Back to Journals » Infection and Drug Resistance » Volume 15
Extended-Spectrum Beta-Lactamases Producing Escherichia coli in South America: A Systematic Review with a One Health Perspective
Authors Bastidas-Caldes C , Romero-Alvarez D , Valdez-Vélez V , Morales RD, Montalvo-Hernández A , Gomes-Dias C, Calvopiña M
Received 22 April 2022
Accepted for publication 4 August 2022
Published 30 September 2022 Volume 2022:15 Pages 5759—5779
DOI https://doi.org/10.2147/IDR.S371845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Carlos Bastidas-Caldes,1,2 Daniel Romero-Alvarez,3,4 Victor Valdez-Vélez,1 Roberto D Morales,1 Andrés Montalvo-Hernández,1 Cicero Gomes-Dias,5 Manuel Calvopiña3
1One Health Research Group, Faculty of Engineering and Applied Sciences, Universidad de las Américas, Quito, Ecuador; 2Doctoral Program in Public and Animal Health, Faculty of Veterinary Medicine, University of Extremadura, Cáceres, Spain; 3One Health Reserch Group, Faculty of Medicine, Universidad de las Américas, Quito, Ecuador; 4Biodiversity Institute and Department of Ecology & Evolutionary Biology, The University of Kansas, Lawrence, KS, USA; 5Department of Basic Health Sciences, Federal University of Health Sciences of Porto Alegre, Porto Alegre, Brazil
Correspondence: Carlos Bastidas-Caldes, One Health Research Group, Faculty of Engineering and Applied Sciences, Universidad de las Américas, Quito, 170124, Ecuador, Tel +593 983 174949, Email [email protected]
Purpose: Extended-spectrum beta-lactamase-producing (ESBL) Enterobacteriaceae, which includes Escherichia coli, has emerged as a global health threat. ESBL enzymes including CTX-M, TEM, and SHV are the most detected. Here, a systematic review was developed to assess the status of ESBLs in E. coli considering studies performed in the human, animal, food, and environmental realms in South America.
Methods: Following PRISMA guidelines, a systematic review was performed using the PubMed database as a primary source to identify studies containing data on ESBL-producing E. coli in South America. To obtain a comprehensive sample, studies in English, Spanish, and Portuguese were included from 1990 to April 2021. Inclusion such as the reporting of sample origin and diagnostic method and exclusion criteria such as review/letter articles were established to complete data extraction steps.
Results: Amongst 506 articles retrieved, 130 met the inclusion criteria. Brazil reported 65 (50%) of publications, followed by Argentina, and Ecuador with 11.5% each. According to the category of studies, human studies represented the 56%, animals the 20%, environmental the 11%, and food studies the 6%. Interestingly, studies assessing more than one category (ie, interdisciplinary) represented the 7%. Prevalence of ESBL producing E. coli in animal, food, and environmental studies was widely superior compared to human sources. In clinical studies, Brazil presented the greatest diversity in terms of ESBLs, featuring CTX-M, TEM, SHV, TOHO, OXA, and AmpC. CTX-M enzymes were the most frequent variants with 89.4% detections.
Conclusion: The present One Health review of 130 studies conducted over the past 21 years found ESBLs producing E. coli distributed across human, animal, food, and environmental samples across South America. There is a need to increment studies in underrepresented countries and to strengthen multi-sectoral antimicrobial resistance research and surveillance. This information can be used as basis for subsequent implementation of monitoring programs, targeting potential critical points of transmission sources.
Keywords: extended-spectrum beta-lactamase, Escherichia coli, South America, One Health
Introduction
The antimicrobial resistance phenomena existed long time before humans were implementing antibiotics.1 Bacteria have several mechanisms to evade the action of antimicrobials. One of the most important in humans, animals, and the environment is the enzyme-mediated breaking of the beta-lactam ring of penicillin and its derivatives. Penicillins are the widest group of antibiotics.2
Enzyme-mediated resistance is a worldwide public health problem recognized by the World Health Organization (WHO) due to its rapid expansion and the generation of multidrug-resistant (MDR) bacteria that are increasingly difficult to eliminate.3,4 The current increase and dispersion of penicillin, carbapenem, and cephalosporin resistance is driven by a group of enzymes known as beta-lactamases. These enzymes, commonly found in well-known human environments, have been discovered in apparently unsuitable localities such as soils or glaciers in Antarctica, which have probably never encountered beta-lactam antibiotics previously.5
Beta-lactamases enzymes were first described in 1940, England; isolated from an E. coli, which prompted antibiotic resistance research.6,7 In early 1980s, TEM-1, TEM-2 (isolated from a patient in Temoneira in Athens, Greece), and SHV-1 (sulfhydryl variable, active site) circulating beta-lactamases were found capable to hydrolyze the beta-lactamic ring of cephalosporins8 and therefore resistance was soon reported.9 Single-point mutations in these enzymes allowed beta-lactamases to break penicillin and its derivatives, as well as the first, the second, and third generation cephalosporins, and even monobactams.10–13
In 1988 and 1989, the first isolate of SHV-ESBL was found in clinical samples from Argentina and Chile, respectively.14 Since then, different types of enzymes have been detected in South America with different predominating enzymes, namely, TEM and SHV, and CTX-M, the latter currently being the most widespread ESBL group in the region.7
Apart from human detections, beta-lactamases have been found in non-human specimens, animals, and the environment. The presence of ESBL genes in aquatic ecosystems has been studied in E. coli in different parts of the world, for example, in Mur River in Europe (ie, Austria)15 and in Yamato River in Asia (Japan)16 with blaCTX-M-1 and blaCTX-M-14 as the most prevalent ESBL genes, respectively.
Livestock and other animals used as food sources are a well-known reservoir of antibiotic-resistant microorganisms, despite the lack of literature exploring this topic. For example, few studies have explored veterinary sources of ESBLs, in stark contrast with the amount of data from humans.17 In 1988, ESBLs were detected for the first time in a dog in Japan with a strain of CTX-M-3-producing E. coli. ESBL types SHV-1, TEM-1, and OXA have been frequently described in E. coli and Salmonella spp. of animals and food of animal origin in Spain, Germany, the US, and the United Kingdom (UK).17
In South America, as in the rest of the world, human clinical studies of ESBLs in E. coli are abundant.18 Conversely, the current status of beta-lactam resistance in non-clinical scenarios such as their presence in healthy carriers, food matrices, animals, and the environment is scarce.19 Nevertheless, evidence suggests that limited access to public health services and lack of hygiene can contribute to the spread of ESBLs within communities.13,20 Moreover, the use of antibiotics as growth promoters in livestock animals favors the dissemination of different types of beta-lactamases CTX-M in food matrices.2,21 Finally, the poor management of hospital wastewater may result in discharge of multidrug-resistant coliforms (such as E. coli ESBL+) into natural waterbodies.22 All this evidence demonstrates the importance of conducting studies using a One Health approach, namely, understanding the dynamics surrounding human and animal health, and the environment, to develop strategies to monitor and control beta-lactam resistance.23
Due to the aforementioned arguments, in this study we have aimed to develop a systematic review of the current status of ESBLs in one of the most relevant and ubiquitous bacteria, E. coli, in South America, considering studies performed in the human, animal, and environmental realms to present a comprehensive summary offering updated information for practitioners across different fields.
Methods
Protocol and Search Strategy
This systematic review was developed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.24 The scientific literature was obtained from the NCBI-PubMed database on April 5th, 2021, including studies in English, Spanish, and Portuguese published since 1990 until 2021. Search terms included “Escherichia coli” AND “ESBL” OR “beta-lactamase” OR “β-lactamase”, plus the names of countries/territories that belong the South American region: ie, “Ecuador” OR “Peru” OR “Brazil” OR “Argentina” OR “Chile” OR “Colombia” OR “Venezuela” OR “Uruguay” OR “Paraguay” OR “Bolivia” OR “Suriname” OR “Guyana” OR “French Guiana”. All terms included the PubMed Title/Abstract criterion so that only studies that contained the searched keywords in their title and/or in their abstract were considered.
Additional articles found manually in Scopus, SciELO, and latindex databases were also included in this review. These articles were not found in the initial search because their title and/or abstract not included the search terms mentioned above; however, they had other keywords such as “CTX-M”, “resistomes”, “multidrug-resistant” or “multi-resistant”. These studies presented relevant epidemiological information related to CTX-M beta-lactamases.
Study Selection
The selection of the studies was carried out by two separate reviewers (VV and AM) using the Rayyan QCRI bibliographic manager to review only titles and abstracts of the selected articles. The first phase consisted in the removal of duplicated studies and the inclusion of those related to E. coli and South America while excluding reviews/letters and studies not focused on ESBLs.
After the first round of selection, a detailed review of the selected articles was implemented. During this eligibility phase, only those studies conducted in humans, animals, and/or the environment with complete information (ie, sample origin and ESBL positive cases detected either by phenotypic or molecular tests) were included for the final analysis. At this stage, exclusion criteria allowed the rejection of [1] case reports, [2] studies in Enterobacteriaceae and other bacterial families that did not report data on ESBLs in E. coli, [3] articles unavailable in full-text, and [4] studies conducted in regions different from South America.
Data Extraction
Studies selected were tabulated and introduced in a Microsoft Excel 2016 spreadsheet with their general information (ie, author, year, country, and URL). Data to be evaluated included (i) detection methods, (ii) type and origin of samples, (iii) prevalence of E. coli, (iv) prevalence of E. coli with ESBL phenotype, (v) prevalence of E. coli with ESBL genotype, (vi) ESBL types, and (vii) identification of clones by multi-locus sequence typing (MLST) of clinical importance. The three-prevalence parameters (points iii, iv, and v) were independently reported for each South American country; these values were obtained by dividing the number of samples found in each category (E. coli, E. coli with ESBL phenotype, and E. coli with ESBL genotype) with the total sample size (n). Relevant data are presented in statistical graphs.
Data Analysis
Descriptive statistics were obtained for all the studied parameters (eg, sample origin and source, E. coli prevalence, etc.) and are shown with 95% confidence intervals when appropriate. ESBL types and CTX-M variants were further categorized using descriptive statistics and their proportions were depicted per country in maps of the region using barplots and pie charts according to each of the established categories, that is, human clinical cases, human healthy carriers, animal, food, and environmental studies using R programming language version 3.6.3 and QGIS 2.18 “Las Palmas”.
Results
Studies Included
The total number of articles found in PubMed from 1990 to April 2021 was 500; additionally, six articles were also included from SciElo and Latindex databases for a total of 506 articles. During the first screening phase, 321 studies were excluded because they were either reviews/letters or articles that deviate from the theme of this review. During the eligibility phase, 55 studies were discarded due to their lack of detail. A total of 130 articles were included in this review (Figure 1).
 |
Figure 1 PRISMA flow diagram for study categorization and selection of the 130 studies included in this systematic review. Data came from PubMed and additional databases between 1990–2021. Abbreviation: ESBL, extended spectrum beta-lactamases. Notes: Adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89.25 Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/legalcode). |
ESBLs Detection Methods for E. coli
From the 130 articles included, 25 used phenotypic tests, the three commonest being the disc diffusion, the minimum inhibitory concentration (MIC), and the VITEK system; the latter also used for bacterial identification. On the other hand, 14 articles used molecular tests including PCR (ie, either endpoint, multiplex, or quantitative) and sequencing to determine the presence of beta-lactamase coding genes. Ninety-one publications (70%) used both phenotypic and molecular tests for gene and bacteria identification.
Classification of Studies
The studies were classified into six categories according to the origin of the sample: two categories for human studies (clinical cases and healthy carriers), followed by animal, food, environmental, and interdisciplinary studies, here labeled as those analyzing more than one category at the same time. More than half of the studies corresponded to human samples (56%; n=73/130). From them, 52% (n=68/130) corresponded to isolations from human clinical studies and 4% (n=5/130) corresponded to studies on human healthy carriers, the smallest category on our analysis. Samples isolated from animals and the environment corresponded to 20% (n=26/130) and 11% (n=14/130), respectively. Interdisciplinary studies corresponded to 7% (n=9/130). Samples isolated from food corresponded to the 6% of the publications studied (n=8/130).
In addition, animal samples were sub-classified into birds, companion animals, livestock, and wild animals (Figure 2). Birds were investigated in more than half of animal-oriented studies (51%; n=18/35), followed by domestic animals (23%; n=8/35), farm animals (20%; n=7/35), and wild animals (6%; n=2/35; Figure 2A). A subcategorization of birds showed that poultry was the most studied with 61% (n=11/18) of publications, followed by urban species such as doves and pigeons with 28% (n=5/18), and migratory bird data in 11% of studies (n=2/18; Figure 2B). A subcategorization of livestock showed that cattle and pigs represented 46% (n=5/11) and 36% (n=4/11) of the, respectively, farm animals studied (Figure 2C). It is important to mention that in some publications, more than one type of farm animal was studied.
