Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence
Authors Bonora BM , Avogaro A , Fadini GP
Received 5 October 2019
Accepted for publication 24 December 2019
Published 21 January 2020 Volume 2020:13 Pages 161—174
DOI https://doi.org/10.2147/DMSO.S233538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Benedetta Maria Bonora, Angelo Avogaro, Gian Paolo Fadini
Department of Medicine, University of Padova, Padova 35128, Italy
Correspondence: Gian Paolo Fadini
Department of Medicine, University of Padova, Via Giustiniani 2, Padova 35128, Italy
Tel +39 49 8214318
Fax +29 49 8212184
Email [email protected]
Abstract: Patients with type 2 diabetes (T2D) are often overweight/obese and affected by arterial hypertension, dyslipidaemia, and have high serum levels of uric acid. Moreover, T2D patient have a higher risk of developing cardiovascular or renal complications, which are leading causes of morbidity and mortality in this population. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are a new class of glucose-lowering medications that block the reabsorption of glucose in the kidney, thereby increasing urinary glucose excretion, and lowering blood glucose levels. The beneficial effects of SGLT2 inhibition extend beyond glycaemic control, and include improvement in blood pressure, body weight, uric acid concentrations, liver steatosis, oxidative stress, and inflammation. In dedicated cardiovascular outcome trials, SGLT2i treatment was associated with a significant reduction in the rate of cardiovascular events and renal endpoints. In this review, we summarize the evidence for extra-glycemic effects of SGLT2i and the potential mechanisms driving cardiorenal protection exerted by this class of medications.
Keywords: type 2 diabetes, sodium-glucose cotransporter-2 inhibitors, cardiovascular effects, renal effects, review
Introduction
Type 2 diabetes (T2D) is a complex metabolic disease commonly associated with overweight/obesity, hypertension, dyslipidemia, hyperuricemia and non-alcoholic fatty liver disease (NAFLD).1 Diabetes confers a 2–4 fold excess risk for cardiovascular disease (CVD) and, in patients with T2D, CVD is the leading cause of morbidity and mortality.2 Almost 40% of diabetic patients develop diabetic nephropathy during their lifetime.3 Diabetic nephropathy is still a major cause of end-stage renal disease and also an important cause of progressive morbidity and mortality. In addition, patients with diabetic nephropathy have at markedly increased risk of adverse cardiovascular outcomes.4 Therefore, an ideal glucose-lowering medication (GLM) should not only improve glycaemic control but also have favourable impact on weight, blood pressure, dyslipidemia, cardiovascular, and renal outcomes.
Prior studies have shown that targeting HbA1c below 7% was unable to improve cardiovascular outcomes, when compared to less aggressive glucose control.5,6 Therefore, the idea of a more comprehensive approach to cardiovascular risk management in T2D has emerged. This was clearly supported by results of the STENO-2 trial,7 wherein multifactorial intervention improved all diabetes-related outcomes, including cardiovascular disease. Hence, the idea of “STENO-2 in a pill” is particularly attractive.
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) block sodium-dependent glucose transporter-2 (SGLT2) located in the early proximal renal tubule which is responsible for reabsorption of most (80–90%) of the glucose filtered by the glomerulus.8 The resulting increase in urinary glucose excretion lowers plasma glucose concentrations. This mechanism of action is dependent on blood glucose levels and is independent of the action and availability of insulin. Glycosuria results in a significant caloric loss and bodyweight reduction. Glycosuria is also accompanied by osmotic diuresis and reduction in blood pressure.9
In cardiovascular outcomes trials (CVOTs). SGLT2i have shown capacity to reduce major adverse cardiovascular events (MACE) and hospitalization for heart failure, and were associated with slower progression of kidney disease and with reduced rates of renal endpoints, such as progression of albuminuria, doubling of serum creatine, initiation of renal replacement therapy or death due to renal disease.10–13 These effects appeared to be, at least in part, independent of glucose-lowering efficacy. Most of the beneficial effects of SGLT2i on cardiorenal endpoints observed in CVOTs have been confirmed in large observational studies.14–20
In view of the robustness of these findings, current international guidelines recommend that patients with T2D and CVD or at high cardiovascular or renal risk should receive an SGLT2 inhibitor.21–23 It should be noted that, while solid data indicate cardiovascular protection also for GLP-1 receptor agonists (GLP-1RA), this class of medications exert overall no action on hard renal endpoints.24
Interestingly, however, the mechanisms that drive cardiorenal protection by SGLT2i still must be elucidated. Since publication of the ground-breaking results of the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME) trial, subsequently confirmed by other robust CVOTs, the scientific community is struggling to understand why a treatment originally developed as a purely glucose-eliminating strategy exerts so a strong systemic protection against major adverse outcomes of T2D.
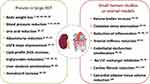
The aim of this review is to provide an update on extra-glycemic effects and clinical advantage of SGLT2i which derive from preclinical and clinical studies. We first summarize results of the studies showing cardiorenal benefits of SGLT2i and then review the evidence for extra-glycemic effects of SGLT2i that may contribute to cardiorenal protection (Table 1).
