am J Physiol Renal Physiol. 2019;317(2):F512–F517.
39. Li S, Zheng L, Zhang J, et al. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic Biol Med. 2021;162:435–449.
40. Kim S, Kang SW, Joo J, et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021;12(2):160.
41. Daskivich LP, Vasquez C, Martinez C, et al. Implementation and Evaluation of a Large-Scale Teleretinal Diabetic Retinopathy Screening Program in the Los Angeles County Department of Health Services. JAMA Intern Med. 2017;177(5):642–649.
42. Sinclair SH, Schwartz SS. Diabetic Retinopathy-An Underdiagnosed and Undertreated Inflammatory, Neuro-Vascular Complication of Diabetes. Front Endocrinol (Lausanne). 2019;10:843.
43. Zhang J, Qiu Q, Wang H, et al. TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Exp Cell Res. 2021;407(2):112800.
44. Pagidipati NJ, Navar AM, Pieper KS, et al. Secondary Prevention of Cardiovascular Disease in Patients With Type 2 Diabetes Mellitus: international Insights From the TECOS Trial (Trial Evaluating Cardiovascular Outcomes With Sitagliptin). Circulation. 2017;136(13):1193–1203.
45. Norhammar A, Lindbäck J, Rydén L, et al. Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admission. Heart. 2007;93(12):1577–1583.
46. Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17(1):83.
47. Poznyak A, Grechko AV, Poggio P, et al. The Diabetes Mellitus-Atherosclerosis Connection: the Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int J Mol Sci. 2020;21(5):56.
48. Li Q, Park K, Xia Y, et al. Regulation of Macrophage Apoptosis and Atherosclerosis by Lipid-Induced PKCδ Isoform Activation. Circ Res. 2017;121(10):1153–1167.
49. Rajapurkar MM, Shah SV, Lele SS, et al. Association of catalytic iron with cardiovascular disease. Am J Cardiol. 2012;109(3):438–442.
50. Paraskevaidis IA, Iliodromitis EK, Vlahakos D, et al. Deferoxamine infusion during coronary artery bypass grafting ameliorates lipid peroxidation and protects the myocardium against reperfusion injury: immediate and long-term significance. Eur Heart J. 2005;26(3):263–270.
51. Bäck M, Yurdagul A, Tabas I, et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406.
52. Meng Z, Liang H, Zhao J, et al. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sci. 2021;284:119935.
53. Wei J, Zhao Y, Liang H, et al. Preliminary evidence for the presence of multiple forms of cell death in diabetes cardiomyopathy. Acta Pharm Sin B. 2022;12(1):1–17.
54. Li W, Li W, Leng Y, et al. Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion Injury Through Endoplasmic Reticulum Stress. DNA Cell Biol. 2020;39(2):210–225.
55. Wang N, Ma H, Li J, et al. HSF1 functions as a key defender against palmitic acid-induced ferroptosis in cardiomyocytes. J Mol Cell Cardiol. 2021;150:65–76.
56. Widlansky ME, Gokce N, Keaney JF, et al. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–1160.
57. Luo EF, Li HX, Qin YH, et al. Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J Diabetes. 2021;12(2):124–137.
58. Shaghaghi Z, Motieian S, Alvandi M, et al. Ferroptosis Inhibitors as Potential New Therapeutic Targets for Cardiovascular Disease. Mini Rev Med Chem. 2022:87.
59. Karim L, Moulton J, Van Vliet M, et al. Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes. Bone. 2018;114:32–39.
60. Alikhani M, Alikhani Z, Boyd C, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40(2):345–353.
61. Ge W, Jie J, Yao J, et al. Advanced glycation end products promote osteoporosis by inducing ferroptosis in osteoblasts. Mol Med Rep. 2022;25(4):23.
62. Yang Y, Lin Y, Wang M, et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 2022;10(1):26.
63. Kose T, Vera-Aviles M, Sharp PA, et al. Curcumin and (-)- Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells Against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals. 2019;12(1):26.
64. Zhang L, Liu D, Li H, et al. Grape seed procyanidin extract inhibited high glucose and high fat-induced ferroptosis through Nrf2 signaling pathway in MIN6 cells. Food Science. 2022;1–10.