ESBL Producing E. coli Studies per Country
Fifty percent of the studies (n=65/130) was carried out in Brazil, followed by Argentina, and Ecuador with 11.5% (n=15/130) each. As it can be seen, Brazil exceeds with 50 studies to all other countries (Figure 3). It is worth noting that a fraction of the studies identified (n=7/130) were developed in more than one country; thus, at least 5.4% of the studies occurred as international multicenter approaches.
As expected, a further categorization of sample type per country showed the predominance of samples from human clinical origin (Figure 3). For example, in Brazil, 46.2% (n=30/65) of the included studies corresponded to clinical isolations; moreover, in Venezuela, 100% of their research (n=12/12) were focused on nosocomial samples. Brazil, despite having the highest number of studies in the region, lacked studies on human healthy carriers. This pattern was similar across the rest of the countries analyzed, namely, predominance of human clinical samples followed by either one or two studies including any of the other studied categories (ie, healthy carriers, animal, or environmental sample types). Exceptions included French Guiana with a unique study focused on human healthy carriers, and Ecuador (n=15 studies) with at least one publication across each sample type category (Figure 3).
Prevalence and Distribution of ESBLs Producing E. coli
In South America, the prevalence of ESBLs at the level of the animal, food, and environment was larger than the prevalence from human sources (ie, either clinical cases or healthy carriers) (Table 1). The same pattern was evident in each country analyzed. Brazil presented the greatest number of samples of each category except for the healthy carriers, where data were absent. Details of these results per country are described in Supplementary Table 1.
ESBL Types and Enzymes Variants
Human Clinical Studies
The total number of ESBLs genes in clinical samples was 3509. The South American distribution of ESBLs based on the selected studies shows that Brazil was the country with the highest number of detections with 21.3% (n=788/3701). Also, Brazil presented the highest diversity of ESBL types reporting CTX-M, TEM, SHV, TOHO, OXA, and AmpC enzymes. Surprisingly, PER-2 enzymes were only found in Uruguay. CTX-M enzymes were the most prevalent in South America with at least 89.4% (n=3309/3701) identifications. CTX-M enzymes variants recognized were usually reported as unspecified across countries/territories with few exceptional publications. For instance, multiple Bolivian studies reported the presence of CTX-M-1 (50.8%; n=67/132). Similarly, studies from Uruguay consistently reported the presence of CTX-M-15 (59.5%, n=47/79). These results are shown in more detail in Figure 4 and Supplementary Table 2.
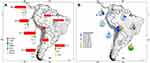 |
Figure 4 Distribution of ESBLs types (A) and CTX-M enzyme variants (B) in clinical human studies developed in South America. Unspecified = CTX-M enzyme present but variant unreported. Pie charts are showing the maximum and minimum percentages for each country. Complete information can be found in the main text and Supplementary Table 2. |
Human Healthy Carriers Studies
Epidemiological data focused on isolations from healthy carriers in South America were limited. Only Ecuador, Peru, Bolivia, and French Guiana reported ESBLs from this category; thus, the total number of ESBLs in this context was 204. The enzymes CTX-M (96%; n=195/204) and TEM (4%; n=9/204) were the only ESBLs found. The CTX-M-2 enzyme variants were most prevalent in Peru (47.7%; n=31/65) and Bolivia (43.7%; n=21/48). In French Guiana, the enzyme CTX-M-1 was the most significant variant with a prevalence of 46.1% (n=6/13). Conversely, only CTX-M-55 enzyme variants were reported in Ecuador (n=69/69). These results are depicted in more detail in Figure 5 and Supplementary Table 2.
 |
Figure 5 Distribution of ESBLs types (A) and CTX-M enzyme variants (B) identified in the context of healthy carriers human studies developed in South America. Pie charts are showing the maximum and minimum percentages for each country; complete information can be found in the main text and Supplementary Table 2. |
Animal Studies
Six South American countries reported epidemiologic data in animals. The total number of ESBLs in animal samples was 1191. ESBL types identified included CTX-M, TEM, SHV, PER-2, and AmpC. CTX-M enzymes were the most prevalent (64.5%; n=768/1191). CTX-M enzyme variants featured included CTX-M-1 in Ecuador (28.4%; n=50/176) and Chile (63.3%; n=124/196); CTX-M-2 in Brazil (40.2%; n=127/316); and CTX-M-8 in Brazil (23.4%; n=74/316), Argentina (56.7%; n=17/30), and Uruguay (56.4%; n=22/39). Many CTX-M variants were recorded in Ecuador although the higher proportion (56.2%, n=99/176) of cases was represented by unknown variants. In Peru, only CTX-M-15 enzymes were reported (n=11/11). These results can be observed in more detail in Figure 6 and Supplementary Table 2.
 |
Figure 6 Distribution of ESBLs types (A) and CTX-M enzyme variants (B) identified in animals and developed in South America. Unspecified = CTX-M enzyme present but variant unreported. **Others = Variants of CTX-M enzymes with <1% of prevalence. Pie charts are showing the maximum and minimum percentages for each country; complete information can be found in the main text and Supplementary Table 2. |
Food Studies
Only two countries (Ecuador and Brazil) published studies about ESBL detection in food. The total number of ESBLs in food samples was 520, among them, CTX-M, TEM, SHV, and AmpC were identified. CTX-M enzymes were the most prevalent (73.6%; n=383/520). Considering CTX-M variants, CTX-M-1 in Ecuador with 66.1% (n=109/165) and CTX-M-2 in Brazil with 63.3% (n=138/218) were found. These results can be observed in detail through Figure 7 and Supplementary Table 2.
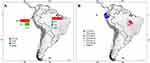 |
Figure 7 Distribution of ESBLs types (A) and CTX-M enzyme variants (B) identified in food and developed in South America. *Others = Variants of CTX-M enzymes with <1% of prevalence. Pie charts are showing the maximum and minimum percentages for each country; complete information can be found in the main text and in Supplementary Table 2. |
Environmental Studies
Environmental studies were found in five South American countries. The total number of ESBLs in environmental samples was 276. ESBL types identified included CTX-M, TEM, SHV, TOHO, OXA, and AmpC. Similar as it was obtained in results from the other categories (ie, human clinical cases, human healthy carriers, animals, and food studies), CTX-M enzymes were the most prevalent (71.4%; n=197/276). Among CTX-M variants, CTX-M-1 in Colombia (68.1%; n=49/72), CTX-M-2 in Peru (72.7%; n=8/11), and CTX-M-55 in Ecuador (50%; n=8/16) were reported. In Brazil, CTX-M variants were unknown in most cases, representing 43.3% (n=42/97) of all detections. In Bolivia, CTX-M-3 was the variant detected in their unique report (n=1/1). These results can be traced in detail in Figure 8 and Supplementary Table 2.
 |
Figure 8 Distribution of ESBLs types (A) and CTX-M enzyme variants (B) identified in the environment and developed in South America. Unspecified = CTX-M enzyme present but variant unreported. *Others = Variants of CTX-M enzymes with <1% of prevalence. Pie charts are showing the maximum and minimum percentages for each country; complete information can be found in the main text and Supplementary Table 2. |
Clones of Epidemiological Importance
A total of 59 different clones were described in 31 studies. The clone E. coli ST131 represented 48.4% of the total of detected clones in the studies (n=15/31), followed by ST10 with 29% (n=9/31). The clones ST405, ST648, and ST38 accounted for 13% (n=4/31) each; ST410 and ST744 for 10% (n=3/31) each; followed by clones ST90, ST117, and ST155 representing the 6.4% (n=2/31). All the remaining clones were represented by a single detection across the reporting studies (3%; n=1/31 each; Supplementary Table 3). It is worth to mention that the presence of these clones was conditioned to their detection per country; thus, Brazil was the country that reported more clones (55%; n=38/59) followed by Peru (17.4%; n=12/59), Ecuador, Uruguay (11.6%; n=8/59 each), Chile (5.8%; n=4/59), and single reports of clones from Colombia, Argentina, and French Guiana (1.4%; n=1/59 each).
Discussion
The present systematic review of the literature provides relevant information on the distributions of ESBLs in E. coli in South America. To our knowledge, this is the first attempt that addresses the presence of ESBLs across different categories, going beyond human-derived clinical samples, to also consider samples from human healthy carriers, animals, environmental, and food isolations, echoing calls of multidisciplinary, One Health approaches, to comprehend the presence of antibiotic resistance mechanisms.
Of the 130 studies included in this review, Brazil contributed with more than half the research of this topic (n=65/130; Figure 3). These results correspond with evidence showing different levels of scientific production in South America where Brazil is recognized as a regional leader.25–28 The other South American countries had at least one publication on ESBLs producing E. coli,29 which is far from ideal and should prompt efforts to understand beta-lactam resistance and their importance in public health.2,3 Despite the low scientific production of Ecuador compared to the rest of South American countries,27,28 Ecuador was the country with the second highest research contribution in the region in this review (n=15/130), together with Argentina (Figure 3).
Considering the limitations of many South American laboratories (eg, logistics, equipment, infrastructure, others),30,31 we expected that the inclusion of molecular tests that require higher costs and increased technical expertise32 would have been lower compared to phenotypic tests. Consequently, in this review, phenotypic tests (n=25/130) were implemented in 11 more publications than molecular tests (n=14/130); however, most of the publications used both phenotypic and molecular tests (70%; n=91/130) for identification, which allows a more precise detection and therefore improvement of ESBLs epidemiological surveillance in E. coli.33 Some human clinical or environmental studies used molecular methods directly for ESBLs genes’ detection. Ideally, both detection methods should be implemented since neither is exempt from limitations. First, methods such as PCR are less effective in the presence of unknown mutations of new unreported ESBL variants, especially when these mutations appear at primer hybridization sites.33 Second, methods based on disk diffusion can report problems with interpretation when co-resistance events occur.34 Thus, new techniques for identifying ESBLs are being developed and have proven to be more sensitive, specific, efficient, and even provide other advantages such as point-of-care detections.35 One of the more recent options is the CRISPR-Cas9-based detection method with optical DNA mapping, which was used to identify blaCTX-M-15 and blaCTX-M-14 genes in E. coli from clinical urinary tract infections in Sweden.36
Up to 52% (n=68/130) of studies corresponded to human clinical samples, which depicts the lack of research about antimicrobial resistance from non-human oriented sources.19 For example, for this review, Venezuela contributed with publications only within this category (Figure 3). Reviews such as that of Guzmán et al37 expose the clinical situation in Venezuela but lack an analysis of antimicrobial resistance from animals, food products, or the environment.
Among the categories established in our review, ESBL detections from human healthy carriers, at the community level, were mostly underrepresented (n=5/130; Figure 3). Onduru et al evidenced a similar pattern in a review for African countries38 where human clinical studies represented the 74%, while studies performed on healthy carriers contributed only with the 15%. It is known that healthy carriers are an important reservoir for the transmission of beta-lactamases and therefore act as spreaders to healthy individuals or environmental settings. Further studies including surveillance at these scales might unveil a hidden pattern for the epidemiology of bacterial resistance in human populations.39,40
For this review, One Health studies were categorized as interdisciplinary, considering that they analyzed samples across different interfaces: human-animal,41–43 human-environment,44,45 human-food,46 animal-environment,47 human-animal-food,48 and animal-food-environment.49 The number of interdisciplinary studies included in this review was low (7%; n=9/130; Figure 3). Similarly, O’Neal et al review for Central America50 and Escher et al review for Africa51 found small numbers of One Health-related studies. Thus, apart from South America, other world regions also struggle to incorporate One Health approaches to their experimental designs; a reality that might be tackled with international cooperation, executing multi-sectorial action plans as proposed by the WHO.52 Many of the clinical studies included in the present review were “international multicenter studies”, which are characterized by promoting joint research across several countries, albeit these alliances are usually focused on human clinical samples.53 These types of studies might be a good example to follow to include a cooperative approach to address questions in the ecology and veterinary fields.
Considering animal publications, those for human consumption were more studied than other groups. Of these, 61% came from poultry in birds and 82% from bovines and swine in livestock. This can be explained due to awareness of the impact of antibiotic use as prophylactic treatment or most commonly as growth promoters for fattening. There is extensive evidence showing how this practice promotes the spread and therefore the risk of zoonotic antibiotic resistance mechanism contaminations through the food chain.54–56 Nevertheless, for this review, urban and migratory birds contributed with the 28% and 11% of ESBLs in E. coli, respectively. Recently, studies of migratory birds have incriminated their feces in the environment as possible contributors to the international spread of antimicrobial resistance.57–59 Studies focusing on this animal group should be encouraged to assess the validity of this hypothesis.
