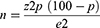Back to Journals » Infection and Drug Resistance » Volume 16
Failure to Attain HIV Viral Suppression After Intensified Adherence Counselling—What Can We Learn About Its Factors?
Authors Mundamshimu JS , Malale K, Kidenya BR, Gunda DW, Bwemelo L, Mwashiuya M, Omar SS, Mlowe N, Kiyumbi M, Ngocho JS, Balandya E , Sunguya B , Mshana SE , Mteta K, Bartlett J, Lyamuya E , Mmbaga BT , Kalluvya S
Received 3 November 2022
Accepted for publication 6 March 2023
Published 30 March 2023 Volume 2023:16 Pages 1885—1894
DOI https://doi.org/10.2147/IDR.S393456
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
James Samwel Mundamshimu,1 Kija Malale,1 Benson R Kidenya,1 Daniel W Gunda,1 Logious Bwemelo,1,* Mwakile Mwashiuya,1,* Salhida Shamnte Omar,1,* Neema Mlowe,1,* Magwa Kiyumbi,1 James S Ngocho,2,3 Emmanuel Balandya,4 Bruno Sunguya,4 Stephen E Mshana,1 Kien Mteta,2 John Bartlett,5,6 Eligius Lyamuya,4 Blandina Theophil Mmbaga,2,3 Samuel Kalluvya1
1Catholic University of Health and Allied Sciences, Mwanza, Tanzania; 2Kilimanjaro Christian Medical University College, Moshi, Tanzania; 3Kilimanjaro Clinical Research Institute, Moshi, Tanzania; 4Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania; 5Kilimanjaro Christian Medical University College, Mosh, Tanzania; 6Duke Global Health Institute, Duke University Medical Center, Durham, NC, USA
*These authors contributed equally to this work
Correspondence: James Samwel Mundamshimu, Tel +255746999249, Email [email protected]
Background: Introduction and expansion of antiretroviral therapy (ART) have turned the tide of HIV pandemic, thus helping people living with HIV (PLHIV) achieve viral suppression. This success may need to be complemented by intensified adherence counseling (IAC) to improve adherence to treatment. However, some PLHIV still face higher than acceptable viral loads despite being on treatment.
Purpose: We investigated the factors associated with the failure to suppress HIV viral load after three months of IAC sessions.
Patients and Methods: This cross-sectional study analyzed secondary data from PLHIV-attended care and treatment clinics in Mwanza between January 2018 and December 2019 who had unsuppressed VL after being on ART for at least six months. We identified PLHIV in first-line ART with viral load evaluation before receiving IAC and had viral load results done at 90 days after IAC. We conducted descriptive statistics to examine the magnitude of viral suppression. Wilcoxon signed-rank test used to compare the median viral load before and after IAC sessions, and logistic regressions predicted the factors associated with failure.
Results: This study included 212 subjects. After intervention, most participants 85.9% (182) had significantly improved adherence compared to baseline. More than half 75.5% (160) of the participants had viral suppression after the intervention. Participants aged 18– 25 years (AOR = 5.6, 95% CI, 1.1– 29.6), unstable client during ART initiation (AOR = 0.3, 95% CI, 0.13– 0.62), and poor adherence to ART (AOR = 4, 95% CI, 1.3– 12.3) remained the main predictors of virological failure after IAC intervention.
Conclusion: Even though virological suppression is influenced by ART adherence, the findings in this study have shown co-existence of other factors to be addressed. Unstable during ART initiation is a new factor identified in this study.
