Back to Journals » International Journal of General Medicine » Volume 16
Expression Pattern of Cytokines in Patients with Chronic Hepatitis B Receiving PEGinterferon Therapy
Authors Chen SL, Xiao H, Li GJ, Shen YJ
Received 9 January 2023
Accepted for publication 27 April 2023
Published 10 May 2023 Volume 2023:16 Pages 1771—1782
DOI https://doi.org/10.2147/IJGM.S402524
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Hyam Leffert
Shao-Long Chen,1 Hong Xiao,2 Guo-Jun Li,3 Yao-Jie Shen4
1Shulan International Medical College, Zhejiang Shuren University, Hangzhou, 310015, People’s Republic of China; 2Department of Infectious Diseases, Shanghai Public Health Clinical Center, Fudan University, Shanghai, 201508, People’s Republic of China; 3Department of Hepatology, The Second Hospital of Yinzhou of Ningbo, Ningbo, 315100, People’s Republic of China; 4Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai, 200040, People’s Republic of China
Correspondence: Shao-Long Chen, Shulan International Medical College, Zhejiang Shuren University, Hangzhou, 310015, People’s Republic of China, Email [email protected]
Purpose: Chronic hepatitis B virus (CHB) infection is a worldwide health problem. Polyethylene glycol (PEG)ylated interferon (PEG-IFN) is an available therapy for CHB that has antiviral and immunomodulatory effects. However, PEG-IFN therapy is limited by the fact that only a subset of patients show a sustained response, its severe side effects, and high cost. The aim of this study was to explore novel biomarkers for the early prediction of PEG-IFN treatment response and to uncover its underlying mechanism.
Patients and Methods: We enrolled 10 paired patients with Hepatitis B e antigen (HBeAg)-positive CHB who received PEG-IFN-α 2a monotherapy. Patient serum samples were collected at 0, 4, 12, 24, and 48 weeks and serum samples were collected from eight healthy people as healthy controls. For confirmation, we enrolled 27 patients with HBeAg-positive CHB receiving PEG-IFN therapy and serum samples at 0 and 12 weeks were obtained. Serum samples were analyzed using Luminex technology.
Results: Among 27 assessed cytokines, 10 cytokines were identified to have high expression levels. Among them, six cytokines had significant differences in their levels between the patients with HBeAg-positive CHB and the healthy controls (P < 0.05). Potentially, treatment response could be predicted using the early time points of 4, 12, and 24 weeks. Moreover, after 12 weeks of PEG-IFN treatment, increased levels of pro-inflammatory cytokines and decreased levels of anti-inflammatory cytokines were observed. The fold change of IP-10 between 12 weeks and 0 weeks correlated with the decrease in ALT levels from 0 to 12 weeks (r = 0.2675, P = 0.0024).
Conclusion: In patients with CHB, we observed a certain pattern in the levels of cytokines during treatment with PEG-IFN, and the cytokine IP-10 might be a potential biomarker for treatment response.
Keywords: chronic hepatitis B, PEGylated interferon, cytokine, chemokine, Luminex
Introduction
Chronic hepatitis B virus (HBV) infection is endemic in Asia, the Pacific islands, Africa, Southern Europe, and Latin America. There are approximately 300 million carriers of chronic HBV surface antigen (HBsAg) worldwide, which have an increased risk of developing liver cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).1 Moreover, there are almost 1 million deaths annually from acute or chronic HBV infection.2,3 Chronic HBV infection is a very dynamic process, involving five phases, characterized by fluctuating levels of inflammation and viral replication. The five phases, which are not necessarily sequential, are the base of patient management and decisions to treat. Progress in our understanding of the pathogenesis and biology of HBV has led to the development of antiviral treatment to inhibit viral replication and decrease liver injury, thereby improving patient survival; however, few patients achieve a functional cure.4
Currently, the available Food and Drug Administration (FDA)-approved therapy regimens for patients with CHB included six nucleos(t)ide analogs (NA) and polyethylene glycol (PEG)ylated interferon (PEG-IFN) alfa-2a and alfa-2b.4 The six approved NAs for HBV treatment are lamivudine (LAM), adefovir (ADV), entecavir (ETV), telbivudine (TBV), tenofovir (TDF), and tenofovir alafenamide (TAF). The two therapeutic regimens have different antiviral mechanisms and treatment responses. NA therapy directly inhibits viral replication, while PEG-IFN has antiviral and immunomodulatory effects. NA therapy can cause rapid viral suppression and normalization of serum transaminases in the vast majority of compliant patients. In addition, NA therapy is convenient to take and its safety profile is favorable. However, serological response rates remain low and NA has to be given lifelong.5 By contrast, PEG-IFN, has a finite course and can induce a long-lasting therapeutic response.6 However, the limitations of PEG-IFN therapy include a sustained response in only a subset of patients, severe side effects, and high costs.7 These disadvantages make a significant number of patients ineligible or unwilling to take PEG-IFN therapy, limiting its use as a first-line treatment for most HBV patients. Thus, the exploration of novel biomarkers that can predict PEG-IFN treatment response early and reveal its underlying mechanism are urgently needed.