Veterinary publications have noticed a zoonotic transmission risk from pet animals, favored by their proximity to their owners.60–62 A similar trend can be stressed for the potential spillover of ESBLs; however, studies from pet animals were also underrepresented in our systematic review with only a 23%. Studies focused on wild animals were even less represented in our review (6%; n=2/35; Figure 2A). Wildlife might be an important source for the spread of resistance mechanisms as they act as bridges between the urban and sylvatic environments, especially mammals.63 Another example is that, in Brazil, fishes have been found to contribute to the spread of ESBLs in natural waterbodies and its marine fauna.64,65
In South America, higher values of ESBL producing E. coli prevalence were obtained from animal, food, and environmental sources compared to human samples (Table 1). In our review, one of these results showed a prevalence of E. coli with ESBL genotype of 18.1% in animals and 12.3% in the environment compared to 1.9% in clinical studies. These results were analogous among the majority of South American countries and similar to observations in Tanzania, the Netherlands, and other regions of the world where ESBLs from animal and environmental sources showed between a 10 to 20% higher prevalence than humans.66–68 Therefore, although apparently many of the E. coli resistance mechanisms are acquired in the clinical setting, prevalence in animals and environmental sources predominate and should be further studied.
CTX-M enzymes were the most prevalent, with more than 50% detections in each country and category analyzed in this review (ie, human clinical samples, human healthy carriers, animal, food, and environment; Figures 4–8). Similar results have been reported in the rest of the world. For example, in Africa CTX-M prevalence reaches an 81.5%.38 In Iran, CTX-M enzyme prevalence reached 31.2% followed by TEM with 27.6%.69 It is worth to mention that the prevalence analyzed here only accounted for E. coli, thus it might be an underestimation if including other bacterial species such as K. pneumoniae, which usually harbor TEM or SHV enzymes.70–72
Apart from the best-known enzymes (ie, TEM, SHV, and CTX-M), TOHO, reported for the first time in Japan in 1993,73 was also found in South America among human clinical and environmental studies (Figures 4A and 8A). Thus, in little less than three decades, TOHO enzymes have spread to a completely different region albeit its low prevalence (<0.1%; n=1/3701). Other types of beta-lactamases that are worth highlighting are the OXA (<0.1%; n=17/3701) and AmpC (<0.1%; n=9/3701) enzymes. Both show a different resistance spectrum to the most common resistant enzymes. OXA enzymes can hydrolyze more effectively antibiotics such as carbapenems,74 while AmpC is not inhibited by clavulanic acid.75 The ESBL enzymes PER-2, with similar spectrum to TEM and SHV enzymes,76 were only found in Uruguay with a low prevalence (Figure 4A). However, Celenza et al76 have reported PER-2 isolated from Enterobacteriaceae in Bolivian hospitals, which suggests the existence of PER-2-carrying E. coli in this country. Although detected, CTX-M enzyme variants were poorly reported (Figure 4B). Detection of variants is one of the most important clinical data because each one has differences in their antibiotic response.77–79 It is worth noting that in other regions of the world such as Nigeria, Tunisia, and the Netherlands, CTX-M-15 variant isolated from clinical settings is usually detected with a prevalence ranging from 67.9% to 83.3%.80–82
Considering animal studies, the presence of CTX-M-8 enzyme variant was frequently described in countries such as Brazil, Argentina, and Uruguay (Figure 6B). Because CTX-M-8 was first isolated from Enterobacteriaceae in Brazil,83 the most likely scenario involves it spread across animals from neighboring countries, despite the lack of reports of CTX-M-8 in Chile, Peru, and Ecuador according to our review. For these countries, the variants CTX-M-1 and CTX-M-15 were detected, as has been seen in goat samples from Tunisia, pigs from Portugal, horses from the UK, and processed beef from Germany.84–87
From food studies, the main types of CTX-M enzymes detected included the CTX-M-1 in Ecuador and the CTX-M-2 in Brazil (Figure 7B); results were consistent with reports from Germany88,89 and Algeria90 where the CTX-M-1 variant is the most prevalent. However, as only few studies address the presence of antibiotic resistant enzymes in food-related sources, more research is granted to confirm their role as a mechanism of spread with public health consequences.
The enzymes of type TOHO, OXA, and AmpC from environmental studies were found only in Brazil (Figure 8A). These types of ESBLs had an overall low prevalence. For CTX-M enzyme variants from environmental sources, there was a great variability of detection across South American countries, namely, the enzyme CTX-M-1 in Colombia, CTX-M-3 enzyme in Ecuador/Bolivia, and the CTX-M-2 enzyme in Peru/Brazil (Figure 8B). Lines of research that go beyond the simple detection of ESBLs in the environment should be encouraged as has been done in other regions of the world. For example, in Nigeria, Lebanon, Vietnam, and India, there are studies assessing the impact of antibiotic release on hospital wastewater correlated with patterns on resistance acquisition in E. coli.91–94 Research from the UK, Tanzania, and the Dominican Republic95–97 have revealed the importance of IncF plasmids found in natural waterbodies. Being a conjugative plasmid, IncF is related to the dissemination of blaCTX-M-15 by horizontal gene transfer.98,99 Finally, other exemplary studies show how activated sludge from wastewater treatment plants can be a source of high prevalence of CTX-M as demonstrated in Japan,100 Austria,101 and India.102
The E. coli clones ST131 and ST10 were found across South America (Supplementary Table 3). These results are consistent with a study conducted in Canada in which 96/209 (46%) E. coli strains corresponded to the clonal complex ST131103 and with one developed in the Netherlands where from 112 strains belonging to E. coli, 21% belonged to ST131 and 17% to ST10,82 demonstrating the worldwide high prevalence of the ST131 clone. These clones are known to be a major reservoir of plasmids carrying resistant genes to multiple antibiotics, including blaTEM, blaSHV, and blaCTX-M.104 The influence of international travel on the dissemination of these clones has been studied in countries such as Germany, where the ST131 clone ranges between 19% and 30%, or the Netherlands, where the ST131 clone prevalence reaches a 21.4%.82,105 For the purposes of this argument, it is worth to mention that Woerther et al106 reported the international clone ST10 in healthy carriers from a remote community at French Guiana, which begs the question on how this clone was acquired within an isolated population.
Limitations
Our results are based on a systematic review of published academic literature. Potentially, there is relevant information among the grey literature (eg, thesis) that might complement the results presented here; however, we rely on the quality of peer-review publications to assess the status of E. coli ESBLs in South America with certainty. Similarly, our analysis excluded articles in the category of “reviews” that sometimes present pieces of original research, but we believe that their overall contribution in the presented information may be negligible. Furthermore, while we comprehensibly reviewed each of the 130 manuscripts included in this study, data heterogeneity across different countries and publications is a challenge that was overcome with some heuristic categorizations, which are the basis of any systematic review.
Finally, it is worth noticing that although manuscripts included in the present review were considered of high quality by the individual assessment of their results, we are aware that potentially some of them might be published in journals considered predatory. We believe that science should be judged by their findings instead of the journal in which it was published, and for this review we can trust in the reliability of the studies analyzed.
Conclusions
ESBLs producing E. coli in South America are widely distributed and show a high diversity of enzyme variants. ESBLs in human samples are the most studied, mainly in those linked to hospital environments (inpatient and outpatient). However, ESBLs in food and animal samples are the most prevalent. Countries such as Brazil present more studies on ESBL surveillance involving various sample sources. CTX-M enzymes are the most common and diverse of the types of beta-lactamases found and show a high prevalence across all the studied categories. ST131 and ST10 are the most widespread clones in the studies included in this review.
In order to fully characterize the situation of E. coli ESBLs in South America, a greater contribution from underrepresented countries of the region should be encouraged. These contributions ideally should emphasize the role of sources different from human clinical settings such as animals, environmental, and food matrices, together with detections from human healthy carriers. Concerns related to transmission of resistant mechanisms among human healthy carriers, zoonotic sources, as well as the spread of ESBLs in aquatic ecosystems, reveal the importance of developing studies beyond the human clinical-centered view of health. Furthermore, the importance of animal and environmental health should be explored since ESBL genes in both realms have been detected in South America and the rest of the world.
Efforts to increase the epidemiological surveillance of the region to detect predominant types of ESBLs and the presence of new variants, as well as the distribution of ST clones, should be a priority considering their different roles in antibiotic resistance and their traceability to detect infection sources. At this point, the inclusion of both phenotypic and molecular tests, as well as the development of new detection techniques, should facilitate surveillance efforts to further the understanding of ESBL distribution in South America.
Finally, the information presented in this review can be used as basis for subsequent implementation of monitoring programs, targeting potential critical points of transmission sources. Following the One Health concept, the development of contingency plans in different areas like hospitals, broiler breeding sites, and wastewater treatment plants might contribute to the identification of resistant enzymes and their spreading, which ideally should be controlled if not completely halted; within this objective, a multi-sectoral and multi-disciplinary cooperation will be of utmost importance.
Acknowledgments
Our thanks to the Universidad de las Américas for financing the APCs of this work. Special thanks to Adriana Gallegos-Ordoñez for her help in proofreading of English language and support in the writing of this work.
Supplementary Materials
To a better description of findings of this work, the following supporting information can be downloaded. Supplementary Table 1: Number of samples identified (frequencies) and % prevalence of E. coli, E. coli with ESBL phenotype, and E. coli with ESBL genotype in South America from human clinical samples, human healthy carriers, animal, food, and environmental studies. Data from 130 studies included in systematic review between 1990 and 2021. Supplementary Table 2: Percentages of different beta-lactamase enzymes identified across South American countries and CTX-M variants for human clinical samples, human healthy carriers, animal, food, and environmental studies. Data from 130 studies included in systematic review between 1990 and 2021. Supplementary Table 3: E. coli ST clones found in studies in South America per country and author. Data from 130 studies included in systematic review between 1990 and 2021. Supplementary Table 4: Summary Data Base and list of studies included in the systematic review and general data from 130 studies included between 1990 and 2021. Supplementary Table 5: List of additional references included in the systematic review.107–225
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Micro Rev. 2001;14:933–951. doi:10.1128/CMR.14.4.933-951.2001
2. Behzadi P, García-Perdomo HA, Karpiński TM, Issakhanian L. Metallo-ß-lactamases: a review. Mol Biol Rep. 2020;47(8):6281–6294. doi:10.1007/s11033-020-05651-9
3. Issakhanian L, Behzadi P. Antimicrobial agents and urinary tract infections. Curr Pharm Des. 2019;25(12):1409–1423. doi:10.2174/1381612825999190619130216
4. Hozzari A, Behzadi P, Kerishchi Khiabani P, Sholeh M, Sabokroo N. Clinical cases, drug resistance, and virulence genes profiling in Uropathogenic Escherichia coli. J Appl Genet. 2020;61(2):265–273. doi:10.1007/s13353-020-00542-y
5. Hernández J, Stedt J, Bonnedahl J, et al. Human-associated extended-spectrum β-lactamase in the Antarctic. Appl Environ Microbiol. 2012;78(6):2056–2058. doi:10.1128/AEM.07320-11
6. Bush K. Past and present perspectives on β-lactamases. Antimicrob Agents Chemother. 2018;62(10). doi:10.1128/AAC.01076-18
7. Villegas MV, Kattan JN, Quinteros MG, Casellas JM. Prevalence of extended-spectrum β-lactamases in South America. Clin Micro Infect. 2008;14:154–158. doi:10.1111/j.1469-0691.2007.01869.x
8. Rada AM, Hernández-Gómez C, Restrepo E, Villegas MV. Distribution and molecular characterization of beta-lactamases in Gram-negative bacteria in Colombia, 2001–2016. Biomédica. 2019;39:199–220. doi:10.7705/biomedica.v39i3.4351
9. Paterson D, Bonomo R. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657. doi:10.1128/CMR.18.4.657-686.2005
10. Mella MS, Zemelman MC, Bello TH, Dominguez YM, Gonzalez RG, Zemelman ZR. Microbiological properties, classification and structure-activity relationship of cephalosporins and importance of fourth generation cephalosporins. Rev Chil Infectol. 2001;18(1):7–19.
11. Polanco-Hinostroza F, Loza-Munarriz R. Resistencia antibiótica en infecciones urinarias en niños atendidos en una institución privada, periodo 2007 – 2011 [Antibiotic resistance in urinary tract infections in children cared for in a private institution, period 2007 – 2011]. Rev Medica Hered. 2013;24(3):210–216.
12. Oliveira C, Amador P, Prudêncio C, Tomaz C, Tavares P, Fernandes R. ESBL and AmpC β-lactamases in clinical strains of Escherichia coli from Serra da Estrela, Portugal. Medicina. 2019;55(6):272. doi:10.3390/medicina55060272
13. Behzadi P, Behzadi E. The microbial agents of urinary tract infections at central laboratory of Dr. Shariati Hospital, Tehran, Iran. Turk Klin Tip Bilim. 2008;28(4):445–449.