Keywords: people living with HIV, virological failure, intensified adherence counseling, factors, Tanzania
Introduction
Since its discovery, antiretroviral therapy (ART) has saved the lives of many people infected with the human immunodeficiency virus.1,2 Prior to the introduction of ART, many people living with HIV (PLHIV) died of HIV-related complications.3 ARTs have reduced the mortality of PLHIV from 1.95 to 0.95 million deaths.4 ART suppresses the viral load and boosts the immunity of the infected individual. The effects of ART are optimized by reasonable accessibility and availability of ART in health facilities.5,6 The approach to ensure good coverage in most countries ART is either donor or government-funded and made available. Even though the availability and accessibility of ART have increased recently, some patients do not attain adequate virological suppression on receipt of their first-line ART globally.7–9 Studies have reported several factors to be associated with non-suppressed viral loads. In South Africa, factors like age of less than 15 years during ART initiation, and being male were reported to be associated with virological failure.10 Male sex, alcoholism, and smoking were reported to be associated with virological failure in Morocco.11 Low CD4 cell count, social isolation, and high stigma were shown to be significantly associated with virological failure in Vietnam.12 In addition, adult patients were reported to have high risk of virological failure in Ethiopia and poor adherence to ART was reported to have significant association with virological failure in Kenya.13,14
About 20% of PLHIV in Haiti switched to second-line regimens after failed first-line regimens.15 Studies in the Lake Zone of Tanzania revealed that approximately 12.18% of PLHIV had virological failure despite being in ART for at least six months.15,16 Poor adherence to the treatment plan mainly causes virological failure, and adherence intervention is highly recommended.17,18 Effort is made in different countries to reach the UNAIDS target which states that by 2030 95% of PLHIV will know their HIV status, 95% of people diagnosed with HIV will receive ART and 95% of PLHIV taking ART will achieve viral suppression.19
As per the national guidelines in Tanzania, adopted by the World Health Organization (WHO), patients with a viral load of more than or equal to 1000 HIV copies per mL of blood after being on ART for at least six months are suspected to have virological suppression failure, and therefore they are enrolled into intensified adherence counseling (IAC) sessions.20 IAC includes a series of adherence counseling sessions offered once every month for three consecutive months, and this intervention maximizes the patients’ commitment to treatment schedules.20 The guidelines affirm that viral load suppression could be achieved after three months of treatment. In addition, the intervention is expected to reduce the number of PLHIV to be switched to the second line, which is very expensive and with many side effects. Even though the ARTs are government or donor-funded, second-line ART is costly, and avoiding switching patients to the second line saves much money. In cases, this intervention demonstrates a failure in improving adherence and virological suppression, switching patients to the second line remains the only option to save the patient.21 The prevalence of switching patients from first-line to second-line ART is 3.1% by three years after the initiation of ART globally.22 In Tanzania, the incidence rate of switching patients from first-line to second-line is 1.7/100 person-years.23 Switching of PLHIV from first line to second line was more higher before the introduction of IAC intervention not only in Uganda but also in Tanzania.24 The prevalence of viral load suppression failure was 62.2%25 in Tanzania and in Uganda.
Despite increased awareness of PLHIV with virological failure after IAC sessions, associated factors are not well studied in Tanzania. Therefore, this study investigated the factors associated with virological failure following three months of IAC to PLHIV.
Materials and Methods
Design and Settings
A multicenter retrospective cross-sectional study was conducted at two centers offering care and treatment clinics (CTCs) in Mwanza city. These centers were Bugando Medical Centre (BMC) and the Sekou-Toure Regional Referral Hospital. BMC is a teaching hospital and the main zonal and referral hospital in Tanzania serving eight regions with a population of over 13 million people.26 Sekou Toure is a regional referral hospital of Mwanza city serving about 4 million people.27 Both hospitals have high-load CTCs clients, serving as referral centers from other CTCs in the Lake Zone. Out of 1.6 million PLHIV on ART in Tanzania, 10,000 of them are registered and attend CTCs at BMC.16 More than 9600 PLHIV are attended at the Sekou-Toure Regional Referral Hospital in a year with an average of 40 clients attending a day.28
Population
The study included PLHIV on first-line ART who were suspected to have virological failure between January, 2018 and December, 2019. According to Tanzania National guideline for management of HIV and AIDs (2019) patients is suspected to have virological failure whenever a patient has been on ART for at least six months and has viral load of more than 1000 copies/mL of blood. Viral suppression according to the WHO consolidated guidelines on the use of antiretroviral drug for treatment and preventing HIV infection recommendation for a public health approach is defined as a VL of less than 1000 copies/mL and undetectable viral load is defined as VL of less than 50 copies/mL of blood.29 We excluded clients whose records missed IAC information, adherence assessment post counseling, and the three months HIV RNA viral load testing post counseling. Out of 267 PLHIVs suspected to have a virological failure, only 212 met the inclusion criteria of this study. Fifty-five (55) subjects were excluded due to missing key information.
Sample Size
The Estimated Sample Size Was Calculated Using Kish-Leslie Formula (1965)
The assumed prevalence (P) of virological failure as from previous study on HIV for adults aged 15 to 49 years 14.7% Z = 1.96, value of standard normal distribution at 95% confidence level and the accepted marginal of error of Ɛ = 5%. We assumed the minimum sample of 212.