Luminex bead array assays are widely used for rapid biomarker quantification, and their most active research areas are in allergy, asthma, autoimmunity, and toxicology.8,9 Currently, the application of Luminex technology in infectious diseases has received much research attention. Several recent studies have reported the use of Luminex technology in HIV10 and HCV.11,12 Meanwhile, there have been a few reports of the involvement of Luminex technology in the area of HBV. In this study, Luminex technology was used to evaluate the expression pattern and levels of cytokines and chemokines in patients with CHB treated with PEG-IFN. We analyzed 27 potential cytokines in 10 paired patients with CHB treated with PEG-IFN and identified highly expressed cytokines. To explore novel biomarkers to predict the response to PEG-IFN therapy, a subset of the highly expressed cytokines were further verified at 0 and 12 weeks.
Materials and Methods
Patients and Serum Samples
In the exploratory experiment, we retrospectively enrolled 10 paired patients with HBV e antigen (HBeAg)-positive CHB who were treated with PEG-IFN-α2a monotherapy between 2010 and 2012 in the Department of Infectious Diseases, Shanghai Public Health Clinical Center, Fudan University. In the 48-week treatment course, five patients achieved a virological response and five did not (virological response was defined as HBeAg loss and HBV loss). Patient serum samples were collected at 0, 4, 12, 24, and 48 weeks and stored at –80 °C. We monitored the levels of HBV DNA, HBeAg, HBsAg, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and Albumin during treatment. Moreover, eight serum samples from healthy people were collected as healthy controls.
In the confirmatory experiment, we prospectively enrolled 41 patients with HBeAg-positive CHB from two participating centers in China (the Department of Infectious Diseases, Huashan Hospital, Fudan University; and the Department of Hepatology, the Second hospital of Yinzhou of Ningbo). These 41 patients received PEG-IFN-α2a or 2b treatment between 2013 and 2015 and their treatment regimens were PEG-IFN monotherapy or PEG-IFN + NA combination therapy. Patient characteristics were monitored during treatment and serum samples were collected and stored at –80 °C. After 48 weeks of treatment, 27 patients were qualified for the confirmatory experiment. This study was carried out according to the ethical standards laid down by the responsible committee on human experimentation and according to the tenets of the Declaration of Helsinki. All participants provided written informed consent.
Cytokine Measurements
All participants provided serum samples, which were stored at –80 °C. A Luminex 100/200 analyzer (Luminex Corp., Austin, TX, USA) and a Milliplex Map Kit Human Cytokine/Chemokine Magnetic Bead Panel (Sigma-Aldrich, St. Louis, MO, USA), and a 96-well Plate Assay (catalog numbers HCYTOMAG-60K, HCYP2MAG-62K, HCYP3MAG-63K, TGFBMAG-64K-03, and HTH17MAG-14) were used in this study. The minimum detection level of each of the 27 cytokines was: granulocyte colony stimulating factor (G-CSF), 2.72 pg/mL; interferon alpha 2 (IFN-α2), 2.99 pg/mL; interferon gamma (IFN-γ), 0.88 pg/mL; interleukin (IL)-10, 2.42 pg/mL; IL-12p70, 2.43 pg/mL; IL-13, 2.42 pg/mL; IL-17, 2.68 pg/mL; IL-1β, 2.96 pg/mL; IL-2, 1.92 pg/mL; IL-3, 2.86 pg/mL; IL-4, 3.30 pg/mL; IL-5, 2.95 pg/mL; IL-6, 2.53 pg/mL; IL-7, 21.24 pg/mL; IL-8, 2.89 pg/mL; IP-10 (also known as C-X-C motif chemokine ligand 10 (CXCL10)), 3.12 pg/mL; macrophage inflammatory protein 1-alpha (MIP-1α), 3.10 pg/mL; tumor necrosis factor alpha (TNF-α), 2.93 pg/mL; IL-29, 189.3 pg/mL; C-X-C motif chemokine ligand 9 (CXCL9), 45.89 pg/mL; IL-16, 7.68 pg/mL; IL-33, 18.80 pg/mL; IL-21, 10.22 pg/mL; IL-27, 0.01 pg/mL; transforming growth factor beta 3 (TGF-β3), 5.77 pg/mL; transforming growth factor beta 2 (TGF-β2), 9.16 pg/mL.