14. Paterson D, Hujer K, Hujer A, et al. Extended-spectrum β-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type β-lactamases. Antimicrob Agents Chemother. 2003;47(11):3554–3560. doi:10.1128/AAC.47.11.3554-3560.2003
15. Zarfel G, Lipp M, Gürtl E, et al. Troubled water under the bridge: screening of River Mur water reveals dominance of CTX-M harboringEscherichia coli and for the first time an environmental VIM-1 producer in Austria. Sci Total Environ. 2017;593-594:399–405. doi:10.1016/j.scitotenv.2017.03.138
16. Gomi R, Matsuda T, Matsumura Y, et al. Whole-genome analysis of antimicrobial-resistant and extraintestinal pathogenic Escherichia coli in river water. Appl Environ Microbiol. 2017;83(5). doi:10.1128/AEM.02703-16
17. Carattoli A. Animal reservoirs for extended spectrum beta-lactamase producers. Clin Microbiol Infect. 2008;14:117–123. doi:10.1111/j.1469-0691.2007.01851.x
18. McEwen SA, Collignon PJ. Antimicrobial resistance: a one health perspective. Microbiol Spectr. 2018;6(2). doi:10.1128/microbiolspec.ARBA-0009-2017
19. Rousham EK, Unicomb L, Islam MA. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: integrating behavioural, epidemiological and One Health approaches. Proc Biol Sci. 2018;285(1876). doi:10.1098/rspb.2018.0332
20. Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One. 2017;12(12):e0189621. doi:10.1371/journal.pone.0189621
21. Aworh MK, Kwaga J, Okolocha E, et al. Extended-spectrum ß-lactamase-producing Escherichia coli among humans, chickens and poultry environments in Abuja, Nigeria. One Health Outlook. 2020;2(8). doi:10.1186/s42522-020-00014-7
22. Alonso CA, Zarazaga M, Sallem RB, Jouini A, Slama KB, Torres C. Antibiotic resistance in Escherichia coli in husbandry animals: the African perspective. Lett Appl Microbiol. 2017;64(5):318–334. doi:10.1111/lam.12724
23. Destoumieux-Garzón D, Mavingui P, Boetsch G, et al. The one health concept: 10 years old and a long road ahead. Front Vet Sci. 2018;5:14. doi:10.3389/fvets.2018.00014
24. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(89). doi:10.1186/s13643-021-01626-4
25. González G, Park J, Huamaní C, Ramos JM. Dominance and leadership in research activities: collaboration between countries of differing human development is reflected through authorship order and designation as corresponding authors in scientific publications. PLoS One. 2017;12(8):e0182513. doi:10.1371/journal.pone.0182513
26. Glänzel W, Leta J, Thijs B. Science in Brazil. Part 1: a macro-level comparative study. Scientometrics. 2006;67:67–86. doi:10.1007/s11192-006-0055-7
27. Carvajal-Tapia AE, Carvajal-Rodríguez E. Status of scientific production in medicine in South America. 1996-2016. Rev Fac Med. 2018;66(4):595–600. doi:10.15446/revfacmed.v66n4.67215
28. Carvajal-Tapia AE, Carvajal-Rodríguez E. Producción científica en ciencias de la salud en los países de América Latina, 2006-2015: análisis a partir de SciELO [Scientific production in health sciences in Latin American countries, 2006–2015: analysis from SciELO]. Revista Interamericana de Bibliotecología. 2019;42(1):15–21. Spanish.
29. Ciocca D, Delgado G. The reality of scientific research in Latin America; an insider’s perspective. Cell Stress Chaperones. 2017;22:847–852. doi:10.1007/s12192-017-0815-8
30. Ashley E, Recht J, Chua A, et al. An inventory of supranational antimicrobial resistance surveillance networks involving low- and middle-income countries since 2000. J Antimicrob Chemother. 2018;73(7):1737–1749. doi:10.1093/jac/dky026
31. Pfaller M, Segreti J. Overview of the epidemiological profile and laboratory detection of extended-spectrum β-lactamases. Clin Infect Dis. 2006;42(Suppl 4):S153–S163. doi:10.1086/500662
32. Thomas S. Antimicrobial Resistance: Global Challenges and Future Interventions.
33. Pitout J, Hossain A, Hanson N. Phenotypic and molecular detection of CTX-M-beta-lactamases produced by Escherichia coli and Klebsiella spp. J Clin Microbiol. 2004;42(12):5715–5721. doi:10.1128/JCM.42.12.5715-5721.2004
34. Calvo J, Cantón R, Cuenca FF, Mirelis B, Navarro F. Procedimientos En Microbiología Clínica [Procedures in Clinical Microbiology].
35. Jia F, Li X, Zhang C, Tang X. The expanded development and application of CRISPR system for sensitive nucleotide detection. Protein Cell. 2020;11:624–629. doi:10.1007/s13238-020-00708-8
36. Müller V, Rajer F, Frykholm K, et al. Direct identification of antibiotic resistance genes on single plasmid molecules using CRISPR/Cas9 in combination with optical DNA mapping. Sci Rep. 2016;6(1). doi:10.1038/srep37938
37. Guzmán M, Labarca JA, Villegas MV, Gotuzzo E; Latin America Working Group on Bacterial Resistance. Extended spectrum β-lactamase producers among nosocomial Enterobacteriaceae in Latin America. Braz J Infect Dis. 2014;18(4):421–433. doi:10.1016/j.bjid.2013.10.005
38. Onduru G, Mkakosya R, Aboud S, Rumisha S. Genetic determinants of resistance among ESBL-producing Enterobacteriaceae in healthy carriers and hospital settings in East, Central, and Southern Africa: a systematic review and meta-analysis of prevalence. Can J Infect Dis Med Microbiol. 2021;2021:1–9. doi:10.1155/2021/5153237
39. Storberg V. ESBL-producing Enterobacteriaceae in Africa – a non-systematic literature review of research published 2008–2012. Infect Ecol Epidemiol. 2014;4(1):20342.
40. Ebrahimi F, Mózes J, Mészáros J, et al. Asymptomatic faecal carriage of ESBL producing Enterobacteriaceae in Hungarian healthy individuals and in long-term care applicants: a shift towards CTX-M producers in the healthy carriers. Infect Dis. 2016;48(7):557–559. doi:10.3109/23744235.2016.1155734
41. Borges CA, Tarlton NJ, Riley LW. Escherichia coli from commercial broiler and backyard chickens share sequence types, antimicrobial resistance profiles, and resistance genes with human extraintestinal pathogenic Escherichia coli. Foodborne Pathog Dis. 2019;16(12):813–822. doi:10.1089/fpd.2019.2680
42. Hernandez J, Johansson A, Stedt J, et al. Characterization and comparison of extended-spectrum β-lactamase (ESBL) resistance genotypes and population structure of Escherichia coli isolated from Franklin’s gulls (Leucophaeus pipixcan) and humans in Chile. PLoS One. 2013;8(9):e76150. doi:10.1371/journal.pone.0076150
43. Murray M, Salvatierra G, Dávila A, et al. Market Chickens as a Source of Antibiotic-Resistant Escherichia coli in a Peri-Urban Healthy carriers in Lima, Peru. Front Microbiol. 2021;12:635871. doi:10.3389/fmicb.2021.635871
44. Dias-Gonçalves V, Meirelles F, Cataldo M, et al. Detection of multidrug-resistant Enterobacteriaceae isolated from river waters flowing to the Guanabara Bay and from clinical samples of hospitals in Rio de Janeiro, Brazil. Biomedica. 2019;39(Suppl 1):S135–S149. doi:10.7705/biomedica.v39i0.4391
45. Pehrsson E, Tsukayama P, Patel S, et al. Interconnected microbiomes and resistomes in low-income human habitats. Nature. 2016;533:212–216. doi:10.1038/nature17672
46. Koga V, Maluta R, da Silveira W, et al. Characterization of CMY-2-type beta-lactamase-producing Escherichia coli isolated from chicken carcasses and human infection in a city of South Brazil. BMC Microbiol. 2019;19:174. doi:10.1186/s12866-019-1550-3
47. Brisola MC, Crecencio RB, Bitner DS, et al. Escherichia coli used as a biomarker of antimicrobial resistance in pig farms of Southern Brazil. Sci Total Environ. 2019;647:362–368. doi:10.1016/j.scitotenv.2018.07.438
48. Ortega D, de Janon S, Villavicencio F, et al. Broiler farms and carcasses are an important reservoir of multi-drug resistant Escherichia coli in Ecuador. Front Vet Sci. 2020;7:547843. doi:10.3389/fvets.2020.547843
49. Gazal L, Medeiros L, Dibo M, et al. Detection of ESBL/AmpC-producing and fosfomycin-resistant Escherichia coli from different sources in poultry production in Southern Brazil. Front Microbiol. 2021;11:604544. doi:10.3389/fmicb.2020.604544
50. O’Neal L, Alvarez D, Mendizábal R, Ramay B, Graham J. Healthy carriers-acquired antimicrobial resistant Enterobacteriaceae in Central America: a one health systematic review. Int J Environ Res Public Health. 2020;17(20):7622. doi:10.3390/ijerph17207622
51. Escher N, Muhummed A, Hattendorf J, Vonaesch P, Zinsstag J. Systematic review and meta-analysis of integrated studies on antimicrobial resistance genes in Africa-a one health perspective. Trop Med Int Health. 2021;26(10):1153–1163. doi:10.1111/tmi.13642
52. World Health Organization [WHO]. Global action plan on antimicrobial resistance; 2016. Available from: http://www.emro.who.int/health-topics/drug-resistance/global-action-plan.html.
53. Bangdiwala S, De Paula C, Ramiro L, Muñoz S. Coordinación de estudios multicéntricos internacionales: estructura administrativa y reglamentación [Coordination of international multicenter studies: administrative structure and regulations]. Salud Pública de México. 2003;45(1):58–66. Spanish. doi:10.1590/S0036-36342003000100008
54. Kumar Y. Antimicrobial Resistance: A Global Threat. London: IntechOpen; 2019.
55. Wang Y, Hu Y, Cao J, et al. Antibiotic resistance gene reservoir in live poultry markets. J Infect. 2019;78(6):445–453. doi:10.1016/j.jinf.2019.03.012
56. Bacanlı M, Başaran N. Importance of antibiotic residues in animal food. Food Chem Toxicol. 2019;125:462–466. doi:10.1016/j.fct.2019.01.033
57. Silva GG, Campana EH, Vasconcelos PC, et al. Ocorrência de Escherichia coli produtora de KPC em psitaciformes resgatados do tráfico na Paraíba, Brasil [Occurrence of KPC-Producing Escherichia coli in Psittaciformes Rescued from Trafficking in Paraíba, Brazil]. Int J Environ Res Public Health. 2021;18(1):95. Portuguese. doi:10.3390/ijerph18010095
58. Islam MS, Nayeem MMH, Sobur MA, et al. Virulence determinants and multidrug resistance of Escherichia coli isolated from migratory birds. Antibiot. 2021;10(2):190. doi:10.3390/antibiotics10020190
59. Wu J, Huang Y, Rao D, Zhang Y, Yang K. Evidence for environmental dissemination of antibiotic resistance mediated by wild birds. Front Microbiol. 2018;9:745. doi:10.3389/fmicb.2018.00745
60. Pomba C, Rantala M, Greko C, et al. Public health risk of antimicrobial resistance transfer from companion animals. J Antimicrob Chemother. 2017;72(4):957–968. doi:10.1093/jac/dkw481
61. Melo LC, Oresco C, Leigue L, et al. Prevalence and molecular features of ESBL/pAmpC-producing Enterobacteriaceae in healthy and diseased companion animals in Brazil. Vet Microbiol. 2018;221:59–66. doi:10.1016/j.vetmic.2018.05.017
62. Riwu KHP, Effendi MH, Rantam FA. A review of extended spectrum β-lactamase (ESBL) producing Klebsiella pneumoniae and multidrug resistant (MDR) on companion animals. Syst Rev Pharm. 2020;11(7):8.