 |
Table 1 Putative Extra-Glycemic Effects of SGLT2i |
Cardiac Effect and Cardiovascular Outcomes
Three SGLT2i, empagliflozin, canagliflozin, and dapagliflozin have so far been evaluated in CVOTs trials.10–13 The EMPA-REG OUTCOME trial, which was conducted in 7020 patients with T2D and established CVD, showed that the primary composite outcome (MACE) of cardiovascular death, non-fatal myocardial infarction (MI), and stroke occurred less frequently in patients randomized to empagliflozin versus those randomized to placebo (HR 0.86, 95% CI 0.74–0.99; p=0.04). This difference was largely driven by significantly lower rates of cardiovascular death in the empagliflozin group (HR 0.62, 95% CI 0.49–0.77; p<0.001). No significant differences were found in the rates of stroke and myocardial infarction. In the empagliflozin group, all-cause death rates were also significantly reduced (HR 0.68, 95% CI 0.57–0.82; p<0.001). These results were partially confirmed in the CVOTs with others SGLT2i.25 In the Canagliflozin Cardiovascular Assessment Study (CANVAS) program, 10,142 patients with T2D and high cardiovascular risk (34.4% of whom had no history of prior CVD) were randomly assigned to receive canagliflozin or placebo. The primary composite endpoint (MACE) was reduced by 14% by canagliflozin (p=0.02). No significant differences were found in individual components of the composite outcome.11 A greater proportion of patients in primary prevention were enrolled in the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial, wherein 59.4% of the 17,160 patients had no established CVD. Dapagliflozin failed to demonstrate significant benefit in terms of reduction of MACE.12 Nonetheless, the meta-analysis performed by Zelniker and colleagues comparing the subgroup of DECLARE patients with known atherosclerotic CVD with the data from the other CVOTs with SGLT2i revealed a non-significant interaction and no heterogeneity between the three CVOTs. A reduction in MACE was observed only in patients with established CVD, whereas no cardiovascular benefits were observed in patients in primary prevention on MACE.25
A secondary analysis of Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial, wherein almost a half of patients was in primary prevention (n=2181, 49.6%), showed that canagliflozin reduced the risk of MACE by 20% (p=0.01) with consistent reduction in both primary and secondary prevention group (HR 0.68, 95% CI 0.61–0.94 and HR 0.85, 95% CI 0.69–1.06, respectively; p interaction 0.25).26
Noteworthy, consistent results have been observed among trials regarding the rates of hospitalization for heart failure (HHF). In all CVOTs HHF was significantly reduced by 30–35%.10–13 The onset of the benefit was very rapid, with the curves diverging within the first weeks after randomization, suggesting a prominent hemodynamic effect rather than anti-atherosclerotic mechanism. Similar results were observed in a large randomized-controlled trial (RCT) investigating dapagliflozin as a treatment for heart failure (HF). In Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF) 4744 patients with New York Heart Association class II-IV HF and an ejection fraction of 40% or less were randomized to receive dapagliflozin or placebo on top of their ongoing medications. The primary endpoint was a composite of worsening HF (hospitalization or an urgent visit resulting in intravenous therapy for HF) or cardiovascular death. The primary endpoint occurred significantly less frequently among those who received dapagliflozin than among those who received placebo regardless of the presence or absence of diabetes (HR 0.74, 95% CI 0.65 to 0.85; p<0.001).27 These data strongly substantiate the extensive benefit provided by SGLT2i on the risk of HF.
Information with respect to the direct effects of SGLT2i on myocardial function in humans is very limited and mostly based on observational studies. These trials seem to suggest that SGLT2i can improve left ventricular (LV) diastolic function.28–30 Recently, in a RCT trial, 97 patients with T2D and coronary artery disease were randomized to empagliflozin or placebo. The primary endpoint was the 6-month change in LV mass measured by cardiac MRI. Empagliflozin was associated with a significant reduction in LV mass, however, did not affect LV ejection fraction, LV end-diastolic volume, LV end-systolic volume.31 Other RCTs investigating the effects of SGLT2i on LV remodelling in patients with T2D and HF are currently ongoing.32,33
A RCT exploring cardiac effects of SGLT2i using impedance cardiography showed that 12 weeks of treatment with dapagliflozin had no significant effects on cardiodynamic parameters related to blood flow (stroke volume, cardiac output, cardiac index), systolic function (ejection fraction, acceleration and velocity indexes, systolic time ratio), circulatory function (systemic vascular resistance index), and fluid status (thoracic fluid content), compared to placebo.34
Several possible mechanisms have been suggested to explain the beneficial effect of SGLT2i on cardiovascular outcomes, but not yet formally proven. Improved glycemic control is unlikely to explain entirely the cardiovascular benefit observed. First, the difference between the reductions of HbA1c in SGLT2i group and in placebo group in the CVOTs was modest (0.3–0.5%) at the end of the studies and similar to that achieved in CVOTs with DPP4i35–38 or lixisenatide39 that failed to show cardiovascular benefit. Second, glycemic control takes many years to translate into cardiovascular protection, as shown in the follow-up extension of Action in Diabetes and Vascular Disease: Preterax and Diamicron Controlled Evaluation (ADVANCE) and Action to Control Cardiovascular Risk in Diabetes (ACCORD) studies.40,41 Instead, in CVOTs on SGLT2i, the event-free survival curves separated soon after randomization, especially in patients with prior CVD. Finally, the recent trial DAPA-HF has demonstrated that benefits are observed also in non-diabetic patients.27
Renal Effects and Renal Outcomes
Glomerular hyperfiltration is a complication of diabetes that can pave the way of nephropathy, by inducing inflammation and fibrosis.42 Inhibition of reabsorption of glucose and sodium in the proximal tubule with consequent increase in sodium delivery to the juxtaglomerular apparatus of the macula densa leads to activation of the tubuloglomerular feedback and consequent vasoconstriction of the afferent arteriole, which reduces intraglomerular pressure. The final result is a mitigation of hyperfiltration.43
The initial reduction in plasma volume and the renal hemodynamic changes occurring during SGLT2i therapy are associated with an initial small decline in estimated glomerular filtration rate (eGFR) by 4–5 mL/min/1.73 m2 followed by a modest increase or stabilization of eGFR. This benefit of SGLT2i in preventing deterioration of kidney function was clearly observed in a post-hoc analysis of the EMPA-REG OUTCOME trial where the eGFR steadily declined over time in the placebo arm and stabilized in the empagliflozin arm.44 As a result, empagliflozin prevented the decline in eGFR that is typically observed in diabetic patients. A meta-analysis of 48 RCTs, including 58,165 patients, has demonstrated that SGLT2i are associated with statistically significant reductions in albuminuria compared to placebo or active comparators. In a meta-regression analysis, the effects of SGLT2i on albuminuria tended to be greater with higher levels of albuminuria at baseline.45
A secondary-prespecified analysis of the EMPA-REG OUTCOME trial has shown that empagliflozin reduced the rate of the composite renal endpoint (progression to macroalbuminuria, doubling of serum creatinine accompanied by an eGFR of ≤45 mL/min/1.73 m2, initiation of renal replacement therapy, or death from renal disease) by 39% (p<0.001). Empagliflozin was associated with a significant reduction of all the individual components of the composite renal outcome. A consistent benefit was seen across eGFR and albuminuria subgroups.44 In the CANVAS program, canagliflozin exerted a 40% reduction in the rate of the prespecified composite renal outcome which comprised a sustained 40% reduction in eGFR, the need for renal replacement therapy, or death from renal causes (HR 0.60, 95% CI 0.47–0.77). Moreover, canagliflozin reduced the rate of progression of albuminuria by 27%. Finally, regression of albuminuria occurred more frequently among participants assigned to canagliflozin (HR 1.70, 95% CI, 1.51 to 1.19).11 Treatment with canagliflozin was also evaluated in patients with impaired kidney function in CREDENCE trial. Patients with T2D who had chronic kidney disease (CKD), defined as those with a decrease in eGFR and macroalbuminuria, and with a background use of an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker. Canagliflozin significantly reduced the rate of the renal composite outcome comprising end-stage renal disease, doubling of serum creatinine, or death from renal causes by 34%, compared with the placebo group (HR 0.66, 95% CI 0.53–0.81, p<0.001). Canagliflozin reduced the slope of the change in eGFR from baseline, despite the initial decrease during the first 3 weeks.13
Consistently, in the DECLARE study, dapagliflozin therapy resulted in a lower rate of renal composite outcome defined as a sustained decrease of 40% or more in eGFR to less than 60 mL/min/1.73 m2, new end-stage renal disease or death from renal causes (HR 0.53, 95% CI, 0.43 to 0.66; p<0·0001) compared to placebo.46
Further information on the nephroprotective properties of SGLT2i will be provided by clinical trials primarily designed to evaluate outcomes in large population with non-diabetic CKD: A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With CKD (Dapa-CKD; ClinicalTrial.gov registration no. NCT03036150), and The Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY; ClinicalTrials.gov registration no. NCT03594110).