Procedures and Measurements
Data of illegible participants in this study were extracted from CTC card number two (CTC-2) and the high viral load adherence assessment form using a checklist adopted and modified to suit the local setting.30 The patient adherence profile was extracted from the high viral load assessment form and was assessed using Bugando Medical Centre adherence tool whereby adherence of 95% and above was considered as good adherence using pill count.31 According to this tool adherence of 95% and above means no missing or missing less than 2 pills during a prescribed time. The opposite of 95% is applicable for poor adherence. Participants’ PCR HIV RNA viral load after three months of IAC was also recorded from CTC-2. Additionally, age, gender, education, and drug regimen were also recorded from CTC-2. The researchers investigated to see how these independent variables interact with the dependent variables which were accepted for adherence and viral suppression.
Data were extracted from the patient medical records into an excel spreadsheet and then transferred to STATA version 15 for analysis. We used the Shapiro–Wilk normality test to assess the distribution of continuous variables. We summarized continuous variables as means ( ) and standard deviation (SD). Categorical variables were summarized using frequencies and percentages. The ART duration among the participants was analyzed to see its effect on adherence and virological suppression. Median years of participants being on ART were calculated to divide them into short use and long use. We compared proportions using the chi-square test and a p-value of less than 0.05 was considered significant at a 95% confidence interval. We also used the Wilcoxon signed-rank test to compare the median viral load before and after IAC. Univariate logistic regression was used to determine the association between independent and dependent variables. Variables were included in the multivariate model if they had a p < 0.2 in univariate logistic regression analysis. Then, multivariate logistic regression was used to establish the factors associated with the viral load suppression failure after the IAC. According to the guidelines by the Tanzania National AIDS Control Program, we considered patients to have virological failure if they did not achieve a viral load of ≤1000 HIV copies/mL of blood following three months of IAC.20
) and standard deviation (SD). Categorical variables were summarized using frequencies and percentages. The ART duration among the participants was analyzed to see its effect on adherence and virological suppression. Median years of participants being on ART were calculated to divide them into short use and long use. We compared proportions using the chi-square test and a p-value of less than 0.05 was considered significant at a 95% confidence interval. We also used the Wilcoxon signed-rank test to compare the median viral load before and after IAC. Univariate logistic regression was used to determine the association between independent and dependent variables. Variables were included in the multivariate model if they had a p < 0.2 in univariate logistic regression analysis. Then, multivariate logistic regression was used to establish the factors associated with the viral load suppression failure after the IAC. According to the guidelines by the Tanzania National AIDS Control Program, we considered patients to have virological failure if they did not achieve a viral load of ≤1000 HIV copies/mL of blood following three months of IAC.20
Ethical Considerations and Participant Consent
This study complies with the Declaration of Helsinki.
The ethical clearance was obtained from the joint Catholic University of Health and Allied Sciences (Bugando Medical Centre Ethics and Review Committee (CREC/410/2019)). Since it is a retrospective study, we received permission to access the patient’s records from Bugando Medical Centre (Ref: AB.317/440/Part. M/) and Sekou Toure regional referral hospital (Ref: FA. 137/264/01/G/32). We used the patient’s special codes instead of the patient’s name for confidentiality purposes. We received a waiver for the patient’s consent as we used secondary data.
Results
Participant’s Demographics and Clinical Characteristics
A total of 212 PLHIVs participated in this study. The mean age and standard deviation (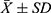 ) of these participants were 43.2 ± 11.8 years. Out of 212 participants, 63.2%18 were female, 38.2% (81) were married, 3.8% (8) were separated, 43.4% (92) were in stage 3 as per WHO clinical staging of HIV, and 50% (106) were underweight (Table 1).