63. Poo-Muñoz DA, Elizondo-Patrone C, Escobar LE, et al. Fleas and ticks in carnivores from a domestic-wildlife interface: implications for public health and wildlife. J Med Entomol. 2016;53(6):1433–1443. doi:10.1093/jme/tjw124
64. Sellera FP, Fernandes MR, Moura Q, Carvalho MPN, Lincopan N. Extended-spectrum-β-lactamase (CTX-M)-producing Escherichia coli in wild fishes from a polluted area in the Atlantic Coast of South America. Mar Pollut Bull. 2018;135:183–186. doi:10.1016/j.marpolbul.2018.07.012
65. Almeida MVA, Cangussú ÍM, Carvalho ALS, Brito ILP, Costa RA. Drug resistance, AmpC-β-lactamase and extended-spectrum β-lactamase-producing Enterobacteriaceae isolated from fish and shrimp. Rev Inst Med Trop Sao Paulo. 2017;59. doi:10.1590/s1678-9946201759070
66. Seni J, Moremi N, Matee M, et al. Preliminary insights into the occurrence of similar clones of extended-spectrum beta-lactamase-producing bacteria in humans, animals and the environment in Tanzania: a systematic review and meta-analysis between 2005 and 2016. Zoonoses Public Health. 2018;65(1):1–10. doi:10.1111/zph.12387
67. Dorado A, Smid JH, van Pelt W, et al. Molecular relatedness of ESBL/AmpC-producing Escherichia coli from humans, animals, food and the environment: a pooled analysis. J Antimicrob Chemother. 2018;73(2):339–347. doi:10.1093/jac/dkx397
68. Pormohammad A, Nasiri MJ, Azimi T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: a systematic review and meta-analysis. Infect Drug Resist. 2019;12:1181. doi:10.2147/IDR.S201324
69. Jabalameli L, Beigverdi R, Ranjbar HH, Pouriran R, Jabalameli F, Emaneini M. Phenotypic and genotypic prevalence of extended-spectrum β-lactamase-producing Escherichia coli: a systematic review and meta-analysis in Iran. Microb Drug Resist. 2021;27(1):73–86. doi:10.1089/mdr.2019.0396
70. Malik T, Naim A, Saeed A. Molecular detection of TEM, SHV and CTX-M genes among gram-negative Klebsiella isolates. Curr Drug Deliv. 2018;15(3):417–423. doi:10.2174/1567201815666180101160108
71. Mondal AH, Siddiqui MT, Sultan I, Haq QMR. Prevalence and diversity of blaTEM, blaSHV and blaCTX-M variants among multidrug resistant Klebsiella spp. from an urban riverine environment in India. Int J Environ Health Res. 2018;29(2):117–129. doi:10.1080/09603123.2018.1515425
72. Ramos DA, Pulgarín JAH, Gómez GAM, et al. Geographic mapping of Enterobacteriaceae with extended-spectrum β-lactamase (ESBL) phenotype in Pereira, Colombia. BMC Infect Dis. 2020;20(1):1–9.
73. Tzouvelekis LS, Tzelepi E, Tassios PT, Legakis NJ. CTX-M-type β-lactamases: an emerging group of extended-spectrum enzymes. Int J Antimicrob Agents. 2000;14(2):137–142. doi:10.1016/S0924-8579(99)00165-X
74. Pitout JDD, Peirano G, Kock MM, Strydom KA, Matsumura Y. The global ascendency of OXA-48-type carbapenemases. Clin Microbiol Rev. 2020;33(1):e00102–e00119.
75. Martinez D. Betalactamasas tipo AmpC: generalidades y métodos para detección fenotípica [AmpC-type beta-lactamases: overview and methods for phenotypic detection]. Rev Soc Ven. 2009;29(2):78–83. Spanish.
76. Celenza G, Pellegrini C, Caccamo M, Segatore B, Amicosante G, Perilli M. Spread of blaCTX-M-type and blaPER-2 β-lactamase genes in clinical isolates from Bolivian hospitals. J Antimicrob Chemother. 2006;57(5):975–978. doi:10.1093/jac/dkl055
77. D’Andrea MM, Arena F, Pallecchi L, Rossolini GM. CTX-M-type β-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol. 2013;303(6–7):305–317. doi:10.1016/j.ijmm.2013.02.008
78. He D, Chiou J, Zeng Z, Chan EWC, Liu JH, Chen S. Comparative characterization of CTX-M-64 and CTX-M-14 provides insights into the structure and catalytic activity of the CTX-M class of enzymes. Antimicrob Agents Chemother. 2016;60(10):6084–6090. doi:10.1128/AAC.00917-16
79. Lubna M, Shamsi K, Abid A, Khan A. Significant role of Asn-247 and Arg-64 residues in close proximity of the active site in maintaining the catalytic function of CTX-M-15 type β-lactamase. RSC Adv. 2019;9(10):5325–5337. doi:10.1039/C8RA10313E
80. Ogbolu DO, Alli OAT, Webber MA, Oluremi AS, Oloyede OM. CTX-M-15 is established in most multidrug-resistant uropathogenic Enterobacteriaceae and Pseudomonaceae from hospitals in Nigeria. Eur J Microbiol Immunol. 2018;8(1):20–24. doi:10.1556/1886.2017.00012
81. Hassen B, Abbassi MS, Benlabidi S, et al. Genetic characterization of ESBL-producing Escherichia coli and Klebsiella pneumoniae isolated from wastewater and river water in Tunisia: predominance of CTX-M-15 and high genetic diversity. Environ Sci Pollut Res. 2020;27(35):44368–44377. doi:10.1007/s11356-020-10326-w
82. Louka C, Ravensbergen SJ, Ott A, et al. Predominance of CTX-M-15-producing ST131 strains among ESBL-producing Escherichia coli isolated from asylum seekers in the Netherlands. J Antimicrob Chemother. 2021;76(1):70–76. doi:10.1093/jac/dkaa395
83. Bonnet R, Sampaio JLM, Labia R, et al. A novel CTX-M β-lactamase (CTX-M-8) in cefotaxime-resistant Enterobacteriaceae isolated in Brazil. Antimicrob Agents Chemother. 2000;44(7):1936–1942. doi:10.1128/AAC.44.7.1936-1942.2000
84. Hassen B, Saloua B, Abbassi MS, et al. mcr-1 encoding colistin resistance in CTX-M-1/CTX-M-15- producing Escherichia coli isolates of bovine and caprine origins in Tunisia. First report of CTX-M-15-ST394/D E. coli from goats. Comp Immunol Microbiol Infect Dis. 2019;67:101366. doi:10.1016/j.cimid.2019.101366
85. Fournier C, Aires S, Nordmann P, Poirel L. Occurrence of CTX-M-15- and MCR-1-producing Enterobacterales in pigs in Portugal: evidence of direct links with antibiotic selective pressure. Int J Antimicrob Agents. 2020;55(2):105802. doi:10.1016/j.ijantimicag.2019.09.006
86. Isgren CM, Edwards T, Pinchbeck GL, et al. Emergence of carriage of CTX-M-15 in faecal Escherichia coli in horses at an equine hospital in the UK; increasing prevalence over a decade (2008–2017). BMC Vet Res. 2019;15(1):1–8. doi:10.1186/s12917-019-2011-9
87. Irrgang A, Falgenhauer L, Fischer J, et al. CTX-M-15-producing E. coli isolates from food products in Germany are mainly associated with an IncF-type plasmid and belong to two predominant clonal E. coli lineages. Front Microbiol. 2017;8:2318.
88. Kaesbohrer A, Bakran-Lebl K, Irrgang A, et al. Diversity in prevalence and characteristics of ESBL/pAmpC producing E. coli in food in Germany. Vet Microbiol. 2019;233:52–60. doi:10.1016/j.vetmic.2019.03.025
89. Schill F, Abdulmawjood A, Klein G, Reich F. Prevalence and characterization of extended-spectrum β-lactamase (ESBL) and AmpC β-lactamase producing Enterobacteriaceae in fresh pork meat at processing level in Germany. Int J Food Microbiol. 2017;257:58–66. doi:10.1016/j.ijfoodmicro.2017.06.010
90. Yaici L, Haenni M, Métayer V, et al. Spread of ESBL/AmpC-producing Escherichia coli and Klebsiella pneumoniae in the healthy carriers through ready-to-eat sandwiches in Algeria. Int J Food Microbiol. 2017;245:66–72. doi:10.1016/j.ijfoodmicro.2017.01.011
91. Adelowo OO, Caucci S, Banjo OA, et al. Extended Spectrum Beta-Lactamase (ESBL)-producing bacteria isolated from hospital wastewaters, rivers and aquaculture sources in Nigeria. Environ Sci Pollut Res. 2017;25(3):2744–2755. doi:10.1007/s11356-017-0686-7
92. Daoud Z, Farah J, Sokhn S, et al. Multidrug-resistant Enterobacteriaceae in Lebanese hospital wastewater: implication in the one health concept. Microb Drug Resist. 2018;24(2):166–174. doi:10.1089/mdr.2017.0090
93. Lien LTQ, Lan PT, Chuc NTK, et al. Antibiotic resistance and antibiotic resistance genes in Escherichia coli isolates from hospital wastewater in Vietnam. Int J Environ Res Public Health. 2017;14(7):699. doi:10.3390/ijerph14070699
94. Lamba M, Graham DW, Ahammad SZ. Hospital wastewater releases of carbapenem-resistance pathogens and genes in Urban India. Environ Sci Technol. 2017;51(23):13906–13912. doi:10.1021/acs.est.7b03380
95. Dinatale A. Prevalence of Plasmid Genetic Elements Among ESBL-Producing E. coli Isolated from a UK River and the Effects of Waste Water Effluent Release [dissertation]. UK: University of Lincoln; 2017.
96. Baniga Z, Hounmanou YMG, Kudirkiene E, Kusiluka LJM, Mdegela RH, Dalsgaard A. Genome-based analysis of extended-spectrum β-lactamase-producing Escherichia coli in the aquatic environment and Nile Perch (Lates niloticus) of Lake Victoria, Tanzania. Front Microbiol. 2020;11:108. doi:10.3389/fmicb.2020.00108
97. Calderón V, Bonnelly R, Del Rosario C, et al. Distribution of beta-lactamase producing gram-negative bacterial isolates in isabela river of Santo Domingo, Dominican Republic. Front Microbiol. 2021;11. doi:10.3389/fmicb.2020.519169
98. Mattila S, Ruotsalainen P, Ojala V, Tuononen T, Hiltunen T, Jalasvuori M. Conjugative ESBL plasmids differ in their potential to rescue susceptible bacteria via horizontal gene transfer in lethal antibiotic concentrations. J Antibiot. 2017;70(6):805–808. doi:10.1038/ja.2017.41
99. Cortés G, Kim J, Mira P, Mota L, Barlow M, Camps M. Conjugative transfer of ESBL and aminoglycoside antibiotic resistance genes in Extraintestinal E. coli: implications for multidrug resistance evolution: in proceedings of the 1st International Electronic Conference on Antibiotics—the equal power of antibiotics and antimicrobial resistance. Sci Forum. 2021;2021:9747.