Body Weight
By promoting excretion of 60–80 g of glucose per day through the urine, SGLT2i lead to a significant loss of 240–320 calories per day, resulting in a consequent substantial weight loss. SGLT2i treatment is associated with an average 2–4 kg reduction of body weight.47 This reduction is consistent across all studies, and for all molecules, when SGLT2i were used in monotherapy or in combination with other GLM. Notably, data resulting from dual-energy x-ray absorptiometry studies of body composition showed that 60–70% of the total weight loss observed with the SGLT2i dapagliflozin was fat mass.48 However, this result is not always consistent with different methods for the analysis of body composition.49 In a magnetic resonance imaging (MRI) sub-study, dapagliflozin reduced visceral as well as subcutaneous adipose tissue.48
As observed in Ferrannini et al, although glycosuria is persistent over time, weight loss reaches a plateau and is much less than calorie loss expected by the amount of daily glycosuria assuming no changes in energy intake. The curves of weight loss observed and that predicted by model started to diverge at 24 weeks when observed body weight stabilized. Therefore, caloric intake must have increased to explain this discrepancy.50 This effect differentiates the bodyweight reduction effect of SGLT2i from the bodyweight reduction of GLP-1RA. Although the effects are quantitatively similar,51 weight reduction is achieved with a normal/increased caloric intake during SGLT2i therapy, whereas GLP-1RA induce satiety and reduce caloric intake. Nevertheless, the weight loss achieved in the first weeks of treatment with SGLT2i appears to persist over time.10
Blood Pressure
In the proximal tubule of kidney, the reabsorption of glucose is coupled with the reabsorption of sodium. Inhibition of SGLT2 results in natriuresis and osmotic diuresis and, subsequently, in reduction of plasma and extracellular volume. This reduction takes account for the 5–6 mm Hg decrease in systolic and the 1–2 mm Hg decrease in diastolic blood pressure (BP) observed in Phase III studies.47 Greater reductions generally occurring in individuals with higher baseline systolic BP. The results were consistent across different SGLT2i and where similar in trials wherein SGLT2i were compared to placebo or to others GLM event in patients already receiving antihypertensive therapy.47,52 The BP reduction seems independent from the improvement in glycemic control. In fact, in studies conducted in diabetic patients with CKD, in whom the anti-hyperglycemic effect of SGLT2i was reduced to negligible levels, SGLT2i therapy was still effective in improving BP.53 In contrast with GLP-1RA,54 SGLT2i reduce BP without sympathetic nervous system activation and no increasing heart rate.
Reduction in preload and afterload results in a reduction of cardiac workload and myocardial oxygen demand and in an improved LV function. Nevertheless, BP reduction is unlikely to explain all cardiovascular benefit. In fact, other anti-hypertensive agents appear to be associated with a smaller and later cardiovascular benefit.55 Moreover, reduction in BP is commonly associated with reduction in the risk of stroke, whereas in CVOTs the risk of non-fatal stroke was not reduced with the three SGLT2i.
Uric Acid
Hyperuricemia is commonly observed in T2D patients and is related to insulin resistance.56 High uric acid concentrations are associated with increased risk of hypertension, CVD and CKD.57 Therefore, lowering serum uric acid concentrations may contribute to reduce cardio-renal risk.
SGLT2i increase renal uric acid excretion, thereby reducing serum uric acid concentrations. SGLT2i increase glucose concentrations in the proximal tubules, wherein glucose competes with urates for the transporter GLUT9b, reducing urate reabsorption.58 In a study conducted in patients with type 1 diabetes (T1D), after induction of glycosuria with SGLT2i, plasma uric acid decreased while uric acid excretion significantly increased.59 In a meta-analysis of 62 RCT, comprising 34,941 patients with T2D, treatment with an SGLT2i resulted in a superior reduction in serum uric acid compared with placebo or active comparator. This reduction became modest or absent in patients with CKD.60
Ketone Bodies
The decreased plasma glucose levels caused by glycosuria during SGLT2i therapy force cells to shift their metabolism to enhanced fatty acid oxidation and lipolysis for their energy requirement. Lipid oxidation generates acetyl-CoA that is converted in ketone bodies when glucose oxidation is reduced. Lower plasma glucose level stimulates glucagon secretion and suppression of insulin production leading to an increase in the glucagon: insulin ratio. These metabolic changes overall promote ketogenesis.61 Moreover, some studies have suggested that SGLT2i may exert a direct stimulatory effect on alfa cells,62,63 although this finding is controversial and has not been consistently replicated in all studies.64,65 Oxidation of ketone bodies produces more amounts of ATP per molecule of oxygen than glucose or fatty acids oxidation does and may provide a more efficient energy source for the myocardium.61 In fact, enhanced production of ketone bodies has been proposed as one mechanism driving protection from cardiovascular death and heart failure observed during SGLT2i therapy. Notably, however, the ability to increase ketone bodies concentrations also lead to one feared adverse reaction of SGLT2i, namely diabetic ketoacidosis (DKA).66 DKA during SGT2i therapy was previously considered to be “euglycemic”, which was not subsequently confirmed, although DKA in T2D treated with SGLT2i can present with lower glucose levels than typical DKA in T1D. Risk of DKA appeared to be particularly related to autoimmune diabetes misdiagnosed for T2D, abrupt insulin withdrawal, alcoholism, and/or advanced age.66,67
Oxidative Stress and Inflammation
Oxidative stress is involved in the development of diabetic complications and plays a key role in the progression of atherosclerosis.68,69 Therefore, blunting oxidative stress might improve cardiovascular outcomes and the overall diabetic complication burden. SGLT2i have been recently recognized to be able to reduce oxidative stress and to restore the balance between pro- and anti-inflammatory adipokines and cytokine, as briefly summarized below.