) of these participants were 43.2 ± 11.8 years. Out of 212 participants, 63.2%18 were female, 38.2% (81) were married, 3.8% (8) were separated, 43.4% (92) were in stage 3 as per WHO clinical staging of HIV, and 50% (106) were underweight (Table 1).
 |
Table 1 Participants’ Demographic and Clinical Characteristics (N = 212) |
Antiretral Viral Adherence and Median Viral Load Before and After IAC
Before IAC, only 66.5% (141) participants had good adherence of 95% and above using pill count. After IAC, we observed a significant increase in participants who achieved good adherence 85.9% (182) with a p-value of 0.001 using the same scale. Viral load data normality check was performed using swilk test (p-value=0.001) and therefore median was opted for further analysis. We also observed a significant decrease in median viral load after IAC using the Wilcoxon signed-rank test. However, with this significant reduction in the median viral load but some of the participants 24.5% (52), their viral load reduced but did not reach viral suppression and were confirmed to have virological failure after IAC (Table 2 and Table 3).
 |
Table 2 ARTs Adherence and Virological Status Before and After IAC (N = 212) |
 |
Table 3 Prevalence of Virological Suppression After IAC (N = 212) |
Factors Associated with ART Adherence Before and After IAC
In univariate analysis factors like being single, age group, ART duration, and use of local medicine qualified to be subjected to multivariate analysis. Being single shown to have significant contribution to ART adherence before the intervention. After IAC factors such as being divorced, being single, being on ART for more than 11 years, using local medicine, and living in Nyamagana had p-value of less than 0.2 in univariate analysis qualifying to be subjected to multivariate analysis. Being single remained the only factor that significantly associate with adherence before and after IAC in the univariate and multivariate analysis.(See Table 4).
 |
Table 4 Factors Associated with Adherence Before and After Intensified Adherence Counselling (N = 212) |
Factors Associated with Virological Failure Following Three Months of Intensified Adherence Counseling
During univariate logistic regression analysis, we discovered that being single (OR = 3.6 95% CI = 1.6–7.8), young (18–25 years) (OR = 7.9, 95% CI = 2.6–23.8), not disclosing the HIV status (OR = 3.3, 95% CI = 1.1–10.9) and being unstable (OR = 2.5, 95% CI = 2.3–4.7) during the start of ART were significantly associated with virological failure after IAC. All variables that had a p-value of less than 0.2 were subjected to multivariate logistic regression analysis.
In this regression analysis factors such as young age (AOR = 5.6, 95% CI = 1.1–29.6), poor adherence to ART after IAC (AOR = 4, 95% CI = 1.3–12.3), and unstable when starting ART (AOR = 0.3, 95% CI = 0.13–0.62) were associated with virological failure post-IAC (Table 5).
 |
Table 5 Factors Associated with Virological Failure After Intensified Adherence Counselling (N = 212) |
Discussion
This study has demonstrated promising results of improving ART adherence to PLHIV suspected to have virological failure while on first line ART. The findings from this study show that 85.9% and 75.5% PLHIV achieved more than 95% adherence and virological suppression respectively following IAC. Youngness and poor medication adherence increase the risk of virological failure after 3-months of intensification.
The improvement in adherence to medication observed in this study was probably because during the adherence counseling sessions, barriers like not disclosing information to partners, alcohol use, and herbal medicines were identified by the nurse counselors and discussed with the clients. The nurse counselors advised them to use mobile phone reminders, identifying a supporting person and peer clubs and these might have contributed to the improvement in medication adherence. Barriers for non-improved medication adherence reported being missing some counseling sessions, poor quality of counseling, and lack of remainder. In addition, another study by Lee et al32 reported chaotic home situations and busy work schedules as the main barriers for most clients not achieving medication adherence.32 Pill burden and forgetfulness among clients were the barriers for not achieving optimal medication adherence.33 In China, clients report poor medication adherence due to forgetting, being away from home, being busy, and feeling worse after taking drugs.34 Poor adherence to medication can cause treatment failure and even drug resistance35 These findings were similar to what we observed in this study barriers like not disclosing information to partners, alcohol use, and herbal medicines as the main reasons for not adhering to medications.
In this study, we recorded the viral load before and after IAC. Unfortunately, 24.5% of the participants did not achieve viral suppression after the intervention. There was an increased risk of virological failure after IAC among single compared to those married, separated, or divorced. Single individuals have no support from partners who can remind them. This finding was different from studies done in Kombolcha town and South Wollo Zone Ethiopia, where they reported that being divorced or separated had increased risk of virological failure.36,37 The possible reason for this discrepancy is that in their study, single was kept constant during analysis. Again, we found that a unit decrease in age increases the risk of virological failure after IAC among PLHIV, while a unit increase in age decreases the risk. Being young was significantly associated with virological failure after IAC sessions 7.9 times more odds. Similar findings from studies conducted in Ethiopia and Rwanda.38,39 The risk is higher at a young age since young individuals have difficulty achieving accepted medication adherence.