100. Urano N, Okai M, Tashiro Y, et al. Behavior of antibiotic-resistant fecal coliforms in the stream of a sewage treatment plant in Tokyo. Adv Microbiol. 2020;10(7):318–330. doi:10.4236/aim.2020.107023
101. Galler H, Feierl G, Petternel C, et al. Multiresistant bacteria isolated from activated sludge in Austria. Int J Environ Res Public Health. 2018;15(3):479. doi:10.3390/ijerph15030479
102. Ali A, Sultan I, Mondal AH, Siddiqui MT, Gogry FA, Haq QMR. Lentic and effluent water of Delhi-NCR: a reservoir of multidrug-resistant bacteria harbouring blaCTX-M, blaTEM and blaSHV type ESBL genes. J Water Health. 2021;19(4):592–603. doi:10.2166/wh.2021.085
103. Peirano G, Richardson D, Nigrin J, et al. High prevalence of ST131 isolates producing CTX-M-15 and CTX-M-14 among extended-spectrum-β-lactamase-producing Escherichia coli isolates from Canada. Antimicrob Agents Chemother. 2010;54(3):1327–1330. doi:10.1128/AAC.01338-09
104. Jouini A, Klibi A, Elarbi I, et al. First detection of human ST131-CTX-M-15-O25-B2 clone and high-risk clonal lineages of ESBL/pAmpC-producing E. coli isolates from diarrheic poultry in Tunisia. Antibiot. 2021;10(6):670. doi:10.3390/antibiotics10060670
105. Ehlkes L, Pfeifer Y, Werner G, et al. No evidence of carbapenemase-producing Enterobacteriaceae in stool samples of 1544 asylum seekers arriving in Rhineland-Palatinate, Germany, April 2016 to March 2017. Eurosurveillance. 2019;24(8):1800030. doi:10.2807/1560-7917.ES.2019.24.8.1800030
106. Woerther PL, Angebault C, Jacquier H, et al. Characterization of fecal extended-spectrum-β-lactamase-producing Escherichia coli in a remote healthy carriers during a long time period. Antimicrob Agents Chemother. 2013;57(10):5060–5066. doi:10.1128/AAC.00848-13
107. Daga AP, Koga VL, Soncini JGM, et al. Escherichia coli bloodstream infections in patients at a university hospital: virulence factors and clinical characteristics. Front Cell Infect Microbiol. 2019;9:191. doi:10.3389/fcimb.2019.00191
108. Palmeira JD, Haenni M, Metayer V, Madec JY, Ferreira HMN. Epidemic spread of IncI1/pST113 plasmid carrying the Extended-Spectrum Beta-Lactamase (ESBL) blaCTX-M-8 gene in Escherichia coli of Brazilian cattle. Vet Microbiol. 2020;243:108629. doi:10.1016/j.vetmic.2020.108629
109. Ortega-Paredes D, Haro M, Leoro-Garzón P, et al. Multidrug-resistant Escherichia coli isolated from canine faeces in a public park in Quito, Ecuador. J Glob Antimicrob Resist. 2019;18:263–268. doi:10.1016/j.jgar.2019.04.002
110. Faccone D, Moredo FA, Giacoboni GI, et al. Multidrug-resistant Escherichia coli harbouring mcr-1 and blaCTX-M genes isolated from swine in Argentina. J Glob Antimicrob Resist. 2019;18:160–162. doi:10.1016/j.jgar.2019.03.011
111. Vinueza-Burgos C, Ortega-Paredes D, Narváez C, De Zutter L, Zurita J. Characterization of cefotaxime resistant Escherichia coli isolated from broiler farms in Ecuador. PLoS One. 2019;14(4):e0207567. doi:10.1371/journal.pone.0207567
112. Cunha MPV, Oliveira MCV, Oliveira MGX, Menão MC, Knöbl T. CTX-M-producing Escherichia coli Isolated from urban pigeons (Columba livia domestica) in Brazil. J Infect Dev Ctries. 2019;13:1052–1056. doi:10.3855/jidc.11441
113. Aristizábal-Hoyos AM, Rodríguez EA, Arias L, Jiménez JN. High clonal diversity of multidrug-resistant and extended spectrum beta-lactamase-producing Escherichia coli in a wastewater treatment plant. J Environ Manage. 2019;245:37–47. doi:10.1016/j.jenvman.2019.05.073
114. Rumi MV, Mas J, Elena A, et al. Co-occurrence of clinically relevant β-lactamases and MCR-1 encoding genes in Escherichia coli from companion animals in Argentina. Vet Microbiol. 2019;230:228–234. doi:10.1016/j.vetmic.2019.02.006
115. Hoepers PG, Silva PL, Rossi DA, et al. The association between extended spectrum beta-lactamase (ESBL) and ampicillin C (AmpC) beta-lactamase genes with multidrug resistance in Escherichia coli isolates recovered from turkeys in Brazil. Br Poult Sci. 2018;59(4):396–401. doi:10.1080/00071668.2018.1468070
116. Chavez MV, Caicedo LD, Castillo JE. Occurrence of β-lactamase-producing gram-negative bacterial isolates in water sources in Cali City, Colombia. Int J Microbiol. 2019;2019:1375060. doi:10.1155/2019/1375060
117. De carvalho MPN, Fernandes MR, Sellera FP, et al. International clones of extended-spectrum β-lactamase (CTX-M)-producing Escherichia coli in peri-urban wild animals, Brazil. Transbound Emerg Dis. 2020;67(5):1804–1815.
118. Benavides JA, Shiva C, Virhuez M, et al. Extended-spectrum beta-lactamase-producing Escherichia coli in common vampire bats Desmodus rotundus and livestock in Peru. Zoonoses Public Health. 2018;65(4):454–458. doi:10.1111/zph.12456
119. Guzmán M, Salazar E, Cordero V, et al. Multirresistencia a medicamentos y factores de riesgo asociados con infecciones urinarias por Escherichia coli adquiridas en la comunidad, Venezuela [Multidrug resistance and risk factors associated with healthy carriers-acquired urinary tract infections caused by Escherichia coli in Venezuela]. Biomedica. 2019;39(Suppl1):S96–S107. Spanish.
120. Zurita J, Solís MB, Ortega-Paredes D, et al. High prevalence of B2-ST131 clonal group among extended-spectrum β-lactamase-producing Escherichia coli isolated from bloodstream infections in Quito, Ecuador. J Glob Antimicrob Resist. 2019;19:216–221. doi:10.1016/j.jgar.2019.04.019
121. Sfaciotte RAP, Parussolo L, Melo FD, et al. Identification and characterization of multidrug-resistant extended-spectrum beta-lactamase-producing bacteria from healthy and diseased dogs and cats admitted to a veterinary hospital in Brazil. Microb Drug Resist. 2021;27(6):855–864. doi:10.1089/mdr.2020.0043
122. Crecencio RB, Brisola MC, Bitner D, et al. Antimicrobial susceptibility, biofilm formation and genetic profiles of Escherichia coli isolated from retail chicken meat. Infect Genet Evol. 2020;84:104355. doi:10.1016/j.meegid.2020.104355
123. Fernandes MR, Sellera FP, Moura Q, Esposito F, Sabino CP, Lincopan N. Identification and genomic features of halotolerant extended-spectrum-β-lactamase (CTX-M)-producing Escherichia coli in urban-impacted coastal waters, Southeast Brazil. Mar Pollut Bull. 2020;150:110689. doi:10.1016/j.marpolbul.2019.110689
124. Freitas DY, Araújo S, Folador ARC, et al. Extended spectrum beta-lactamase-producing gram-negative bacteria recovered from an Amazonian lake near the City of Belém, Brazil. Front Microbiol. 2019;10:364. doi:10.3389/fmicb.2019.00364
125. Fuentes-Castillo D, Farfán-López M, Esposito F, et al. Wild owls colonized by international clones of extended-spectrum β-lactamase (CTX-M)-producing Escherichia coli and Salmonella infantis in the Southern Cone of America. Sci Total Environ. 2019;674:554–562. doi:10.1016/j.scitotenv.2019.04.149
126. Larson A, Hartinger SM, Riveros M, et al. Antibiotic-resistant Escherichia coli in drinking water samples from rural Andean households in Cajamarca, Peru. Am J Trop Med Hyg. 2019;100(6):1363–1368. doi:10.4269/ajtmh.18-0776
127. Batalha de Jesus AA, Freitas AAR, de Souza JC, et al. High-level multidrug-resistant Escherichia coli isolates from wild birds in a large urban environment. Microb Drug Resist. 2019;25(2):167–172. doi:10.1089/mdr.2018.0180
128. Lentz SAM, Adam FC, Rivas PM, et al. High levels of resistance to cephalosporins associated with the presence of extended-spectrum and AmpC β-lactamases in Escherichia coli from broilers in Southern Brazil. Microb Drug Resist. 2020;26(5):531–535. doi:10.1089/mdr.2019.0050
129. Zurita J, Yánez F, Sevillano G, Ortega-Paredes D, Paz Y, Miño A. Ready-to-eat street food: a potential source for dissemination of multidrug-resistant Escherichia coli epidemic clones in Quito, Ecuador. Lett Appl Microbiol. 2020;70(3):203–209. doi:10.1111/lam.13263
130. Miranda J, Pinto J, Faustino M, Sánchez-Jacinto B, Ramirez F. Resistencia antimicrobiana de uropatógenos en adultos mayores de una clínica privada de Lima, Perú [Antimicrobial resistance of uropathogens in older adults in a private clinic in Lima, Peru]. Rev Peru Med Exp Salud Publica. 2019;36(1):87–92. Spanish. doi:10.17843/rpmesp.2019.361.3765
131. Ortega-Paredes D, Barba P, Mena-López S, Espinel N, Zurita J. Escherichia coli hyperepidemic clone ST410-A harboring blaCTX-M-15 isolated from fresh vegetables in a municipal market in Quito-Ecuador. Int J Food Microbiol. 2018;280:41–45. doi:10.1016/j.ijfoodmicro.2018.04.037
132. Pavez M, Troncoso C, Osses I, et al. High prevalence of CTX-M-1 group in ESBL-producing Enterobacteriaceae infection in intensive care units in southern Chile. Braz J Infect Dis. 2019;23(2):102–110. doi:10.1016/j.bjid.2019.03.002
133. Umpiérrez A, Bado I, Oliver M, et al. Zoonotic potential and antibiotic resistance of Escherichia coli in neonatal calves in Uruguay. Microbes Environ. 2017;32(3):275–282. doi:10.1264/jsme2.ME17046
134. Coppola N, Freire B, Umpiérrez A, et al. Transferable resistance to highest priority critically important antibiotics for human health in Escherichia coli strains obtained from livestock feces in Uruguay. Front Vet Sci. 2020;7:588919. doi:10.3389/fvets.2020.588919
135. Cyoia PS, Koga VL, Nishio EK, et al. Distribution of ExPEC virulence factors, blaCTX-M, fosA3, and mcr-1 in Escherichia coli isolated from commercialized chicken carcasses. Front Microbiol. 2019;9:3254. doi:10.3389/fmicb.2018.03254
136. Nogueira S, Conte D, Maia FV, Dalla-Costa LM. Distribution of extended-spectrum β-lactamase types in a Brazilian tertiary hospital. Rev Soc Bras Med Trop. 2015;48(2):162–169. doi:10.1590/0037-8682-0009-2015
137. Bartley PS, Domitrovic TN, Moretto VT, et al. Antibiotic resistance in Enterobacteriaceae from surface waters in Urban Brazil highlights the risks of poor sanitation. Am J Trop Med Hyg. 2019;100(6):1369–1377. doi:10.4269/ajtmh.18-0726
138. Araque M, Labrador I. Prevalence of fecal carriage of CTX-M-15 beta-lactamase-producing Escherichia coli in healthy children from a Rural Andean Village in Venezuela. Osong Public Health Res Perspect. 2018;9(1):9–15. doi:10.24171/j.phrp.2018.9.1.03
139. Pereira JL, Volcão LM, Klafke GB, et al. Antimicrobial resistance and molecular characterization of extended-spectrum β-lactamases of Escherichia coli and Klebsiella spp. isolates from urinary tract infections in Southern Brazil. Microb Drug Resist. 2019;25(2):173–181. doi:10.1089/mdr.2018.0046
140. Palma N, Pons MJ, Gomes C, et al. Resistance to quinolones, cephalosporins and macrolides in Escherichia coli causing bacteraemia in Peruvian children. J Glob Antimicrob Resist. 2017;11:28–33. doi:10.1016/j.jgar.2017.06.011
141. Botelho LAB, Kraychete GB, Rocha PB, et al. CTX-M- and pAmpC-encoding genes are associated with similar mobile genetic elements in Escherichia coli isolated from different brands of Brazilian chicken meat. Microb Drug Resist. 2020;26(1):14–20. doi:10.1089/mdr.2019.0043
142. Faccone D, Rapoport M, Albornoz E, et al. Plasmidic resistance to colistin mediated by mcr-1 gene in Escherichia coli clinical isolates in Argentina: a retrospective study, 2012–2018. Rev Panam Salud Publica. 2020;44:e55. doi:10.26633/RPSP.2020.55
143. Beirão EM, Rodrigues SDS, Andrade TK, et al. Activity of ceftolozane-tazobactam and comparators against gram-negative bacilli: results from the study for monitoring antimicrobial resistance trends (SMART - Brazil; 2016–2017). Braz J Infect Dis. 2020;24(4):310–321. doi:10.1016/j.bjid.2020.05.010
144. Villar HE, Aubert V, Baserni MN, Jugo MB. Maternal carriage of extended-spectrum beta-lactamase-producing Escherichia coli isolates in Argentina. J Chemother. 2013;25(6):324–327. doi:10.1179/1973947813Y.0000000081
145. Colquechagua-Aliaga F, Sevilla-Andrade C, Gonzales-Escalante E. Enterobacterias productoras de betalactamasas de espectro extendido en muestras fecales en el Instituto Nacional de Salud del Niño, Perú [Extended-spectrum beta-lactamase (esbl)-producing Enterobacteriaceae in fecal samples at the National Institute of Child Health, Peru]. Rev Peru Med Exp Salud Publica. 2015;32(1):26–32. Spanish.