Tahara and colleagues reported that 12 weeks of treatment with the SGLT2i ipragliflozin significantly reduced liver levels of oxidative stress biomarkers (thiobarbituric acid reactive substances and protein carbonyl), and plasma levels of inflammation markers (interleukin 6 [IL-6], tumor necrosis factor α [TNF-α], monocyte chemotactic protein-1 [MCP-1] and C-reactive protein [CRP]) in T1D rats.70
Empagliflozin reduced oxidative stress in the streptozotocin-diabetic rat model by interfering with NADPH oxidase activity. The expression of Nox1 and Nox2 enzymes was reduced and reactive oxygen species production was reduced in response to different stimuli.71
In db/db mice, dapagliflozin attenuated the expression of Nox4, isoform of NADPH oxidase, and the expression of several proinflammatory genes, including transforming growth factor-β (TGF-β), MCP-1, osteopontin and intercellular adhesion molecule-1 (ICAM-1) in the kidney. Furthermore, dapagliflozin suppresses the proinflammatory macrophage infiltration into the glomeruli and in the interstitial, mesangial matrix accumulation and interstitial fibrosis in kidney.72
Empagliflozin therapy has been associated with reduced levels of cardiac oxidative stress, inflammation, and fibrosis and in animal model of pre-diabetes and diabetes.73,74
Other studies conducted in animal model of diabetes or in cultured human cells showed that SGLT2i may lower free-radical production via advanced glycation end products (AGEs) generation, improving mitochondrial function or other mechanisms.75
In a RCT conducted in 200 patients with T2D, treatment with canagliflozin was associated with a 22% reduction in median serum IL-6 and a 7% increase in median serum tumor necrosis factor-α (TNF-α) compared with glimepiride. A trend for a decrease in CRP concentrations was observed in the canagliflozin group but did not achieve statistical significance. Small changes in plasminogen activator inhibitor-1 (PAI-1), vascular cell adhesion molecule-1 (VCAM-1) and MCP-1, were seen with both treatment but were not statistically significant.76
Lipid Profile
Data on the effects of SGLT2i on lipids are not consistent among various studies. SGLT2i treatment was associated with small increases in HDL cholesterol and decreases in triglyceride levels.77 These favourable effects, however, were accompanied by a small increase in LDL cholesterol.10 Whether the increase in HDL cholesterol is accompanied by an improvement in reverse cholesterol transport (HDL capacity for cholesterol efflux from macrophages) is unclear. In a placebo-controlled randomized trial, dapagliflozin was unable to significantly modify HDL cholesterol levels and reverse cholesterol transport was not improved.49
In diabetic mice expressing human cholesterol ester transfer protein and human apolipoprotein B100, the SGLT2i canagliflozin and a specific antisense oligonucleotide against SGLT2 increased circulating levels of LDL cholesterol and reduced triglycerides, as well as increased lipoprotein lipase activity, decreased postprandial lipemia, accelerated clearance of radiolabeled VLDL, and delayed turnover of labeled LDL from circulation.78 Overall, changes in lipid profile observed in humans are however modest and are therefore unlikely to contribute substantially to cardiovascular protection of SGLT2i.
Effects on the Liver
NAFLD may precede, coexist or follow the occurrence of the metabolic syndrome and its individual features, including T2D.79 In addition, NAFLD contributes to the pathophysiology of T2D by modulating insulin resistance, systemic inflammation and driving cardiovascular risk.80
Recent randomized controlled and open-label trials have shown that SGLT2i can reduce liver steatosis and improve biological markers of NAFLD in T2D patients. In a randomised placebo-controlled double-blind trial, Eriksson and colleagues reported that dapagliflozin reduced liver fat content assessed by MRI compared to placebo in T2D patients with MRI-defined NAFLD.81 Dapagliflozin treatment was also associated with a significant reduction in hepatocyte injury biomarkers alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT). Similar results were observed in open-label trial conducted in 50 T2D patients with NAFLD randomized to receive empagliflozin or standard treatment. Empagliflozin performed significantly better in reducing liver fat content assessed by MRI and ALT level, but not AST or GGT.82
In other studies exploring the effects of SGLT2i on this endpoint, NAFLD was diagnosed using computed tomography (CT) or ultrasound and primary outcome was change versus baseline in liver-to-spleen attenuation ratio. Ipragliflozin exerted equally beneficial effects on NAFLD compare to pioglitazone.83 Luseogliflozin was found superior in increased liver-to-spleen attenuation ratio compared to metformin.84
All these results come from studies with small sample size. Therefore, further studies with larger sample size will be needed to confirm the beneficial effects of SGLT2i on NAFLD. So far, data coming from large RCTs have reported that treatment with SGLT2i was associated with a significant reduction of liver enzymes (ALT, AST, GGT) comparing with placebo or active comparator.85,86
Hematocrit
SGLT2i treatment is associated with a modest increase in hematocrit by 2–4%. The decrease in plasma volume occurring early after initiation of SGLT2i is partly responsible for such haemoconcentration. However, it has been postulated that the increased hematocrit may also be a consequence of direct stimulation of erythropoiesis through the increase in erythropoietin secretion and induction of reticulocytosis.87 Indeed, chronic hyperglycemia stimulates reabsorption of glucose via SGLT2 that increases energy consumption by Na+K+ pump and oxygen consumption for ATP production. This results in a relative tubulointerstitial hypoxia, leading to transdifferentiation of erythropoietin-producing fibroblasts into myofibroblasts that lose the capacity to produce erythropoietin and actively produce fibrogenic molecules instead. SGLT2i has the potential to reduce activity of Na+K+ pump, ATP consumption, and hypoxic microenvironment. Myofibroblasts would thus recover their original capacity of producing erythropoietin.88 Increased hematocrit may lead to enhance delivery of oxygen to tissues and can contribute to the beneficial effect of SGLT2i on cardiovascular outcomes.61 In a post hoc analysis performed by Inzucchi and colleagues, based on evidence from previous studies, the authors identified potential mediators empagliflozin benefits on cardiovascular death among the variables measured in the trial. The variables chosen for the mediation analysis included: HbA1c, systolic and diastolic BP, lipids, weight, albuminuria, eGFR, hematocrit, hemoglobin, and uric acid. In analyses adjusting for change from baseline of each variable, models showed that increase in hematocrit and hemoglobin were associated with a reduced risk of cardiovascular death. Changes in hematocrit and hemoglobin mediated 51.8% and 48.9%, respectively, of the effect of empagliflozin versus placebo on the reduction in cardiovascular death rates. Changes in albumin and uric acid mediated 25.5% and 24.6% of the effect, respectively. The other mediators had no effects in these analyses.89
