Back to Journals » ImmunoTargets and Therapy » Volume 12
Extended-Release Tofacitinib Therapy for a MDA5 Antibody-Positive Amyopathic Dermatomyositis Patient with Early-Stage Interstitial Lung Disease
Authors Wang CR , Lin WC, Wong TW
Received 23 October 2023
Accepted for publication 13 December 2023
Published 20 December 2023 Volume 2023:12 Pages 187—192
DOI https://doi.org/10.2147/ITT.S445971
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Michael Shurin
Chrong-Reen Wang,1 Wei-Chieh Lin,2 Tak-Wah Wong3
1Division of Rheumatology and Immunology, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan, Taiwan; 2Division of Critical Care Medicine, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan, Taiwan; 3Department of Dermatology, National Cheng Kung University Hospital, Tainan, Taiwan
Correspondence: Chrong-Reen Wang, Department of Internal Medicine, National Cheng Kung University Hospital, 138 Sheng-Li Road, Tainan, 70403, Taiwan, Email [email protected]
Introduction: In East Asia, more than half of patients with amyopathic dermatomyositis (ADM) have interstitial lung disease (ILD). There is up to 50% 6-month mortality in MDA5-positive ILD refractory to corticosteroid (CS) combined with immunosuppressant therapy.
Patient Details: A 39-year-old local woman had a 1-month history of reddish-purple discoloration around the eyelids (heliotrope rash), and erythematous areas on the upper back and posterior neck (shawl sign) as well as on the front of her chest (V sign), followed by dry cough and mild dyspnea for 1 week. She had normal muscle strength, muscle-enzyme concentrations, and muscular magnetic resonance images. Laboratory tests showed hypoxemia, increased ferritin and CRP levels, and positive MDA5 antibodies. High-resolution chest computed tomography revealed bilateral ground-glass opacity. She received a diagnosis of anti-MDA5-positive ADM with early-stage ILD.
Intervention: Pulse methylprednisolone and cyclophosphamide therapies were initiated, followed by high-dose CS treatment. Immediate-release twice-daily 5 mg tofacitinib (Tof) has been demonstrated to be effective induction therapy for early-stage ILD in anti-MDA5-positive ADM. Owing to the patient’s preference for once-daily therapy, 11 mg extended-release Tof was prescribed 4 weeks after starting the initial pulse CS treatment for ILD.
Outcomes: Respiratory symptoms and cutaneous manifestations were absent and the use of CS spared 5 months after initiating Tof therapy. Laboratory examinations exhibited normalized ferritin/oxygen levels, and chest images displayed completely resolved pulmonary infiltration. ILD remains under adequate control with Tof monotherapy without recurrence at 5 months.
Lessons: Owing to a rapid decline in higher mortality in anti-MDA5-positive ADM patients with ILD, early detection with prompt initiation of extended-release Tof induction therapy might achieve a beneficial outcome.
Keywords: amyopathic dermatomyositis, positive MDA5 antibody, interstitial lung disease, extended-release tofacitinib, Janus kinase inhibitor
Introduction
In East Asia, more than half of amyopathic dermatomyositis (ADM) patients have interstitial lung disease (ILD).1 There is up to 50% 6-month mortality in MDA5-positive cases with ILD refractory to treatment with corticosteroid (CS) in combination with immunosuppressants (ISs), including cyclosporin A, cyclophosphamide (CYC), and/or tacrolimus.1,2 The therapeutic potential of 5 mg twice-daily immediate-release tofacitinib (Tof), a small-molecule pan-JAK inhibitor (JAKi), was firstly shown in two anti-MDA5-positive ADM patients with ILD receiving additional Tof for 18 weeks and 7 months after failure on triple therapy of pulse/high-dose CS, cyclosporin A, and CYC.3 Despite the significant infection complications with cytomegalovirus, herpes zoster, and bacterial pneumonia, they showed decreased ferritin levels and improved respiratory conditions. Later, therapeutic efficacy was demonstrated in 18 patients with anti-MDA5-positive ADM and early-stage ILD, ie, confirmation by chest computed tomography (CT) <3 months with predicted forced vital capacity (FVC) of at least 50%.4 Despite their medication history of ISs, after enrollment into this trial, all patients received only Tof 5 mg twice daily combined with CS. Six months after therapy, there were lower ferritin levels, increased percentages of predicted FVC and diffusion capacity, and longer survival than 32 historical control patients under the conventional CS/IS therapeutic regimen (100% versus 78%), indicating that Tof treatment is an effective induction therapy in early-stage ILD.2,4
An extended-release formulation of Tof with a once-daily 11 mg dosage can achieve comparable pharmacokinetic parameters to the twice-daily 5 mg immediate-release formulation.5 Nevertheless, the therapeutic efficacy of an extended-release formulation has not been demonstrated in anti-MDA5-positive ILD yet. Herein, we report a an MDA5 antibody–positive ADM patient with early-stage ILD under extended-release Tof therapy of 10 months. She had resolved pulmonary infiltration, normalized ferritin, CRP and oxygen levels, and discontinued CS use 5 months after initiating the Tof treatment. Furthermore, the ILD was under adequate control with Tof monotherapy without recurrence for 5 months.