In the exploratory experiment, we evaluated the levels of the 27 Cytokines in the 10 paired patients with HBeAg-positive CHB at 0, 4, 12, 24, and 48 weeks. In the confirmatory experiment, baseline and week 12 serum levels of eight cytokines (TGF-β2, TGF-β1, IL-16, TNF-α, IP-10, IL-8, IFN-γ, and G-CSF), were determined in the 27 patients. All experiments were carried out following the manufacturer’s instructions.
Statistical Analysis
Statistical analyses were performed using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA). Before analysis, log transformation of skewed laboratory values was performed. Data are shown as the mean ± SD or frequency (%). Pearson correlation, Chi-squared, and Student’s t-tests (or their non-parametric equivalents when appropriate) were used to test the associations between variables. Statistical significance was accepted at P < 0.05.
Results
Characteristics of the Patients
Table 1 and Table 2 summarize the clinical characteristics of the patients in the exploratory experiment and the confirmatory experiment. In the exploratory experiment, 10 paired patients with HBeAg-positive CHB were treated with PEG-IFN monotherapy for 48 weeks: Five achieved virological response and five did not. The dynamic levels of HBV DNA, HBeAg, HBsAg, ALT, AST, and Albumin in the response group and non-response group are shown in Figure 1. There were no significant differences in the clinical characteristics between the two paired groups (P > 0.05). In the confirmatory experiment, among 27 patients treated for 48 weeks with PEG-IFN monotherapy or PEG-IFN and NA combination therapy, 6 patients reached virological response and 21 did not. The clinical characteristics were not significantly different between the two groups (P > 0.05).
 |
Table 1 Baseline Characteristics of the 10 Paired Patients with HBeAg-Positive CHB Receiving 48 Weeks of PEG-IFN Monotherapy |
 |
Table 2 Baseline Characteristics of 27 Patients with HBeAg-Positive CHB Who Received PEG-IFN Monotherapy or PEG-IFN and Nucleos(t)ide Analog Combination Therapy |
The Levels of 27 Cytokines in the 10 Paired Patients with HBeAg-Positive CHB Receiving PEG-IFN Monotherapy
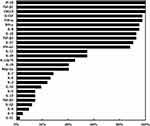
Serum samples of the 10 paired patients with HBeAg-positive CHB treated with PEG-IFN monotherapy were acquired 0, 4, 12, 24, and 48 weeks, and serum cytokine levels were measured. Eight serum samples were lost; therefore, 42 serum samples were processed for Luminex detection. Serum samples with lower than the minimum detection levels for a cytokine were considered to be negative at that time point. The detection rate of a particular cytokine was calculated as the number of samples detected as positive/the total number of samples. The detection rates of the 27 cytokines are shown in Figure 2. Among them, we chose 10 cytokines with high detection rates (high levels) for further study, which included IP-10, TGF-β1, CXCL9, G-CSF, TNF-α, IFN-γ, IL-8, IL-16, TGF-β2, and IL-27.
Comparison of the 10 Cytokines with High Levels in the 10 Paired Patients with HBeAg-Positive CHB and Healthy Controls
We evaluated the differences in the levels of the 10 highly abundant cytokines between the 10 patients with HBeAg-positive CHB and the healthy controls. Among the 10 cytokines, there were significant differences in 6 cytokines between the two groups (IL-27, TGF-β2, IL-16, IFN-γ, CXCL9 and IP-10) (Figure 3). The levels of IP-10, CXCL9, IFN-γ, and IL-27 were higher in the patients than in the healthy controls. The levels of IL-16 and TGF-β2 were lower in patients than in the healthy controls.
The Levels of the 10 Highly Abundant Cytokines in Paired Patients with HBeAg-Positive CHB Receiving 48-Weeks of PEG-IFN Treatment
The levels of the 10 cytokines in the virological responders and non-responders among the 10 patients with HBeAg-positive CHB during PEG-IFN treatment are shown in Figures 4 and 5. PEG-IFN treatment induced the expression of certain cytokines in the virological responders, such as IP-10, IFN-γ, G-CSF, TNF-α, and IL-8, compared with those in the non-responders. The early time points, such as 4, 12, and 24 weeks are potential time points to predict the treatment response.