Arterial Stiffness and Endothelial Dysfunction
Arterial stiffness is a surrogate of vascular aging and it has been validated as a strong predictor of cardiovascular events and mortality.90 Endothelial dysfunction is considered the primum movens of the atherosclerotic process and its contribution to the development of cardiovascular and renal disease is well established.91 Therefore, both these surrogates are considered reliable markers of cardiovascular risk.92
Several studies have documented a significant reduction in arterial stiffness and an improvement in endothelial dysfunction after acute or chronic use of SGLT2i. In a trial involving 40 T1D patients, empagliflozin significantly reduced arterial stiffness parameters, such as carotid radial pulse wave velocity and radial augmentation index.93 Furthermore, an acute treatment with dapagliflozin, but not hydrochlorothiazide, significantly improved systemic endothelial function measured as flow-mediated dilation (FMD) of the brachial artery and arterial stiffness parameters in 16 patients with T2D.94 This effect was independent of changes in BP. Similarly, canagliflozin improved carotid-femoral pulse wave velocity in 30 patients with T2D after 6 months of treatment.95 Shigiyama and colleagues reported that, in Japanese T2D patients, a treatment with dapagliflozin plus metformin for 16 weeks was associated with a significant improvement in FMD compared to patients treated only with metformin.96 Nevertheless, a recent multicenter study conducted in Japan suggested that a treatment with empagliflozin is not associated with an improvement in endothelial function. One hundred and five patients with T2D and established CVD were randomized to receive empagliflozin or placebo for 24 weeks. Reactive hyperemia index was used as a marker of endothelial function. No significant difference in change in reactive hyperemia index was observed in the two groups.97
Na+/H+ Exchanger
Increased of cytosolic sodium concentration and upregulated Na+/H+ exchanger activity have been shown to contribute to heart failure and may contribute to arrhythmogenesis and oxidative stress in diabetic hearts.98–100
Studies in experimental models have documented that SGLT2i inhibited myocardial Na+/H+ exchanger. In the first experiment, cardiomyocytes were isolated from hearts of healthy rabbits and rats. Na+/H+ exchanger activity, sodium, and calcium concentrations were measured fluorometrically before and treatment with empagliflozin. Empagliflozin treatment reduced cytoplasmic sodium and calcium concentration and increased mitochondrial calcium concentration. After a concomitant treatment with an inhibitor of Na+/H+ exchanger (cariporide), these effects were strongly reduced suggesting a direct effect of empagliflozin on Na+/H+exchanger.98
Similar results were observed in mouse cardiomyocytes after a treatment with canagliflozin and dapagliflozin, thus suggesting a class effect.101
The increased concentration of mitochondrial calcium is not only crucial for cardiomyocytes excitation-contraction, but also for preserving mitochondrial antioxidative capacity.102,103
Cardiac Fibrosis
Myocardial fibrosis contributes to impairment in cardiac contractile function, to LV dysfunction leading to the development of heart failure.104
Lee and colleagues conducted animal experiment in healthy rats in which the ligation of the anterior descending coronary artery resulted in infarction of the LV wall. In rats treated with dapagliflozin, myocardial superoxide production was significantly decreased, the presence of myofibroblast was significantly reduced, along with fibrosis of the LV. Moreover, dapagliflozin significantly increased anti-inflammatory (M2) macrophages in the infarcted heart.105
A recent study showed that treatment with empagliflozin reduced the expression of TGF-β, the levels of collagen I and III and cardiac fibrosis in diabetic mice compared to healthy mice and diabetic mice with no treatment. Furthermore, empagliflozin significantly reduced the expression of Nox4, lipid hydroperoxide concentration, and malondialdehyde level.106
Epicardial Adipose Tissue Volume
Another mechanism that has been proposed for cardiac benefit of SGLT2i is the reduction of epicardial adipose tissue (EAT) mass. EAT volume was found to be strongly correlated with the severity of coronary artery disease.107
In a RCT conducted in 40 patients with T2D and coronary artery disease, EAT was measured with CT al baseline and after 6 months of treatment with dapagliflozin or others GLMs excluded SGLT2i (conventional therapy). After 6 months, EAT volume decreased significantly compared to baseline in the dapagliflozin group whereas remained stable in the conventional therapy arm. In addition, TNF-α levels decreased significantly in the dapagliflozin group compared to the other arm.108
Circulating Stem/Progenitor Cells
Diabetes affects the bone marrow (BM) microenvironment, leading to a shortage of BM-derived stem and progenitor cells in the circulation.109 These include hematopoietic CD34+/CD133+ stem cells (HSC) and endothelial progenitor cells (EPC). Since these cells are endowed with vascular regenerative properties, their pauperization reflects an impaired endogenous repair capacity. In fact, shortage of HSC predicts future onset or deterioration of micro- and macro-angiopathy in patients with T2D.110,111 Based on this premise, we tested whether a therapy with SGLT2i improved circulating stem/progenitor cell levels. In a randomized placebo-controlled trial in T2D, dapagliflozin add-on therapy for 3 months failed to improve HSC or EPC levels. Same results were found in an observational study on patients receiving empagliflozin.112 Upon longer observation, optimization of glucose control resulted in an increase in EPCs, but this was observed in both the dapagliflozin and placebo group and is consistent with prior studies on T2D patients treated with insulin.113 Conversely, Hess and colleagues have recently reported that a therapy with empagliflozin can increase Aldehyde dehydrogenase-expressing cells.114 Unfortunately, the identify of these cells is still unclear, as is their role in T2D complications, such that there is so far no evidence that cardiorenal protection by SGLT2i can be attributed to effects on regenerative circulating stem/progenitor cells.