Case Presentation
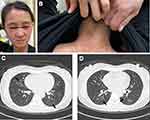
A 39-year-old local woman visited the outpatient rheumatology clinic with a 1-month history of reddish-purple discoloration around the eyelids with swelling (heliotrope rash, Figure 1A), and erythematous areas on the upper back and posterior neck (shawl sign, Figure 1B), as well as on the front of the chest (V sign), followed by dry cough and mild dyspnea for 1 week. Physical examination showed normal muscle strength, and laboratory tests disclosed hypoxemia (80 mmHg, 95% oxygen saturation), elevated ferritin (279 ng/mL) and CRP (10.1 mg/L) levels, and normal muscle enzyme concentrations (ALT, AST, CK, LDH). Muscular gadolinium-enhanced magnetic resonance images revealed no edematous changes or abnormal enhancement, further confirming the amyopathic character.6 Autoantibody profiles exhibited positive MDA5 antibodies (myositis profile: IgG 16 antigens, Euroimmun), negative aminoacyl-tRNA synthetase (ARS) antibodies (EJ, Jo-1, OJ, PL7, PL12), and absent antinuclear antibodies. Despite normal predicted FVC and diffusion capacity, high-resolution chest CT demonstrated bilateral ground-glass opacity (GGO) in the lungs (Figure 1C and D). She received a diagnosis of MDA5 antibody-positive ADM with early-stage ILD. Pulse CS (methylprednisolone 1 g/day × 3) and CYC (0.5 g/m2 × 1) therapies were initiated, followed by high-dose CS (prednisolone 1 mg/kg/day) treatment. Despite lessened skin lesions after this therapy, she still had respiratory symptoms with impaired oxygen saturation (96%). In addition, there were CYC/CS-related side effects with infection and hyperglycemia.
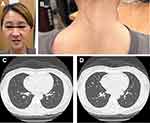
Immediate-release Tof has been demonstrated to be an effective induction therapy for early-stage ILD in anti-MDA5-positive ADM.4 Owing to the patient’s preference for once-daily therapy, 11 mg extended-release Tof was prescribed 4 weeks after starting the initial pulse CS treatment for ILD. She had resolved respiratory symptoms and cutaneous manifestations (Figure 2A and B), while the use of CS was discontinued, 5 months after initiating Tof therapy. Laboratory examinations showed normalized serum ferritin concentrations (21 ng/mL), CRP values (<3 mg/L), and arterial oxygen levels (104 mmHg, 98% oxygen saturation). Follow-up CT displayed complete resolution of bilateral pulmonary GGO infiltration (Figure 2C and D).
At the time of writing, ILD is under adequate control with Tof monotherapy without using CS or ISs for 5 months, as verified by absent respiratory symptoms, unremarkable ferritin, CRP, and oxygen levels and normal chest images, implicating a potential role for this JAKi as a remission-maintenance medication. In addition, there were no observed adverse effects, such as infection complications, during the Tof-therapy period.