The Levels of Eight Highly Abundant Cytokines in Virological Responders and Non-Responders at Week 0 and Week 12 in the Two Experiments
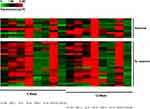
The error rate between the same detection kits of Luminex technology is basically negligible; therefore, we combined the experimental results of 10 patients in the exploratory experiment and 27 patients in the confirmatory experiment together for analysis. A heat map of 8 highly abundant cytokines in the 37 patients at week 0 and week 12 is presented in Figure 6. After 12 weeks of PEG-IFN treatment, IL-16, IP-10, IFN-γ, G-CSF, TNF-α, and IL-8 levels increased in most of the 37 patients. However, the levels of TGF-β1 and TGF-β2 were downregulated. At week 0, there were no significant differences for the eight cytokines between the virological responders and non-responders (P > 0.05). To further explore the difference between the virological responder and non-responder groups, the fold change in cytokine levels at week 12/week 0 was used as a parameter. The results showed that between the two groups, there were no significant differences in the fold changes of the eight cytokines (P > 0.05).
Correlation of the Eight Highly Abundant Cytokines with Patient Clinical Characteristics
We evaluated the correlation between the fold changes of cytokine levels at week 12/week 0 and HBV DNA, HBsAg, HBeAg, and ALT levels. The results showed that there was no association between the fold change of the eight highly abundant cytokines with the decreasing values of HBV DNA, HBsAg, and HBeAg from week 0 to week 12. However, there was a correlation between the fold change of IP-10 week at 12/week 0 and the decreasing level of ALT from week 0 to week 12 (r = 0.2675, P = 0.0024) (Figure 7).
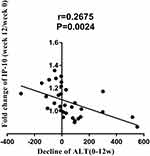 |
Figure 7 The correlation between the fold change in the C-X-C motif chemokine ligand 10 (IP-10) level from week 12 to week 0 and the decreasing aminotransferase (ALT) value week 0 to week 12. |
Discussion
Although PEG-IFN has a wide range of side effects and is administered parenterally for 48 weeks, the advantages of this treatment are its a finite duration of administration, sustained virological response, and its higher rate of HBeAg and HBsAg clearance compared with that of NA monotherapy.13,14 Thus, further exploration of the underlying mechanisms and the identification of response predictors for PEG-IFN treatment are urgently needed. Luminex is a technology developed by Luminex corporation and is based on Luminex xMAP.15,16 Luminex technology has been widely used in pre-clinical and clinical research applications for the past 20 years and has attracted increased research attention in the area of infectious diseases, such as HIV10 and HCV.11 However, the application of Luminex technology in HBV is rare. In this study, this technology was used to explore cytokines that are potentially associated with HBV infection and PEG-IFN treatment, to uncover the mechanisms and predictive factors of the response to PEG-IFN treatment.
Our study enrolled 10 paired patients with HBeAg positive CHB treated with PEG-IFN monotherapy for 48 weeks, among which five were responders and five were non-responders. We assessed the levels of 27 cytokines, including 5 proinflammatory cytokines (IL-1β, IL-8, IL-6, IL-12p70, and IL-7), 4 Th1-related cytokines (IL-2, TNF-α, IFN-α2, and IFN-γ), 3 Th2-related cytokines (IL-4, IL-5, and IL-13), 2 Th17 related cytokines (IL-17 and IL-21), 4 regulatory T cell (Treg)-related cytokines (IL-10, TGF-β1, and TGF-β2, and TGF-β3), 3 chemokines (CXCL9 (MIG), CXCL10 (IP-10), and MIP-1α), and 6 other cytokines (G-CSF, IL-29, IL-16, IL-27, IL-3, and IL-33). The results revealed that 10 cytokines were highly abundant in patient serum: IP-10, TGF-β1, CXCL9, G-CSF, TNF-α, IFN-γ, IL-8, IL-16, TGF-β2, and IL-27. Among them, G-CSF,17 IFN-γ,18 IL-8,19 IP-10,20 CXCL9,21 and IL-1622 were reported to be associated with PEG-IFN therapy, which was confirmed in the present study. However, IL-21,23,24 which has attracted research interest in chronic hepatitis B infection, was hardly detected in the serum samples from our PEG-IFN treatment cohort. This could be because the level of IL-21 in the PEG-IFN treatment cohort was very low or because different technologies were used to detect the IL-21 level in the other studies. During PEG-IFN treatment, IP-10, IFN-γ, G-CSF, TNF-α, and IL-8 levels were higher in the virological responders and at early time points, such as 4, 12, and 24 weeks, which were thus identified potential time points for the early prediction of the treatment response. A previous study showed that pre-treatment and on-treatment IP-10 levels were associated with the treatment response.25 Moreover, Yang et al had reported that IL-8 had a major function in CHB pathogenesis and was related to IFN-α antiviral activity.26 Thus, additional experiments are required to verify these cytokines as predictive factors of the response to PEG-IFN treatment.