146. Chiluisa-Guacho C, Escobar-Perez J, Dutra-Asensi M. First detection of the CTXM-15 producing Escherichia coli O25-ST131 pandemic clone in Ecuador. Pathogens. 2018;7(2):42. doi:10.3390/pathogens7020042
147. Palma N, Gomes C, Riveros M, et al. Virulence factors profiles and ESBL production in Escherichia coli causing bacteremia in Peruvian children. Diagn Microbiol Infect Dis. 2016;86(1):70–75. doi:10.1016/j.diagmicrobio.2016.05.017
148. Sarmiento AF, Mejia MN, Rozas AB, Giraldo CA, Málaga G. Frecuencia y factores de riesgo para bacteriemia por enterobacterias productoras de betalactamasa de espectro extendido en pacientes de un hospital público de Lima, Perú [Frequency and risk factors for bacteremia caused by extended spectrum beta-lactamase-producing Enterobacteriaceae in patients of a public hospital in Lima, Peru]. Rev Peru Med Exp Salud Publica. 2018;35(1):62–67. Spanish. doi:10.17843/rpmesp.2018.351.3601
149. Blanco VM, Maya JJ, Correa A, et al. Prevalencia y factores de riesgo para infecciones del tracto urinario de inicio en la comunidad causadas por Escherichia coli productor de betalactamasas de espectro extendido en Colombia [Prevalence and risk factors for extended-spectrum β-lactamase-producing Escherichia coli causing healthy carriers-onset urinary tract infections in Colombia]. Enferm Infecc Microbiol Clin. 2016;34(9):559–565. Spanish. doi:10.1016/j.eimc.2015.11.017
150. Chagas TP, Seki LM, Cury JC, et al. Multiresistance, beta-lactamase-encoding genes and bacterial diversity in hospital wastewater in Rio de Janeiro, Brazil. J Appl Microbiol. 2011;111(3):572–581. doi:10.1111/j.1365-2672.2011.05072.x
151. Vignoli R, García-Fulgueiras V, Cordeiro NF, et al. Extended-spectrum β-lactamases, transferable quinolone resistance, and virulotyping in extra-intestinal E. coli in Uruguay. J Infect Dev Ctries. 2016;10(1):43–52. doi:10.3855/jidc.6918
152. Campos ACC, Andrade NL, Ferdous M, et al. Corrigendum: comprehensive molecular characterization of Escherichia coli isolates from urine samples of hospitalized patients in Rio de Janeiro, Brazil. Front Microbiol. 2020;11:599031. doi:10.3389/fmicb.2020.599031
153. Gonçalves LF, De oliveira P, de Melo ABF, et al. Multidrug resistance dissemination by extended-spectrum β-lactamase-producing Escherichia coli causing healthy carriers-acquired urinary tract infection in the Central-Western Region, Brazil. J Glob Antimicrob Resist. 2016;6:1–4. doi:10.1016/j.jgar.2016.02.003
154. Martinez P, Garzón D, Mattar S. CTX-M-producing Escherichia coli and Klebsiella pneumoniae isolated from healthy carriers-acquired urinary tract infections in Valledupar, Colombia. Braz J Infect Dis. 2012;16(5):420–425. doi:10.1016/j.bjid.2012.05.001
155. Peirano G, Asensi MD, Pitondo-Silva A, Pitout JD. Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli from Rio de Janeiro, Brazil. Clin Microbiol Infect. 2011;17(7):1039–1043. doi:10.1111/j.1469-0691.2010.03440.x
156. Andrade LN, Minarini LA, Pitondo-Silva A, et al. Determinants of beta-lactam resistance in meningitis-causing Enterobacteriaceae in Brazil. Can J Microbiol. 2010;56(5):399–407. doi:10.1139/W10-020
157. Abreu AG, Marques SG, Monteiro-Neto V, Carvalho RM, Gonçalves AG. Infecção hospitalar e caracterização de Enterobactérias produtoras de β-lactamases de espectro ampliado no Nordeste do Brasil [Nosocomial infection and characterization of extended-spectrum β-lactamases-producing Enterobacteriaceae in Northeast Brazil]. Rev Soc Bras Med Trop. 2011;44(4):441–446. Portuguese. doi:10.1590/S0037-86822011000400008
158. Villar HE, Baserni MN, Jugo MB. Faecal carriage of ESBL-producing Enterobacteriaceae and carbapenem-resistant Gram-negative bacilli in healthy carriers settings. J Infect Dev Ctries. 2013;7(8):630–634. doi:10.3855/jidc.2900
159. Garrido D, Garrido S, Gutiérrez M, et al. Clinical characterization and antimicrobial resistance of Escherichia coli in pediatric patients with urinary tract infection at a third level hospital of Quito, Ecuador. Bol Med Hosp Infant Mex. 2017;74(4):265–271. doi:10.1016/j.bmhimx.2017.02.004
160. Koga VL, Rodrigues GR, Scandorieiro S, et al. Evaluation of the antibiotic resistance and virulence of Escherichia coli strains isolated from chicken carcasses in 2007 and 2013 from Paraná, Brazil. Foodborne Pathog Dis. 2015;12(6):479–485. doi:10.1089/fpd.2014.1888
161. Wollheim C, Guerra IM, Conte VD, et al. Nosocomial and healthy carriers infections due to class A extended-spectrum β-lactamase (ESBLA)-producing Escherichia coli and Klebsiella spp. in southern Brazil. Braz J Infect Dis. 2011;15(2):138–143.
162. Borges CA, Maluta RP, Beraldo LG, et al. Captive and free-living urban pigeons (Columba livia) from Brazil as carriers of multidrug-resistant pathogenic Escherichia coli. Vet J. 2017;219:65–67. doi:10.1016/j.tvjl.2016.12.015
163. Soria-Segarra C, Soria-Baquero E, Cartelle-Gestal M. High prevalence of CTX-M-1-like enzymes in urinary isolates of Escherichia coli in Guayaquil, Ecuador. Microb Drug Resist. 2018;24(4):393–402. doi:10.1089/mdr.2017.0325
164. Viana ALM, Cayô R, Avelino CC, Gales AC, Franco MC, Minarini LAR. Extended-spectrum β-lactamases in Enterobacteriaceae isolated in Brazil carry distinct types of plasmid-mediated quinolone resistance genes. J Med Microbiol. 2013;62(Pt 9):1326–1331. doi:10.1099/jmm.0.055970-0
165. Bado I, Gutiérrez C, García-Fulgueiras V, et al. CTX-M-15 in combination with aac(6’)-Ib-cr is the most prevalent mechanism of resistance both in Escherichia coli and Klebsiella pneumoniae, including K. pneumoniae ST258, in an ICU in Uruguay. J Glob Antimicrob Resist. 2016;6:5–9. doi:10.1016/j.jgar.2016.02.001
166. Botelho LA, Kraychete GB, Costa JL, et al. Widespread distribution of CTX-M and plasmid-mediated AmpC β-lactamases in Escherichia coli from Brazilian chicken meat. Mem Inst Oswaldo Cruz. 2015;110(2):249–254. doi:10.1590/0074-02760140389
167. Dropa M, Lincopan N, Balsalobre LC, et al. Genetic background of novel sequence types of CTX-M-8- and CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae from public wastewater treatment plants in São Paulo, Brazil. Environ Sci Pollut Res Int. 2016;23(5):4953–4958. doi:10.1007/s11356-016-6079-5
168. García-Fulgueiras V, Bado I, Mota MI, et al. Extended-spectrum β-lactamases and plasmid-mediated quinolone resistance in enterobacterial clinical isolates in the paediatric hospital of Uruguay. J Antimicrob Chemother. 2011;66(8):1725–1729. doi:10.1093/jac/dkr222
169. Pallecchi L, Malossi M, Mantella A, et al. Detection of CTX-M-type beta-lactamase genes in fecal Escherichia coli isolates from healthy children in Bolivia and Peru. Antimicrob Agents Chemother. 2004;48(12):4556–4561. doi:10.1128/AAC.48.12.4556-4561.2004
170. Quijada-Martínez P, Flores-Carrero A, Labrador I, Araque M. Estudio clínico y microbiológico de la infección urinaria asociada a catéter, en los servicios de medicina interna de un hospital universitario venezolano [Clinical and microbiological study of catheter-associated urinary tract infections in internal medicine services of a Venezuelan university hospital]. Rev Peru Med Exp Salud Publica. 2017;34(1):52–61. Spanish. doi:10.17843/rpmesp.2017.341.2766
171. Minarini LA, Gales AC, Palazzo IC, Darini AL. Prevalence of healthy carriers-occurring extended spectrum beta-lactamase-producing Enterobacteriaceae in Brazil. Curr Microbiol. 2007;54(5):335–341. doi:10.1007/s00284-006-0307-z
172. Sartori L, Fernandes MR, Ienne S, et al. Draft genome sequences of two fluoroquinolone-resistant CTX-M-15-producing Escherichia coli ST90 (ST23 complex) isolated from a calf and a dairy cow in South America. J Glob Antimicrob Resist. 2017;11:145–147. doi:10.1016/j.jgar.2017.10.009
173. Marusinec R, Kurowski KM, Amato HK, et al. Caretaker knowledge, attitudes, and practices (KAP) and carriage of extended-spectrum beta-lactamase-producing E. coli (ESBL-EC) in children in Quito, Ecuador. Antimicrob Resist Infect Control. 2021;10(1):2. doi:10.1186/s13756-020-00867-7
174. Jones RN, Guzman-Blanco M, Gales AC, et al. Susceptibility rates in Latin American nations: report from a regional resistance surveillance program (2011). Braz J Infect Dis. 2013;17(6):672–681. doi:10.1016/j.bjid.2013.07.002
175. Adrianzén D, Arbizu A, Ortiz J, Samalvides F. Mortalidad por bacteriemia causada por Escherichia coli y Klebsiella spp. productoras de beta lactamasas de espectro extendido: cohorte retrospectiva en un hospital de Lima, Perú [Mortality caused by bacteremia Escherichia coli and Klebsiella spp. extended-spectrum beta-lactamase- producers: a retrospective cohort from a hospital in Lima, Peru]. Rev Peru Med Exp Salud Publica. 2013;30(1):18–25. Spanish. doi:10.1590/s1726-46342013000100004
176. Tedesco-Maiullari RM, Guevara A. Enterobatteri produttori di beta-lattamasi a spettro esteso in un ospedale in Venezuela [Enterobacteria producers of extended-spectrum beta-lactamase in a hospital from Venezuela]. Infez Med. 2012;20(2):93–99. Italian.