Discussion
In patients with idiopathic inflammatory myopathy, such as ADM, DM, and polymyositis, ILD manifestation is associated with ARS or MDA5 antibodies.7–10 Chronic ILD is frequently observed in anti-ARS-positive DM/polymyositis with satisfactory responses to CS treatment, whereas rapidly progressive ILD with a pathological finding of diffuse alveolar damage has been identified in anti-MDA5-positive ADM. MDA5 functions as a pattern-recognition receptor capable of detecting invading virus-derived RNAs, and participates in innate immunoresponses through inducing the production of IFN/other cytokines and activating immune cells.11 Although the pathogenesis of anti-MDA5-positive ILD has been suspected to be related to a cytokine storm, its exact mechanism remains to be elucidated. Nevertheless, anti-MDA5-positive ADM patients with ILD have been shown to have higher circulating levels of miscellaneous cytokines.12 Notably, IL2, IL6, IL10, IFNα, and IFNγ can bind to their cytokine receptors associated with JAKs, further recruiting STAT proteins to modulate the downstream gene transcription in pathogenic cells involved in ILD development.13
Immediate-release Tof was the first JAKi approved by the US Food and Drug Administration (FDA) in November 2012 for treating rheumatoid arthritis (RA) patients with moderate or high activity and an inadequate response to methotrexate use.14 Individual cytokine receptors can recruit specially combined JAKs to activate distinct downstream signals in targeted cells, while antagonizing a specific JAK can suppress more than one cytokine pathway, further expanding the therapeutic efficacy of JAKi.15 Currently, based on these action mechanisms, Tof is prescribed in treating various autoimmune and inflammatory diseases and provides beneficial effects by minimizing dosages or sparing the use of CS and/or ISs.16
There is equivalence in the area under the plasma concentration–time curve between two formulations of Tof, while evidence on the exposure–response relationship indicates that this area is the relevant parameter for clinical responses.17 The extended-release formulation firstly obtained approval from the FDA for rheumatoid arthritis (RA) therapy in February 2016.18 There were subsequent approved indications for this formulation to treat patients with psoriatic arthritis, ulcerative colitis, and ankylosing spondylitis in December 2017, December 2019, and December 2021, respectively. In particular, despite not being an indicated use, the effects of extended-release Tof have been demonstrated in patients with systemic vasculitis, including Behçet’s disease and Takayasu’s arteritis refractory to CS/IS or with single-cytokine inhibitors like anti-IL6 and anti-TNFα.19–22 Collectively, for treating inflammatory rheumatology disorders, these findings indicate that the extended-release formulation has the convenience of less frequent dosing and corresponding efficacy compared with the immediate-release formulation.
Although there were no observed infection episodes during the therapeutic period in our reported anti-MDA5-positive ADM case with ILD, Tof use has been reported to be associated with an increased risk of herpes zoster infection, with an incidence of three to four per 100 person-years in Tof-treated RA patients.23 Nonetheless, this JAKi has no known damage on antiviral immunity against severe COVID-19 infection.24 Prescribing high-dose Tof as a twice-daily 10 mg immediate-release formulation in patients with COVID-2019 pneumonia can prevent deterioration to respiratory failure.25
Conclusion
In this reported anti-MDA5 positive ADM patient with early-stage ILD, after pulse CS/CYC and high-dose CS therapies, there were persistent respiratory symptoms with impaired oxygen saturation, as well as overt CYC/CS-related side effects, requiring additional IS agents with therapeutic efficacy to reduce or spare the use of CS/CYC. She had resolved pulmonary infiltration, normalized ferritin, CRP, and oxygen levels, and totally discontinued CS prescription after initiating extended-release Tof induction therapy for 5 months. Furthermore, ILD was under adequate control with Tof monotherapy without recurrence for 5 months. Owing to a rapid decline with higher mortality in such patients, early detection of ILD with prompt initiation of extended-release Tof induction therapy might achieve a beneficial outcome.
Abbreviations
ARS, aminoacyl-tRNA synthetase; CS, corticosteroid; CT, computed tomography; CYC, cyclophosphamide; DM, dermatomyositis; FDA, Food and Drug Administration; FVC, forced vital capacity; GGO, ground-glass opacity; ILD, interstitial lung disease; IS, immunosuppressant; RA, rheumatoid arthritis; Tof, tofacitinib.
Data Sharing
The data of this study can be provided to researchers from the corresponding author upon reasonable request.
Ethics
The Institutional Review Board of National Cheng Kung University Hospital approved this study (approval B-ER-105-108).