65. Liu D, Li X, Zhou T, et al. Protective effect of proanthocyanidin on pancreatic iron metabolism disorder in diabetic rats. Chin J Public Health. 2021;37(10):1517–1520.
66. Li D, Jiang C, Mei G, et al. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients. 2020;12(10):53.
67. Zhou Y. The Protective Effects of Cryptochlorogenic Acid on β-Cells Function in Diabetes in vivo and vitro via Inhibition of Ferroptosis. Diabetes Metab Syndr Obes. 2020;13:1921–1931.
68. Ding Y, Wang L. Mechanism Exploration of Sennoside A in Treating DN Based on Nrf2/HMOX-1 Ferroptosis Signaling Pathway. Info Traditional Chine Med. 2021;38(07):36–39.
69. Guan X, Xie Y, Ni W, et al. Effect of Nrf2/HO-1/GPX4 on ferroptosis of podocytes induced by high glucose and the intervention mechanism of berberine. Chinese Pharmacological Bulletin. 2021;37(03):396–403.
70. Jin T, Chen C. Umbelliferone delays the progression of diabetic nephropathy by inhibiting ferroptosis through activation of the Nrf-2/HO-1 pathway. Food Chem Toxicol. 2022;112892.
71. Huang J, Chen G, Wang J, et al. Platycodin D regulates high glucose-induced ferroptosis of HK-2 cells through glutathione peroxidase 4 (GPX4). Bioengineered. 2022;13(3):6627–6637.
72. Ma Z, Jiang D, Hu B, et al. Resveratrol ameliorates diabetic myocardial injury through HSF1-mediated ferroptosis. J Hainan Med Univ. 2022;28(6):406–411+419.
73. Yu J, Lin Y, Zhou F, et al. Effect of Gegen Qinlian Decoction on cardiac diastolic function of diabetic mice with damp-heat syndrome. China J Chine Materia Medica. 2022;47(10):2705–2711.
74. Wang X, Chen X, Zhou W, et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 2022;12(2):708–722.
75. Tang X, Li X, Zhang D, et al. Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered. 2022;13(4):8240–8254.
76. Lee BK, Kim Y, Kim YI. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism. 2011;60(10):1416–1424.
77. Eshak ES, Iso H, Maruyama K, et al. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: a large population-based prospective cohort study. Clin Nutr. 2018;37(2):667–674.
78. Marku A, Galli A, Marciani P, et al. Iron Metabolism in Pancreatic Beta-Cell Function and Dysfunction. Cells. 2021;10(11):65.
79. Kocaadam B, Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57(13):2889–2895.
80. Jiao Y, Wilkinson J, Christine Pietsch E, et al. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40(7):1152–1160.
81. Wang Y, Xu X, Chen J, et al. Improving Regulation of Polymeric Proanthocyanidins and Tea Polyphenols against Postprandial Hyperglycemia via Acid-Catalyzed Transformation. J Agric Food Chem. 2022;70(16):5218–5227.
82. Zhang J, Mao W, Bai Q. Research progress on quercetin and its derivatives in prevention and treatment of liver injury. Chine Traditional Herbal Drugs. 2021;52(23):7348–7357.
83. Bardy G, Virsolvy A, Quignard JF, et al. Quercetin induces insulin secretion by direct activation of L-type calcium channels in pancreatic beta cells. Br J Pharmacol. 2013;169(5):1102–1113.
84. Wang Z, Zhai D, Zhang D, et al. Quercetin Decreases Insulin Resistance in a Polycystic Ovary Syndrome Rat Model by Improving Inflammatory Microenvironment. Reprod Sci. 2017;24(5):682–690.
85. Varghese SM, Thomas J. Polyphenolic constituents in mulberry leaf extract (M. latifolia L. cv. BC259) and its antidiabetic effect in streptozotocin induced diabetic rats. Pak J Pharm Sci. 2019;32(1):69–74.
86. Meng Q, Qi X, Chao Y, et al. IRS1/PI3K/AKT pathway signal involved in the regulation of glycolipid metabolic abnormalities by Mulberry (Morus alba L.) leaf extracts in 3T3-L1 adipocytes. Chin Med. 2020;15:1.