Not achieving acceptable adherence of 95% and above, being unstable during admission to treatment had increased risk and was significantly associated with virological failure compared to their counterparts. This finding is consistent with findings reported by Lailulo et al.40 In addition to peer-to-peer counselling, peer club or support groups, disclosure of HIV status, and ART mobile phone reminders by SMS reported in the previous studies, IAC could also be used as an alternative to improving ART adherence.
Furthermore, 66.5% of participants who had good adherence before IAC but suspected to have virological failure 68.8% suppressed their viral load and 59.6% retained their viral load of more than or equal to 1000 copies/mL even after the intervention. This could be contributed to the reason that IAC is a client centered intervention and aims to optimize client adherence to all guidelines including ART use probably those who suppressed virologic ally may be benefited from this. Those who failed virologic ally regardless of their good adherence may be due to drug resistance or may necessitate the use of other tools in assessing adherence apart from pill count. Study has reported several factors apart from adherence to be associated with virological failure. In the study conducted in Ethiopia revealed that participant who experience drug toxicity has high risk of virological failure.41 A study in children and adolescents discovered mismatch between pill count and viral load suppression.42
In this study, we used pill count to assess medication adherence as these patients’ received ART during their CTC visit. The strength of this measure is that it is more objective than self-report adherence. However, due to retrospective nature of the study, small sample size, involving subjects from a single region, and exclusion of participants with missing key information limit generalizability of the findings.
Conclusion
Despite the fact that virological suppression is influenced by ART adherence, the findings in this study have shown existence of other factors including young age being unstable during ART initiation. Unstable during ART initiation is a new factor that has never being reported.
Abbreviations
ART, antiretroviral therapy; BMC, Buganda Medical Centre; CTCs, care and treatment clinics; HIV, human immunodeficiency virus; IRB, Institutional Review Board; PLHIV, people living with HIV.
Acknowledgments
The authors wish to thank the members of the Community of Young Research Peers (CYRP) for their contribution to the conduct of this study: Alex Mremi, Baraka Morris, Delfina Msanga, Dorah Mrema, Evangelista Malindisa, Godwin Pancras, Honest Massawe, Maryam Amor, Matiko Mwita, Nicholas Ngowi, and Tosi Mwakyandile.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health, US under Award Number R25 TW011227.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Global A, Sedai A, Ankita K. Statistics, global information and education on HIV and AIDS. JMIR Med Inform. 2019;7(1). doi:10.2196/medinform.9291
2. Quinn TC. HIV epidemiology and the effects of antiviral therapy on long-term consequences. AIDS. 2008;22(Suppl 3):S7. doi:10.1097/01.aids.0000327510.68503.e8
3. Slaymaker E, Todd J, Marston M, et al. How have ART treatment programmes changed the patterns of excess mortality in people living with HIV? Estimates from four countries in East and Southern Africa. Glob Health Action. 2014;7(1):22789. doi:10.3402/gha.v7.22789
4. Frank TD, Carter A, Jahagirdar D, et al. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6(12):e831–e859. doi:10.1016/S2352-3018(19)30196-1
5. Oguntibeju OO. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV/AIDS Res Palliat Care. 2012;117–124. doi:10.2147/HIV.S32321
6. Marsh K, Eaton JW, Mahy M, et al. Global, regional and country-level 90–90–90 estimates for 2018: assessing progress towards the 2020 target. AIDS. 2019;33(Suppl 3):S213. doi:10.1097/QAD.0000000000002355
7. Kyaw NTT, Harries AD, Kumar AMV, et al. High rate of virological failure and low rate of switching to second-line treatment among adolescents and adults living with HIV on first-line ART in Myanmar, 2005–2015. PLoS One. 2017;12(2):e0171780. doi:10.1371/journal.pone.0171780
8. Leng X, Liang S, Ma Y, et al. HIV virological failure and drug resistance among injecting drug users receiving first-line ART in China. BMJ open. 2014;4(10):e005886. doi:10.1136/bmjopen-2014-005886
9. Yuan D, Liu M, Jia P, et al. Prevalence and determinants of virological failure, genetic diversity and drug resistance among people living with HIV in a minority area in China: a population-based study. BMC Infect Dis. 2020;20(1):1–10.