Consent for Publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Acknowledgments
The authors are indebted to the physicians and nurses who were involved in managing this reported patient.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. McPherson M, Economidou S, Liampas A, Zis P, Parperis K. Management of MDA-5 antibody positive clinically amyopathic dermatomyositis associated interstitial lung disease: a systematic review. Semin Arthritis Rheum. 2022;53(4):151959. doi:10.1016/j.semarthrit.2022.151959
2. Takanashi S, Kaneko Y, Takeuchi T. Tofacitinib in interstitial lung disease complicated with anti-MDA5 antibody-positive dermatomyositis: a literature review. Mod Rheumatol. 2022;32(1):231–237. doi:10.1080/14397595.2021.1906505
3. Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology. 2018;57(12):2114–2119. doi:10.1093/rheumatology/key188
4. Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med. 2019;381(3):291–293. doi:10.1056/NEJMc1900045
5. Lamba M, Wang R, Fletcher T, Alvey C, Kushner J 4th, Stock TC. Extended-release once-daily formulation of tofacitinib: evaluation of pharmacokinetics compared with immediate-release tofacitinib and impact of food. J Clin Pharmacol. 2016;56(3):1362–1371. doi:10.1056/NEJMc1900045
6. Wang CR. Successful treatment of refractory juvenile dermatomyositis with Adalimumab. J Clin Rheumatol. 2017;23(3):174–175. doi:10.1097/RHU.0000000000000514
7. Labirua A, Lundberg IE. Interstitial lung disease and idiopathic inflammatory myopathies: progress and pitfalls. Curr Opin Rheumatol. 2010;22(6):6338. doi:10.1097/BOR.0b013e32833f1970
8. Nakashima R, Imura Y, Kobayashi S, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology. 2010;49(3):43340. doi:10.1093/rheumatology/kep375
9. Mimori T, Nakashima R, Hosono Y. Interstitial lung disease in myositis: clinical subsets, biomarkers, and treatment. Curr Rheumatol Rep. 2012;14(3):26474. doi:10.1093/rheumatology/kep375
10. Sun WC, Sun YC, Lin H, Yan B, Shi GX. Dysregulation of the type I interferon system in adult-onset clinically amyopathic dermatomyositis has a potential contribution to the development of interstitial lung disease. Br J Dermatol. 2012;167(6):123644. doi:10.1111/j.1365-2133.2012.11145.x
11. Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20(9):537–551. doi:10.1038/s41577-020-0288-3
12. Gono T, Kaneko H, Kawaguchi Y, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology. 2014;53(12):2196–2203. doi:10.1093/rheumatology/keu258
13. Schwartz DM, Bonelli M, Gadina M, O’Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12(1):25–36. doi:10.1038/nrrheum.2015.167
14. Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(1):843–862. doi:10.1038/nrrheum.2015.167
15. Virtanen AT, Haikarainen T, Raivola J, Silvennoinen O. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs. 2019;33(1):15–32. doi:10.1007/s40259-019-00333-w
16. Tanaka Y, Luo Y, O’Shea JJ, Nakayamada S. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18(3):133–145. doi:10.1038/s41584-021-00726-8
17. Lamba M, Hutmacher MM, Furst DE, et al. Model-informed development and registration of a once-daily regimen of extended release tofacitinib. Clin Pharmacol Ther. 2017;101(6):745–753. doi:10.1002/cpt.576
18. Taylor PC. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology. 2019;58(Suppl 1):i17–i26. doi:10.1093/rheumatology/key225
19. Kuwabara S, Tanimura S, Matsumoto S, Nakamura H, Horita T. Successful remission with tofacitinib in a patient with refractory Takayasu arteritis complicated by ulcerative colitis. Ann Rheum Dis. 2020;79(8):1125–1126. doi:10.1136/annrheumdis-2019-216606
20. Liu J, Hou Y, Sun L, et al. A pilot study of tofacitinib for refractory Behçet’s syndrome. Ann Rheum Dis. 2020;79(11):1517–1520. doi:10.1136/annrheumdis-2020-217307
21. Wang CR, Tsai YS, Liu YW, Li YH. Extended-release tofacitinib improves refractory Takayasu’s arteritis. Scand J Rheumatol. 2022;51(1):72–75. doi:10.1080/03009742.2021.1911054
22. Wang CR, Wong TW, Hsu SM. Extended-release tofacitinib for refractory Behçet disease: a case report. Medicine. 2022;101(15):e29189. doi:10.1097/MD.0000000000029189
23. Wang CR, Tsai HW. Immediate-release tofacitinib reduces insulin resistance in non-diabetic active rheumatoid arthritis patients: a single-center retrospective study. World J Diabetes. 2022;13(6):454–465. doi:10.4239/wjd.v13.i6.454
24. Wang CR, Lin WC. Severe COVID-19 pneumonia in patients with rheumatoid arthritis under B-cell depletion therapy. J Formos Med Assoc. 2023;122(8):807–808. doi:10.1016/j.jfma.2023.03.002
25. Ferrarini A, Vacca A, Solimando AG, et al. Early administration of tofacitinib in COVID-19 pneumonitis: an open randomized controlled trial. Eur J Clin Invest. 2023;53(2):e13898. doi:10.1111/eci.13898
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.