87. Tian S, Wang M, Liu C, et al. Mulberry leaf reduces inflammation and insulin resistance in type 2 diabetic mice by TLRs and insulin Signalling pathway. BMC Complement Altern Med. 2019;19(1):326.
88. Tang G, Li S, Zhang C, et al. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm Sin B. 2021;11(9):2749–2767.
89. Xiong Z. Effect of rhubarb on lipid and TGF-β1 level in patients with diabetic nephropathy. J Hainan Med Univ. 2012;18(08):1066–1068.
90. Khan M, Maryam A, Zhang H, et al. Killing cancer with platycodin D through multiple mechanisms. J Cell Mol Med. 2016;20(3):389–402.
91. Wang G, Guo H, Wang X. Platycodin D protects cortical neurons against oxygen-glucose deprivation/reperfusion in neonatal hypoxic-ischemic encephalopathy. J Cell Mol Med. 2019;120(8):14028–14034.
92. Shi C, Li Q, Zhang X. Platycodin D Protects Human Fibroblast Cells from Premature Senescence Induced by H2O2 through Improving Mitochondrial Biogenesis. Pharmacology. 2020;105(9–10):598–608.
93. Zhang A, Zheng Y, Que Z, et al. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol. 2014;140(11):1883–1890.
94. Yang L, Han X, Yuan J, et al. Early astragaloside IV administration attenuates experimental autoimmune encephalomyelitis in mice by suppressing the maturation and function of dendritic cells. Life Sci. 2020;249:117448.
95. Nie Q, Zhu L, Zhang L, et al. Astragaloside IV protects against hyperglycemia-induced vascular endothelial dysfunction by inhibiting oxidative stress and Calpain-1 activation. Life Sci. 2019;232:116662.
96. Qiao Y, Fan CL, Tang MK. Astragaloside IV protects rat retinal capillary endothelial cells against high glucose-induced oxidative injury. Drug Des Devel Ther. 2017;11:3567–3577.
97. Wang T, Zhang Z, Song C, et al. Astragaloside IV protects retinal pigment epithelial cells from apoptosis by upregulating miR‑128 expression in diabetic rats. Int J Mol Med. 2020;46(1):340–350.
98. Weng CJ, Wu CF, Huang HW, et al. Evaluation of anti-invasion effect of resveratrol and related methoxy analogues on human hepatocarcinoma cells. J Agric Food Chem. 2010;58(5):2886–2894.
99. Ma X, Sun Z, Han X, et al. Neuroprotective Effect of Resveratrol via Activation of Sirt1 Signaling in a Rat Model of Combined Diabetes and Alzheimer’s Disease. Front Neurosci. 2019;13:1400.
100. Prysyazhna O, Wolhuter K, Switzer C, et al. Blood Pressure-Lowering by the Antioxidant Resveratrol Is Counterintuitively Mediated by Oxidation of cGMP-Dependent Protein Kinase. Circulation. 2019;140(2):126–137.
101. Liu B, Zhao C, Li H, et al. Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem Biophys Res Commun. 2018;497(1):233–240.
102. Li Q, Li -Q-Q, Jia J-N, et al. Baicalein Exerts Neuroprotective Effects in FeCl3-Induced Posttraumatic Epileptic Seizures via Suppressing Ferroptosis. Front Pharmacol. 2019;10:638. doi:10.3389/fphar.2019.00638
103. Xin Y, Bai Y, Jiang X, et al. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ß/Fyn pathway. Redox Biol. 2018;15:405–417.
104. Gu J, Cheng Y, Wu H, et al. Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy. Diabetes. 2017;66(2):529–542.
105. Sha W, Hu F, Xi Y, et al. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J Diabetes Res. 2021;2021:9999612.
106. Chen GQ, Benthani FA, Wu J, et al. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020;27(1):242–254.
107. Shao C, Yuan J, Liu Y, et al. Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis. Proc Natl Acad Sci U S A. 2020;117(19):10155–10164.
108. Li D, Liu B, Fan Y, et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br J Pharmacol. 2021;178(5):1182–1199.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