10. Joseph Davey D, Abrahams Z, Feinberg M, et al. Factors associated with recent unsuppressed viral load in HIV-1-infected patients in care on first-line antiretroviral therapy in South Africa. Int J STD AIDS. 2018;29(6):603–610. doi:10.1177/0956462417748859
11. Hicham T, Ilyas E, Tarik H, et al. Risk factors associated with unsuppressed viral load in HIV-1 infected patients at the first antiretroviral therapy in Morocco. Int J Mycobacteriol. 2019;8(2):113. doi:10.4103/ijmy.ijmy_41_19
12. Rangarajan S, Colby DJ, Giang LT, et al. Factors associated with HIV viral load suppression on antiretroviral therapy in Vietnam. J Virus Erad. 2016;2(2):94–101. doi:10.1016/S2055-6640(20)30466-0
13. Waju B, Dube L, Ahmed M, et al. Unsuppressed viral load level in public health facilities: nonvirological predictors among adult antiretroviral therapy users in southwestern Ethiopia. HIV/AIDS Res Palliat Care. 2021;Volume 13:513–526. doi:10.2147/HIV.S304653
14. Mwangi A, van Wyk B. Factors associated with viral suppression among adolescents on antiretroviral therapy in Homa Bay County, Kenya: a retrospective cross-sectional study. HIV/AIDS Res Palliat Care. 2021;Volume 13:1111–1118. doi:10.2147/HIV.S345731
15. Wang Y, Barnhart S, Francois K, et al. Expanded access to viral load testing and use of second line regimens in Haiti: time trends from 2010–2017. BMC Infect Dis. 2020;20(1):1–13.
16. Gunda DW, Kilonzo SB, Mtaki T, et al. Magnitude and correlates of virological failure among adult HIV patients receiving PI based second line ART regimens in north western Tanzania; a case control study. BMC Infect Dis. 2019;19(1):1–7. doi:10.1186/s12879-019-3852-3
17. Kazooba P, Mayanja BN, Levin J, et al. Virological failure on first-line antiretroviral therapy; associated factors and a pragmatic approach for switching to second line therapy–evidence from a prospective cohort study in rural South-Western Uganda, 2004–2011. Pan Afr Med J. 2018;29(1):1–16. doi:10.11604/pamj.2018.29.191.11940
18. Kiweewa F, Esber A, Musingye E, et al. HIV virologic failure and its predictors among HIV-infected adults on antiretroviral therapy in the African Cohort Study. PLoS One. 2019;14(2):e0211344. doi:10.1371/journal.pone.0211344
19. Heath K, Levi J, Hill A. The Joint United Nations Programme on HIV/AIDS 95–95–95 targets: worldwide clinical and cost benefits of generic manufacture. AIDS. 2021;35(1):S197–S203. doi:10.1097/QAD.0000000000002983
20. Ramadhani A, Josiah RM, Rwebembera A, et al. National Guidelines for the Management of HIV and AIDS. Ministry of Health and Social Welfare; 2012.
21. Kroidl A, Burger T, Urio A, et al. High turnaround times and low viral resuppression rates after reinforced adherence counselling following a confirmed virological failure diagnostic algorithm in HIV‐infected patients on first‐line antiretroviral therapy from Tanzania. Trop Med Int Health. 2020;25(5):579–589. doi:10.1111/tmi.13373
22. Collins IJ, Wools-Kaloustian K, Goodall R, et al. Incidence of switching to second-line antiretroviral therapy and associated factors in children with HIV: an international cohort collaboration. Lancet HIV. 2019;6(2):e105–e115. doi:10.1016/S2352-3018(18)30319-9
23. Hawkins C, Hertzmark E, Spiegelman D, et al. Switching to second-line ART in relation to mortality in a large Tanzanian HIV cohort. J Antimicrob Chemother. 2017;72(7):2060–2068. doi:10.1093/jac/dkx098
24. Ssempijja V, Nakigozi G, Chang L, et al. Rates of switching to second-line antiretroviral therapy and impact of delayed switching on immunologic, virologic, and mortality outcomes among HIV-infected adults with virologic failure in Rakai, Uganda. BMC Infect Dis. 2017;17(1):1–10. doi:10.1186/s12879-017-2680-6
25. Ramadhani HO, Bartlett JA, Thielman NM, et al. The effect of switching to second-line antiretroviral therapy on the risk of opportunistic infections among patients infected with human immunodeficiency virus in northern Tanzania. In: Open Forum Infectious Diseases. Oxford University Press; 2016.