Conclusions
Despite all the data so far accumulated in years of intensive research, the mechanisms of cardio-renal protection SGLT2i remain elusive. Figure 1 shows that many putative mechanisms remain speculative or are only supported by studies in preclinical models or small studies in humans. In the absence of a single major effect or a unifying hypothesis, we suggest that multiple diverse mechanisms could contribute to the overall cardio-/nephroprotective effects of SGLT2i in patients with diabetes. Whichever the exact molecular pathway is, ancillary extra-glycemic effects of this class of medications decreased risk of cardiovascular events, HHF, and diabetic kidney disease progression. This strong evidence needs to be taken accurately into consideration by clinicians treating patients with T2D, especially those with cardiovascular or renal risk factors, established CVD or renal disease.
Abbreviations
T2D, type 2 diabetes; SGLT2i, sodium-glucose cotransporter-2 inhibitors; NAFLD, non-alcoholic fatty liver disease; CVD, cardiovascular disease; GLM, glucose-lowering medication; SGLT2, sodium-glucose cotransporter-2; CVOT, cardiovascular outcomes trial; MACE, major adverse cardiovascular events; GLP-1 receptor agonists, GLP-1RA; MI, myocardial infarction; HHF, hospitalization for heart failure; RCT, randomized-controlled trial; HF, heart failure; LV, left ventricular; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; MRI, magnetic resonance imaging; BP, blood pressure; T1D, type 1 diabetes; DKA, diabetic ketoacidosis; IL-6, interleukin 6; TNF-α, tumor necrosis factor α; MCP-1, monocyte chemotactic protein-1; CPR, C-reactive protein; TGF-β, transforming growth factor-β; ICAM-1, intercellular adhesion molecule-1; AGEs, advanced glycation end products; TNF-α, tumor necrosis factor-α; PAI-1, plasminogen activator inhibitor-1; VCAM-1, vascular cell adhesion molecule-1; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; CT, computed tomography; FMD, flow-mediated dilation; EAT, epicardial adipose tissue; BM, bone marrow; HSC, hematopoietic stem cells; EPC, endothelial progenitor cells.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
No funding was provided for writing this paper.
Disclosure
B.M.B. received lecture or advisory board fees from Astra Zeneca, Eli Lilly, Boehringer Ingelheim, Novo Nordisk, and Novartis, G.P.F. received grant support, lecture fees, or advisory board fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi, Genzyme, Abbott, Novartis, and Merck Sharp & Dohme. A.A. received research grants, lecture fees, or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boehringer Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Eli Lilly, Servier, and Takeda. The authors report no other conflicts of interest in this work.
References
1. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Supplement 1):S13LP–S28LP. doi:10.2337/dc19-S002
2. Sarwar N, Gao P, Kondapally Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi:10.1016/S0140-6736(10)60484-9
3. de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi:10.1001/jama.2011.861
4. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886. doi:10.1053/j.ajkd.2012.07.005
5. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in Type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi:10.1056/NEJMoa0802743
6. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with Type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi:10.1056/NEJMoa0802987
7. Gæde P, Lund-Andersen H, Parving H-H, Pedersen O. Effect of a multifactorial intervention on mortality in Type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi:10.1056/NEJMoa0706245
8. Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66(1):255–270. doi:10.1146/annurev-med-051013-110046
9. Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8(8):495–502. doi:10.1038/nrendo.2011.243
10. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi:10.1056/NEJMoa1504720
11. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi:10.1056/NEJMoa1611925
12. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in Type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi:10.1056/NEJMoa1812389
13. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in Type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi:10.1056/NEJMoa1811744
14. Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709–717. doi:10.1016/S2213-8587(17)30258-9
15. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation. 2017;136(3):249–259. doi:10.1161/CIRCULATIONAHA.117.029190
16. Raschi E, Poluzzi E, Fadini GP, Marchesini G, De Ponti F. Observational research on sodium glucose co-transporter-2 inhibitors: a real breakthrough? Diabetes Obes Metab. 2018;20(12):2711–2723. doi:10.1111/dom.13468
17. Fadini GP, Solini A, Manca ML, et al. Effectiveness of dapagliflozin versus comparators on renal endpoints in the real world: a multicentre retrospective study. Diabetes Obes Metab. 2019;21(2):252–260. doi:10.1111/dom.13508
18. Cavender MA, Norhammar A, Birkeland KI, et al. SGLT-2 inhibitors and cardiovascular risk: an analysis of CVD-REAL. J Am Coll Cardiol. 2018;71(22):2497–2506. doi:10.1016/j.jacc.2018.01.085
19. Patorno E, Pawar A, Franklin J, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019;139(25):2822–2830. doi:10.1161/CIRCULATIONAHA.118.039177
20. Udell J, Yuan Z, Rush T, Sicignano N, Galitz M, Rosenthal N. Cardiovascular outcomes and risks after initiation of a sodium glucose cotransporter 2 inhibitor. Circulation. 2018;137(14):1450–1459. doi:10.1161/CIRCULATIONAHA.117.031227
21. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461–2498. doi:10.1007/s00125-018-4729-5
22. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Supplement 1):S90LP–S102LP. doi:10.2337/dc19-S009
23. Cosentino F, Grant PJ, Aboyans V, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Associ. Eur Heart J. 2019:2019. doi:10.1093/eurheartj/ehz486
24. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi:10.1016/S2213-8587(19)30249-9
25. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi:10.1016/S0140-6736(18)32590-X
26. Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in Type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019;140(9):739–750. doi:10.1161/CIRCULATIONAHA.119.042007
27. McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019. doi:10.1056/NEJMoa1911303
28. Verma S, Garg A, Yan AT, et al. Effect of Empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care. 2016;39(12):e212LP–e213LP. doi:10.2337/dc16-1312
29. Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):73. doi:10.1186/s12933-018-0717-9
30. Soga F, Tanaka H, Tatsumi K, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17(1):132. doi:10.1186/s12933-018-0775-z
31. Verma S, Mazer DC, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with Type 2 diabetes mellitus and coronary artery disease. Circulation. 2019;140(21):1693–1702. doi:10.1161/CIRCULATIONAHA.119.042375