In our data set, the levels of IL-16, IP-10, IFN-γ, G-CSF, TNF-α, and IL-8 increased and those of TGF-β1 and TGF-β2 decreased after PEG-IFN treatment for 12 weeks. This indicated that PEG-IFN therapy could induce an early inflammatory cytokine storm with via pro-inflammatory cytokine upregulation and anti-inflammatory cytokine downregulation. We also evaluated the correlation between the cytokine levels and clinical parameters. The results suggested that there were no correlations between the fold changes of the eight highly abundant cytokines and the clinical characteristics HBV DNA, HBsAg, and HBeAg. Interestingly, the IP-10 fold change between week 12/week 0 correlated with the decreasing of ALT levels from 0 to 12 weeks, which indicated that IP-10 might be associated with the extent of liver inflammation. This observation was consistent with the fact that IP-10 could be a potential marker for the response to PEG-IFN therapy.20,25
Conclusion
In conclusion, we identified 10 highly abundant cytokines during treatment with PEG-IFN and observed that early time points might be potential time points to predict the treatment response. After treatment with PEG-IFN for 12 weeks, pro-inflammatory cytokines were upregulated and anti-inflammatory cytokines were downregulated. Moreover, our data identified an association between IP-10 and ALT, indicating the potential role of IP-10 as a serum marker for the PEG-IFN treatment response. Taken together, our study provided novel insights into the responses of patients with CHB to PEG-IFN treatment. This study had limitations. First, the number of patients enrolled in our study for the application of Luminex was small. Thus, we did not find any specific cytokine that could distinguish responders from non-responders. This limitation could be solved by a large cohort study in the future. In addition, the potential underlying mechanism of the cytokines identified this study and their biological functions during HBV infection require further study.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This study was approved by the Ethics Committee of the Second hospital of Yinzhou of Ningbo, Shanghai Public Health Clinical Center, Fudan University and Huashan Hospital, Fudan University. Written informed consent was obtained from all participants. Our study was carried out in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the Chinese Foundation for Hepatitis Prevention and Control-Tianqing Liver Disease Research Fund Subjects (grant number TQGB20140064) and the Second Scientific Project of Yinzhou District of Ningbo (grant number 2014-57).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Fung S, Choi HSJ, Gehring A, Janssen HLA. Getting to HBV cure: the promising paths forward. Hepatology. 2022;76(1):233–250. doi:10.1002/hep.32314
2. Lampertico P, Agarwal K, Berg T. European Association for the Study of the Liver. Electronic address eee, European Association for the study of the L. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021
3. Kramvis A, Chang K-M, Dandri M, et al. A roadmap for serum biomarkers for hepatitis B virus: current status and future outlook. Nat Rev Gastroenterol Hepatol. 2022;19(11):727–745. doi:10.1038/s41575-022-00649-z
4. Wong GLH, Gane E, Lok ASF. How to achieve functional cure of HBV: stopping NUCs, adding interferon or new drug development? J Hepatol. 2022;76(6):1249–1262. doi:10.1016/j.jhep.2021.11.024
5. Chien RN, Liaw YF. Current trend in antiviral therapy for chronic hepatitis B. Viruses. 2022;14(2):434. doi:10.3390/v14020434
6. Ye J, Chen J. Interferon and Hepatitis B: current and future perspectives. Front Immunol. 2021;12:733364. doi:10.3389/fimmu.2021.733364
7. Tseng TC, Kao JH, Chen DS. Peginterferon alpha in the treatment of chronic hepatitis B. Expert Opin Biol Ther. 2014;14(7):995–1006. doi:10.1517/14712598.2014.907784
8. Graham H, Chandler DJ, Dunbar SA. The genesis and evolution of bead-based multiplexing. Methods. 2019;158:2–11. doi:10.1016/j.ymeth.2019.01.007
9. Cui M, Cheng C, Zhang L. High-throughput proteomics: a methodological mini-review. Lab Invest. 2022;102(11):1170–1181. doi:10.1038/s41374-022-00830-7
10. Kong W, Li Y, Cheng S, et al. Luminex xMAP combined with Western blot improves HIV diagnostic sensitivity. J Virol Methods. 2016;227:1–5. doi:10.1016/j.jviromet.2015.10.007
11. Han ZQ, Huang T, Deng YZ, Zhu GZ. Expression profile and kinetics of cytokines and chemokines in patients with chronic hepatitis C. Int J Clin Exp Med. 2015;8(10):17995–18003.