177. Di Conza JA, Badaracco A, Ayala J, Rodríguez C, Famiglietti A, Gutkind GO. β-lactamases produced by amoxicillin-clavulanate-resistant enterobacteria isolated in Buenos Aires, Argentina: a new blaTEM gene. Rev Argent Microbiol. 2014;46(3):210–217. doi:10.1016/S0325-7541(14)70075-6
178. Da Silva KC, Cunha MP, Cerdeira L, et al. High-virulence CMY-2- and CTX-M-2-producing avian pathogenic Escherichia coli strains isolated from commercial turkeys. Diagn Microbiol Infect Dis. 2017;87(1):64–67. doi:10.1016/j.diagmicrobio.2016.10.001
179. Fernandes MR, Sellera FP, Moura Q, Gaspar VC, Cerdeira L, Lincopan N. International high-risk clonal lineages of CTX-M-producing Escherichia coli F-ST648 in free-roaming cats, South America. Infect Genet Evol. 2018;66:48–51. doi:10.1016/j.meegid.2018.09.009
180. Da Silva-dias RC, Borges-Neto AA, D’Almeida-Ferraiuoli GI, De-oliveira MP, Riley LW, Moreira BM. Prevalence of AmpC and other beta-lactamases in enterobacteria at a large urban university hospital in Brazil. Diagn Microbiol Infect Dis. 2008;60(1):79–87. doi:10.1016/j.diagmicrobio.2007.07.018
181. Ferreira JC, Penha Filho RA, Andrade LN, Berchieri A, Darini AL. Detection of chromosomal bla(CTX-M-2) in diverse Escherichia coli isolates from healthy broiler chickens. Clin Microbiol Infect. 2014;20(10):623–626. doi:10.1111/1469-0691.12531
182. Quiles MG, Menezes LC, Bauab C, et al. Diagnosis of bacteremia in pediatric oncologic patients by in-house real-time PCR. BMC Infect Dis. 2015;15:283. doi:10.1186/s12879-015-1033-6
183. Dias RCB, Vieira MA, Moro AC, et al. Characterization of Escherichia coli obtained from patients undergoing peritoneal dialysis and diagnosed with peritonitis in a Brazilian centre. J Med Microbiol. 2019;68(9):1330–1340. doi:10.1099/jmm.0.001043
184. Ferreira CM, Ferreira WA, Almeida NC, Naveca FG, Barbosa M. Extended-spectrum beta-lactamase-producing bacteria isolated from hematologic patients in Manaus, State of Amazonas, Brazil. Braz J Microbiol. 2011;42(3):1076–1084. doi:10.1590/S1517-83822011000300028
185. Rugini CL, Sobottka AM, Fuentefria DB. Occurrence and sensitivity profile of extended spectrum beta-lactamase-producing Enterobacteriaceae at a tertiary hospital in Southern Brazil. Rev Soc Bras Med Trop. 2015;48(6):692–698. doi:10.1590/0037-8682-0211-2015
186. Oplustil CP, Nunes R, Mendes C; RESISTNET Group. Multicenter evaluation of resistance patterns of Klebsiella pneumoniae, Escherichia coli, Salmonella spp and Shigella spp isolated from clinical specimens in Brazil: RESISTNET Surveillance Program. Braz J Infect Dis. 2001;5(1):8–12. doi:10.1590/S1413-86702001000100002
187. Tosin I, Silbert S, Sader HS. The use of molecular typing to evaluate the dissemination of antimicrobial resistance among Gram-negative rods in Brazilian hospitals. Braz J Infect Dis. 2003;7(6):360–369. doi:10.1590/S1413-86702003000600002
188. Guevara N, Guzmán M, Merentes A, et al. Patrones de susceptibilidad antimicrobiana de bacterias gramnegativas aisladas de infecciones del tracto urinario en Venezuela: resultados del estudio SMART 2009-2012 [Antimicrobial susceptibility patterns of Gram-negative bacteria isolated in urinary tract infections in Venezuela: results of the SMART study 2009-2012]. Rev Chilena Infectol. 2015;32(6):639–648. Spanish. doi:10.4067/S0716-10182015000700005
189. Casellas JM, Tomé G, Bantar C, et al. Argentinean collaborative multicenter study on the in vitro comparative activity of piperacillin-tazobactam against selected bacterial isolates recovered from hospitalized patients. Diagn Microbiol Infect Dis. 2003;47(3):527–537. doi:10.1016/S0732-8893(03)00131-7
190. Motta RN, Oliveira MM, Magalhães PS, et al. Plasmid-mediated extended-spectrum beta-lactamase-producing strains of Enterobacteriaceae isolated from diabetes foot infections in a Brazilian diabetic center. Braz J Infect Dis. 2003;7(2):129–134. doi:10.1590/S1413-86702003000200006
191. Rossi F, García P, Ronzon B, Curcio D, Dowzicky MJ. Rates of antimicrobial resistance in Latin America (2004-2007) and in vitro activity of the glycylcycline tigecycline and of other antibiotics. Braz J Infect Dis. 2008;12(5):405–415. doi:10.1590/S1413-86702008000500012
192. Casella T, Rodríguez MM, Takahashi JT, et al. Detection of blaCTX-M-type genes in complex class 1 integrons carried by Enterobacteriaceae isolated from retail chicken meat in Brazil. Int J Food Microbiol. 2015;197:88–91. doi:10.1016/j.ijfoodmicro.2014.12.001
193. De Oliveira CF, Salla A, Lara VM, Rieger A, Horta JA, Alves SH. Prevalence of extended-spectrum beta-lactamases-producing microorganisms in nosocomial patients and molecular characterization of the SHV-type isolates. Braz J Microbiol. 2010;41(2):278–282. doi:10.1590/S1517-83822010000200002
194. Seki LM, Pereira PS, de Souza-conceição M, et al. Molecular epidemiology of CTX-M producing Enterobacteriaceae isolated from bloodstream infections in Rio de Janeiro, Brazil: emergence of CTX-M-15. Braz J Infect Dis. 2013;17(6):640–646. doi:10.1016/j.bjid.2013.03.012
195. Salgado-Caxito M, Benavides JA, Munita JM, et al. Risk factors associated with faecal carriage of extended-spectrum cephalosporin-resistant Escherichia coli among dogs in Southeast Brazil. Prev Vet Med. 2021;190:105316. doi:10.1016/j.prevetmed.2021.105316
196. Vignoli R, Varela G, Mota MI, et al. Enteropathogenic Escherichia coli strains carrying genes encoding the PER-2 and TEM-116 extended-spectrum beta-lactamases isolated from children with diarrhea in Uruguay. J Clin Microbiol. 2005;43(6):2940–2943. doi:10.1128/JCM.43.6.2940-2943.2005
197. Hernández E, Araque M, Millán Y, Millán B, Vielma S. Prevalencia de beta-lactamasa CTX-M-15 en grupos filogenéticos de Escherichia coli uropatógena aisladas en pacientes de la comunidad en Mérida, Venezuela [Prevalence of beta-lactamase CTX-M-15 in phylogenetic groups of uropathogenic Escherichia coli isolated from patients in the healthy carriers of Merida, Venezuela]. Invest Clin. 2014;55(1):32–43. Spanish.
198. Saba-Villarroel PM, Gutkind GO, Di Conza JA, Radice MA. First survey on antibiotic resistance markers in Enterobacteriaceae in Cochabamba, Bolivia. Rev Argent Microbiol. 2017;49(1):50–54. doi:10.1016/j.ram.2016.10.002
199. Lago A, Fuentefria SR, Fuentefria DB. Enterobactérias produtoras de ESBL em Passo Fundo, estado do Rio Grande do Sul, Brasil [ESBL-producing enterobacteria in Passo Fundo, state of Rio Grande do Sul, Brazil]. Rev Soc Bras Med Trop. 2010;43(4):430–434. Portuguese. doi:10.1590/S0037-86822010000400019
200. Marchetti L, Buldain D, Gortari-Castillo L, Buchamer A, Chirino-Trejo M, Mestorino N. Pet and stray dogs as reservoirs of antimicrobial-resistant Escherichia coli. Int J Microbiol. 2021;2021:6664557. doi:10.1155/2021/6664557
201. Wozniak A, Villagra NA, Undabarrena A, et al. Porin alterations present in non-carbapenemase-producing Enterobacteriaceae with high and intermediate levels of carbapenem resistance in Chile. J Med Microbiol. 2012;61(Pt 9):1270–1279. doi:10.1099/jmm.0.045799-0
202. Queiroz ML, Antunes P, Mourão J, Merquior VL, Machado E, Peixe LV. Characterization of extended-spectrum beta-lactamases, antimicrobial resistance genes, and plasmid content in Escherichia coli isolates from different sources in Rio de Janeiro, Brazil. Diagn Microbiol Infect Dis. 2012;74(1):91–94. doi:10.1016/j.diagmicrobio.2012.05.019
203. Salinas L, Loayza F, Cárdenas P, et al. Environmental spread of Extended Spectrum Beta-Lactamase (ESBL) producing Escherichia coli and ESBL genes among children and domestic animals in Ecuador. Environ Health Perspect. 2021;129(2):27007. doi:10.1289/EHP7729
204. Bartoloni A, Sennati S, Di Maggio T, et al. Antimicrobial susceptibility and emerging resistance determinants (blaCTX-M, rmtB, fosA3) in clinical isolates from urinary tract infections in the Bolivian Chaco. Int J Infect Dis. 2016;43:1–6. doi:10.1016/j.ijid.2015.12.008
205. Vasques MR, Bello AR, Lamas C, Correa J, Pereira JA. β-lactamase producing enterobacteria isolated from surveillance swabs of patients in a cardiac intensive care unit in Rio de Janeiro, Brazil. Braz J Infect Dis. 2011;15(1):28–33.
206. Mendo-Lopez R, Jasso L, Guevara X, et al. Multidrug-resistant microorganisms colonizing lower extremity wounds in patients in a tertiary care hospital, Lima, Peru. Am J Trop Med Hyg. 2017;97(4):1045–1048. doi:10.4269/ajtmh.17-0235
207. Gaitán CSL, Espinal MPA. Caracterización molecular de Escherichia coli y Klebsiella pneumoniae productores de beta-lactamasas de espectro extendido en hospitales de la Región Caribe, Colombia [Molecular characterization of extended-spectrum beta-lactamases-producing Escherichia coli and Klebsiella pneumoniae in hospitals of the Caribean Region, Colombia]. Rev Chilena Infectol. 2009;26(3):239–246. Spanish.
208. Sennati S, Santella G, Di Conza J, et al. Changing epidemiology of extended-spectrum β-lactamases in Argentina: emergence of CTX-M-15. Antimicrob Agents Chemother. 2012;56(11):6003–6005. doi:10.1128/AAC.00745-12
209. Ferreira JC, Penha-Filho RA, Andrade LN, Berchieri Junior A, Darini AL. Evaluation and characterization of plasmids carrying CTX-M genes in a non-clonal population of multidrug-resistant Enterobacteriaceae isolated from poultry in Brazil. Diagn Microbiol Infect Dis. 2016;85(4):444–448. doi:10.1016/j.diagmicrobio.2016.05.011
210. Abreu AG, Marques SG, Monteiro-Neto V, Gonçalves AG. Extended-spectrum β-lactamase-producing Enterobacteriaceae in healthy carriers-acquired urinary tract infections in São Luís, Brazil. Braz J Microbiol. 2013;44(2):469–471. doi:10.1590/S1517-83822013005000038
211. Hawser SP, Bouchillon SK, Hoban DJ, et al. Low frequency of ertapenem-resistant intra-abdominal isolates of Escherichia coli from Latin America: susceptibility, ESBL-occurrence, and molecular characterisation (SMART 2008–2009). J Chemother. 2012;24(1):6–11. doi:10.1179/1120009X12Z.0000000003
212. Quinteros M, Radice M, Gardella N, et al. Extended-spectrum beta-lactamases in Enterobacteriaceae in Buenos Aires, Argentina, public hospitals. Antimicrob Agents Chemother. 2003;47(9):2864–2867. doi:10.1128/AAC.47.9.2864-2867.2003
213. Flamm RK, Sader HS, Jones RN. Ceftaroline activity tested against contemporary Latin American bacterial pathogens (2011). Braz J Infect Dis. 2014;18(2):187–195. doi:10.1016/j.bjid.2013.11.005
214. Nordberg V, Quizhpe-Peralta A, Galindo T, et al. High proportion of intestinal colonization with successful epidemic clones of ESBL-producing Enterobacteriaceae in a neonatal intensive care unit in Ecuador. PLoS One. 2013;8(10):e76597. doi:10.1371/journal.pone.0076597
215. Fernandes MR, Sellera FP, Esposito F, Sabino CP, Cerdeira L, Lincopan N. Colistin-resistant mcr-1-positive Escherichia coli on public beaches, an infectious threat emerging in recreational waters. Antimicrob Agents Chemother. 2017;61(7):e00234–17. doi:10.1128/AAC.00234-17
216. Redondo C, Chalbaud A, Alonso G. Frequency and diversity of CTX-M enzymes among extended-spectrum beta-lactamase-producing Enterobacteriaceae isolates from Caracas, Venezuela. Microb Drug Resist. 2013;19(1):42–47. doi:10.1089/mdr.2012.0079
217. Villar HE, Jugo MB, Macan A, Visser M, Hidalgo M, Maccallini GC. Frequency and antibiotic susceptibility patterns of urinary pathogens in male outpatients in Argentina. J Infect Dev Ctries. 2014;8(6):699–704. doi:10.3855/jidc.3766
218. Moreno A, Bello H, Guggiana D, Domínguez M, González G. Extended-spectrum beta-lactamases belonging to CTX-M group produced by Escherichia coli strains isolated from companion animals treated with enrofloxacin. Vet Microbiol. 2008;129(1–2):203–208. doi:10.1016/j.vetmic.2007.11.011
219. Pallecchi L, Bartoloni A, Fiorelli C, et al. Rapid dissemination and diversity of CTX-M extended-spectrum beta-lactamase genes in commensal Escherichia coli isolates from healthy children from low-resource settings in Latin America. Antimicrob Agents Chemother. 2007;51(8):2720–2725. doi:10.1128/AAC.00026-07
220. Báez J, Hernández-García M, Guamparito C, et al. Molecular characterization and genetic diversity of ESBL-producing Escherichia coli colonizing the migratory Franklin’s gulls (Leucophaeus pipixcan) in Antofagasta, North of Chile. Microb Drug Resist. 2015;21(1):111–116. doi:10.1089/mdr.2014.0158
221. Conte D, Palmeiro JK, da Silva Nogueira K, et al. Characterization of CTX-M enzymes, quinolone resistance determinants, and antimicrobial residues from hospital sewage, wastewater treatment plant, and river water. Ecotoxicol Environ Saf. 2017;136:62–69. doi:10.1016/j.ecoenv.2016.10.031
222. Nascimento T, Cantamessa R, Melo L, et al. International high-risk clones of Klebsiella pneumoniae KPC-2/CC258 and Escherichia coli CTX-M-15/CC10 in urban lake waters. Sci Total Environ. 2017;598:910–915. doi:10.1016/j.scitotenv.2017.03.207
223. Flores R, Albornoz C, Hurtado J, Montaño V, Santa-Cruz A. Enterobacterias productoras de B-lactamasas de espectro-extendido y plásmido-AmpC en aguas de riego, zona Maica, Cochabamba [Extended-spectrum B-lactamase and plasmid ampc B-lactamase-producing Enterobacteriaceae in water sources for irrigation in Maica’s zone, Cochabamba]. Rev Cient Cienc Med. 2019;22(2):15–21. Spanish.
224. Guzman-Otazo J, Gonzales-Siles L, Poma V, et al. Diarrheal bacterial pathogens and multi-resistant Enterobacteria in the Choqueyapu River in La Paz, Bolivia. PLoS One. 2019;14(1):e0210735. doi:10.1371/journal.pone.0210735
225. Ortega-Paredes D, Barba P, Mena-López S, Espinel N, Crespo V, Zurita J. High quantities of multidrug-resistant Escherichia coli are present in the Machángara urban river in Quito, Ecuador. J Water Health. 2020;18(1):67–76. doi:10.2166/wh.2019.195
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.