32. Singh JSS, Fathi A, Vickneson K, et al. Research into the effect Of SGLT2 inhibition on left ventricular remodelling in patients with heart failure and diabetes mellitus (REFORM) trial rationale and design. Cardiovasc Diabetol. 2016;15:97. doi:10.1186/s12933-016-0419-0
33. Natali A, Nesti L, Fabiani I, Calogero E, Di Bello V. Impact of empagliflozin on subclinical left ventricular dysfunctions and on the mechanisms involved in myocardial disease progression in type 2 diabetes: rationale and design of the EMPA-HEART trial. Cardiovasc Diabetol. 2017;16(1):130. doi:10.1186/s12933-017-0615-6
34. Bonora BM, Vigili de Kreutzenberg S, Avogaro A, Fadini GP. Effects of the SGLT2 inhibitor dapagliflozin on cardiac function evaluated by impedance cardiography in patients with type 2 diabetes. Secondary analysis of a randomized placebo-controlled trial. Cardiovasc Diabetol. 2019;18(1):106. doi:10.1186/s12933-019-0910-5
35. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with Type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi:10.1056/NEJMoa1305889
36. Scirica BM, Bhatt DL, E B, et al. Saxagliptin and cardiovascular outcomes in patients with Type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi:10.1056/NEJMoa1307684
37. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in Type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi:10.1056/NEJMoa1501352
38. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with Type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79. doi:10.1001/jama.2018.18269
39. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with Type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi:10.1056/NEJMoa1509225
40. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year follow-up of intensive glucose control in Type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi:10.1056/NEJMoa0806470
41. Zoungas S, Chalmers J, Neal B, et al. Follow-up of blood-pressure lowering and glucose control in Type 2 diabetes. N Engl J Med. 2014;371(15):1392–1406. doi:10.1056/NEJMoa1407963
42. Ruggenenti P, Porrini EL, Gaspari F, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35(10):2061–2068. doi:10.2337/dc11-2189
43. Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61(10):2098–2107. doi:10.1007/s00125-018-4669-0
44. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in Type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi:10.1056/NEJMoa1515920
45. Bae JH, Park E-G, Kim S, Kim SG, Hahn S, Kim NH. Effects of sodium-glucose cotransporter 2 inhibitors on renal outcomes in patients with Type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2019;9(1):13009. doi:10.1038/s41598-019-49525-y
46. Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–617. doi:10.1016/S2213-8587(19)30180-9
47. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium–glucose cotransporter 2 inhibitors for Type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(4):262–274. doi:10.7326/0003-4819-159-4-201308200-00007
48. Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with Type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–1031. doi:10.1210/jc.2011-2260
49. Fadini GP, Bonora BM, Zatti G, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol. 2017;16(1):42. doi:10.1186/s12933-017-0529-3
50. Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1730–1735. doi:10.2337/dc15-0355
51. Fadini GP, Sciannameo V, Franzetti I, et al. Similar effectiveness of dapagliflozin and GLP-1 receptor agonists concerning combined endpoints in routine clinical practice: a multicentre retrospective study. Diabetes Obes Metab. 2019;21(8):1886–1894. doi:10.1111/dom.13747
52. Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, Phase 3 study. Lancet Diabetes Endocrinol. 2016;4(3):211–220. doi:10.1016/S2213-8587(15)00417-9
53. Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–971. doi:10.1038/ki.2013.356
54. Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3(1):e001986. doi:10.1136/bmjopen-2012-001986
55. Olde Engberink RH, Frenkel W, van den Bogaard B, Brewster L, Vogt L, van den Born B. Effects of Thiazide-Type and Thiazide-like Diuretics on cardiovascular events and mortality. Hypertension. 2015;65(5):1033–1040. doi:10.1161/HYPERTENSIONAHA.114.05122
56. Facchini F. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–3011. doi:10.1001/jama.1991.03470210076036
57. Feig DI, Kang D-H, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi:10.1056/NEJMra0800885
58. Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35(7):391–404. doi:10.1002/bdd.1909
59. Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZI. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Physiol. 2014;308(2):F77–F83. doi:10.1152/ajprenal.00555.2014
60. Zhao Y, Xu L, Tian D, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(2):458–462. doi:10.1111/dom.13101
61. Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium–glucose cotransporter 2 inhibition in subjects without diabetes and patients with Type 2 diabetes. Diabetes. 2016;65(5):1190LP–1195LP. doi:10.2337/db15-1356
62. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508. doi:10.1172/JCI72227
63. Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512. doi:10.1038/nm.3828
64. Solini A, Sebastiani G, Nigi L, Santini E, Rossi C, Dotta F. Dapagliflozin modulates glucagon secretion in an SGLT2-independent manner in murine alpha cells. Diabetes Metab. 2017;43(6):512–520. doi:10.1016/j.diabet.2017.04.002
65. Kuhre RE, Ghiasi SM, Adriaenssens AE, et al. No direct effect of SGLT2 activity on glucagon secretion. Diabetologia. 2019;62(6):1011–1023. doi:10.1007/s00125-019-4849-6
66. Bonora BM, Avogaro A, Fadini GP. Sodium-glucose co-transporter-2 inhibitors and diabetic ketoacidosis: an updated review of the literature. Diabetes Obes Metab. 2018;20(1):25–33. doi:10.1111/dom.13012
67. Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA adverse event reporting system. Diabetologia. 2017;60(8):1385–1389. doi:10.1007/s00125-017-4301-8
68. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi:10.1161/CIRCRESAHA.110.223545
69. Ulrich F, Ning X, Huige L. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120(4):713–735. doi:10.1161/CIRCRESAHA.116.309326
70. Tahara A, Kurosaki E, Yokono M, et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol. 2014;66(7):975–987. doi:10.1111/jphp.12223
71. Oelze M, Kröller-Schön S, Welschof P, et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS One. 2014;9(11):e112394. doi:10.1371/journal.pone.0112394
72. Terami N, Ogawa D, Tachibana H, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. 2014;9(6):e100777. doi:10.1371/journal.pone.0100777
73. Kusaka H, Koibuchi N, Hasegawa Y, Ogawa H, Kim-Mitsuyama S. Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome. Cardiovasc Diabetol. 2016;15(1):157. doi:10.1186/s12933-016-0473-7