12. Johansson S, Talloen W, Tuefferd M, et al. High MIG (CXCL9) plasma levels favours response to peginterferon and ribavirin in HCV-infected patients regardless of DPP4 activity. Liver Int. 2016;36(3):344–352. doi:10.1111/liv.12932
13. Kao JH. HBeAg-positive chronic hepatitis B: why do I treat my patients with pegylated interferon? Liver Int. 2014;34(Suppl 1):112–119. doi:10.1111/liv.12400
14. Konerman MA, Lok AS. Interferon treatment for hepatitis B. Clin Liver Dis. 2016;20(4):645–665. doi:10.1016/j.cld.2016.06.002
15. Lynch HE, Sanchez AM, D’Souza MP, et al. Development and implementation of a proficiency testing program for Luminex bead-based cytokine assays. J Immunol Methods. 2014;409:62–71. doi:10.1016/j.jim.2014.04.011
16. Purohit S, Sharma A, She JX. Luminex and other multiplex high throughput technologies for the identification of, and host response to, environmental triggers of type 1 diabetes. Biomed Res Int. 2015;2015:326918. doi:10.1155/2015/326918
17. Ding Y, Zhang H, Chen H, et al. Association of immune response parameters with virological response in hepatitis C virus patients treated with pegylated consensus interferon. Int J Clin Pharmacol Ther. 2016;54(3):163–171. doi:10.5414/CP202439
18. Lu MY, Huang CI, Dai CY, et al. Elevated on-treatment levels of serum IFN-gamma is associated with treatment failure of peginterferon plus ribavirin therapy for chronic hepatitis C. Sci Rep. 2016;6:22995. doi:10.1038/srep22995
19. Mihm U, Herrmann E, Sarrazin U, et al. Association of serum interleukin-8 with virologic response to antiviral therapy in patients with chronic hepatitis C. J Hepatol. 2004;40(5):845–852. doi:10.1016/j.jhep.2004.01.007
20. Fu WK, Cao J, Mi NN, et al. Cytokines predict virological response in chronic hepatitis B patients receiving peginterferon alfa-2a therapy. World J Clin Cases. 2020;8(11):2255–2265. doi:10.12998/wjcc.v8.i11.2255
21. Lee IC, Huang YH, Su CW, et al. CXCL9 associated with sustained virological response in chronic hepatitis B patients receiving peginterferon alfa-2a therapy: a pilot study. PLoS One. 2013;8(10):e76798. doi:10.1371/journal.pone.0076798
22. Hou J, van Oord G, Groothuismink ZM, et al. Gene expression profiling to predict and assess the consequences of therapy-induced virus eradication in chronic hepatitis C virus infection. J Virol. 2014;88(21):12254–12264. doi:10.1128/JVI.00775-14
23. Ma SW, Huang X, Li YY, et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol. 2012;56(4):775–781. doi:10.1016/j.jhep.2011.10.020
24. Li Y, Ma S, Tang L, et al. Circulating chemokine (C-X-C Motif) receptor 5(+) CD4(+) T cells benefit hepatitis B e antigen seroconversion through IL-21 in patients with chronic hepatitis B virus infection. Hepatology. 2013;58(4):1277–1286. doi:10.1002/hep.26489
25. Wang Y, Zhao C, Zhang L, et al. Predictive value of interferon-gamma inducible protein 10 kD for hepatitis B e antigen clearance and hepatitis B surface antigen decline during pegylated interferon alpha therapy in chronic hepatitis B patients. Antiviral Res. 2014;103:51–59. doi:10.1016/j.antiviral.2014.01.001
26. Yang K, Guan SH, Zhang H, et al. Enhanced levels of interleukin-8 are associated with hepatitis B virus infection and resistance to interferon-alpha therapy. Int J Mol Sci. 2014;15(11):21286–21298. doi:10.3390/ijms151121286
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.