26. Simon R, Gilyoma JM, Dass RM, et al. Paediatric injuries at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 150 cases. J Trauma Manag Outcomes. 2013;7(1):1–9. doi:10.1186/1752-2897-7-10
27. Moremi N, Claus H, Vogel U, et al. Surveillance of surgical site infections by Pseudomonas aeruginosa and strain characterization in Tanzanian hospitals does not provide proof for a role of hospital water plumbing systems in transmission. Antimicrob Resist Infect Control. 2017;6(1):1–8. doi:10.1186/s13756-017-0216-x
28. Yonah G, Fredrick F, Leyna G. HIV serostatus disclosure among people living with HIV/AIDS in Mwanza, Tanzania. AIDS Res Ther. 2014;11(1):1–5. doi:10.1186/1742-6405-11-5
29. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. World Health Organization; 2016.
30. Wu P, Johnson B, Nachega J, et al. The combination of pill count and self-reported adherence is a strong predictor of first-line ART failure for adults in South Africa. Curr HIV Res. 2014;12(5):366–375. doi:10.2174/1570162X1205141121102501
31. Anuradha S, Joshi A, Negi M, et al. Factors influencing adherence to ART: new insights from a center providing free ART under the national program in Delhi, India. J Int Assoc Provid AIDS Care. 2013;12(3):195–201. doi:10.1177/1545109711431344
32. Lee SB, Valerius J. Valerius, mHealth interventions to promote anti-retroviral adherence in HIV: narrative review. JMIR mHealth and uHealth. 2020;8(8):e14739. doi:10.2196/14739
33. Mwangi A, Wanzala P, Karanja S, Karanja SM. Factors influencing adherence to ARVS among patients attending comprehensive care clinic within Jomo Kenyatta University of Agriculture and Technology, Kiambu County, Kenya. East Afr Med J. 2014;91(4):109–114.
34. Kipsang J, Chen J, Tang C, et al. Self reported adherence to antiretroviral treatment and correlates in Hunan province, the Peoples Republic of China. Int J Nurs Sci. 2018;5(2):162–167. doi:10.1016/j.ijnss.2018.04.008
35. Nachega B, Marconi V, van Zyl G, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets. 2011;11(2):167–174. doi:10.2174/187152611795589663
36. Fentie Wendie T, Workneh BD. Prevalence and predictors of virological failure among adults living with HIV in South Wollo Zone, Northeast Ethiopia: a retrospective cohort study. HIV/AIDS Res Palliat Care. 2020;12:393–402. doi:10.2147/HIV.S266460
37. Meshesha HM, Nigussie ZM, Asrat A, et al. Determinants of virological failure among adults on first-line highly active antiretroviral therapy at public health facilities in Kombolcha town, Northeast, Ethiopia: a case–control study. BMJ open. 2020;10(7):e036223. doi:10.1136/bmjopen-2019-036223
38. Abdullahi IJ, Deybasso HA, Adlo AM. Determinants of virological failure among patients on first-line antiretroviral therapy in central Oromia, Ethiopia: a case–control study. HIV/AIDS Res Palliat Care. 2020;12:931–939. doi:10.2147/HIV.S281672
39. Ndahimana JDA, Riedel DJ, Mwumvaneza M, et al. Drug resistance mutations after the first 12 months on antiretroviral therapy and determinants of virological failure in Rwanda. Trop Med Int Health. 2016;21(7):928–935. doi:10.1111/tmi.12717
40. Lailulo Y, Kitenge M, Jaffer S, et al. Factors associated with antiretroviral treatment failure among people living with HIV on antiretroviral therapy in resource-poor settings: a systematic review and metaanalysis. Syst Rev. 2020;9(1):1–17. doi:10.1186/s13643-020-01524-1
41. Emagnu A, Abay Z, Bulti AB, et al. Determinants of virologic failure among adult HIV patients on first-line antiretroviral therapy at waghimra zone, northern Ethiopia: a case-control study. Adv Public Health. 2020;2020:1–8. doi:10.1155/2020/1929436
42. Martelli G, Antonucci R, Mukurasi A, et al. Adherence to antiretroviral treatment among children and adolescents in Tanzania: comparison between pill count and viral load outcomes in a rural context of Mwanza region. PLoS One. 2019;14(3):e0214014. doi:10.1371/journal.pone.0214014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.