74. Lin B, Koibuchi N, Hasegawa Y, et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. doi:10.1186/s12933-014-0148-1
75. Yaribeygi H, Atkin SL, Butler AE, Sahebkar A. Sodium–glucose cotransporter inhibitors and oxidative stress: an update. J Cell Physiol. 2019;234(4):3231–3237. doi:10.1002/jcp.26760
76. Garvey WT, Van Gaal L, Leiter LA, et al. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metab Clin Exp. 2018;85:32–37. doi:10.1016/j.metabol.2018.02.002
77. Storgaard H, Gluud LL, Bennett C, et al. Benefits and harms of sodium-glucose co-transporter 2 inhibitors in patients with Type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2016;11(11):e0166125. doi:10.1371/journal.pone.0166125
78. Basu D, Huggins L-A, Scerbo D, et al. Mechanism of increased LDL (Low-Density Lipoprotein) and decreased triglycerides with SGLT2 (Sodium-Glucose Cotransporter 2) inhibition. Arterioscler Thromb Vasc Biol. 2018;38(9):2207–2216. doi:10.1161/ATVBAHA.118.311339
79. Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335–352. doi:10.1016/j.jhep.2017.09.021
80. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–1350. doi:10.1056/NEJMra0912063
81. Eriksson JW, Lundkvist P, Jansson P-A, et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61(9):1923–1934. doi:10.1007/s00125-018-4675-2
82. Kuchay MS, Krishan S, Mishra SK, et al. Effect of empagliflozin on liver fat in patients with Type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT trial). Diabetes Care. 2018;41(8):1801LP–1808LP. doi:10.2337/dc18-0165
83. Ito D, Shimizu S, Inoue K, et al. Comparison of Ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with Type 2 diabetes: a randomized, 24-week, open-label, active-controlled trial. Diabetes Care. 2017;40(10):1364LP–1372LP. doi:10.2337/dc17-0518
84. Shibuya T, Fushimi N, Kawai M, et al. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20(2):438–442. doi:10.1111/dom.13061
85. Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia. 2018;61(10):2155–2163. doi:10.1007/s00125-018-4702-3
86. Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42(1):25–32. doi:10.1016/j.diabet.2015.10.003
87. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853–862. doi:10.1111/dom.12127
88. Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8(12):844–847. doi:10.14740/jocmr2760w
89. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(2):356LP–363LP. doi:10.2337/dc17-1096
90. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi:10.1016/j.jacc.2009.10.061
91. Zhang J, Bottiglieri T, McCullough PA. The central role of endothelial dysfunction in cardiorenal syndrome. Cardiorenal Med. 2017;7(2):104–117. doi:10.1159/000452283
92. Anderson TJ. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can J Cardiol. 2006;22(Suppl B):72B–80B. doi:10.1016/s0828-282x(06)70990-4
93. Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi:10.1186/1475-2840-13-28
94. Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. doi:10.1186/s12933-017-0621-8
95. Ramirez AJ, Sanchez MJ, Sanchez RA. Diabetic patients with essential hypertension treated with amlodipine: blood pressure and arterial stiffness effects of canagliflozin or perindopril. J Hypertens. 2019;37:3. doi:10.1097/HJH.0000000000001907
96. Shigiyama F, Kumashiro N, Miyagi M, et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16(1):84. doi:10.1186/s12933-017-0564-0
97. Tanaka A, Shimabukuro M, Machii N, et al. Effect of empagliflozin on endothelial function in patients with Type 2 diabetes and cardiovascular disease: results from the multicenter, randomized, placebo-controlled, double-blind EMBLEM trial. Diabetes Care. 2019;42(10):e159LP–e161LP. doi:10.2337/dc19-1177
98. Baartscheer A, Schumacher CA, Wust RCI, et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60(3):568–573. doi:10.1007/s00125-016-4134-x
99. Darmellah A, Baetz D, Prunier F, Tamareille S, Rücker-Martin C, Feuvray D. Enhanced activity of the myocardial Na+/H+ exchanger contributes to left ventricular hypertrophy in the Goto–kakizaki rat model of type 2 diabetes: critical role of Akt. Diabetologia. 2007;50(6):1335–1344. doi:10.1007/s00125-007-0628-x
100. Lambert R, Srodulski S, Peng X, Margulies KB, Despa F, Despa S. Intracellular Na+ concentration ([Na+]i) is elevated in diabetic hearts due to enhanced Na+-glucose cotransport. J Am Heart Assoc. 2015;4(9):e002183. doi:10.1161/JAHA.115.002183
101. Uthman L, Baartscheer A, Bleijlevens B, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61(3):722–726. doi:10.1007/s00125-017-4509-7
102. Clancy CE, Chen-Izu Y, Bers DM, et al. Deranged sodium to sudden death. J Physiol. 2015;593(6):1331–1345. doi:10.1113/jphysiol.2014.281204
103. Kohlhaas M, Liu T, Knopp A, et al. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121(14):1606–1613. doi:10.1161/CIRCULATIONAHA.109.914911
104. Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–574. doi:10.1007/s00018-013-1349-6
105. Lee T-M, Chang N-C, Lin S-Z. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310. doi:10.1016/j.freeradbiomed.2017.01.035
106. Li C, Zhang J, Xue M, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18(1):15. doi:10.1186/s12933-019-0816-2
107. Jeong J-W, Jeong MH, Yun KH, et al. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71(4):536–539. doi:10.1253/circj.71.536
108. Sato T, Aizawa Y, Yuasa S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17(1):6. doi:10.1186/s12933-017-0658-8
109. Fadini GP, Ciciliot S, Albiero M. Concise review: Perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells. 2017;35(1):106–116. doi:10.1002/stem.2445
110. Fadini GP, Rigato M, Cappellari R, Bonora BM, Avogaro A. Long-term prediction of cardiovascular outcomes by circulating CD34+ and CD34+CD133+ stem cells in patients with Type 2 diabetes. Diabetes Care. 2017;40(1):125LP–131LP. doi:10.2337/dc16-1755
111. Rigato M, Bittante C, Albiero M, Avogaro A, Fadini GP. Circulating progenitor cell count predicts microvascular outcomes in Type 2 diabetic patients. J Clin Endocrinol Metab. 2015;100(7):2666–2672. doi:10.1210/jc.2015-1687
112. Bonora BM, Cappellari R, Albiero M, Avogaro A, Fadini GP. Effects of SGLT2 inhibitors on circulating stem and progenitor cells in patients with Type 2 diabetes. J Clin Endocrinol Metab. 2018;103(10):3773–3782. doi:10.1210/jc.2018-00824
113. Fadini GP, de Kreutzenberg SV, Mariano V, et al. Optimized glycaemic control achieved with add-on basal insulin therapy improves indexes of endothelial damage and regeneration in type 2 diabetic patients with macroangiopathy: a randomized crossover trial comparing detemir versus glargine. Diabetes Obes Metab. 2011;13(8):718–725. doi:10.1111/j.1463-1326.2011.01396.x
114. Hess DA, Terenzi DC, Trac JZ, et al. SGLT2 inhibition with empagliflozin increases circulating provascular progenitor cells in people with Type 2 diabetes mellitus. Cell Metab. 2019;30(4):609–613. doi:10.1016/j.cmet.2019.08.015
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.