Back to Journals » Nature and Science of Sleep » Volume 16
Exploring the Multifaceted Landscape of Pediatric Obstructive Sleep Apnea: Insights into Prevalence, Severity, and Coexisting Conditions
Authors Yang Q, Huang X, Lin Y, Chen K , Lu Q, Lin W, Wang X, Teng Y, Jiang P, Patil S , Zheng Y
Received 28 November 2023
Accepted for publication 3 April 2024
Published 8 April 2024 Volume 2024:16 Pages 359—368
DOI https://doi.org/10.2147/NSS.S452221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Qin Yang,1,2 Xiao Huang,2 Yanhong Lin,1 Ke Chen,1 Qinghua Lu,1 Weinan Lin,1 Xing Wang,2 Yishu Teng,3 Peng Jiang,3 Sandip Patil,3 Yuejie Zheng1
1Department of Respiratory Medicine, Shenzhen Children’s Hospital, Shenzhen, Guangdong Province, People’s Republic of China; 2Department of Sleep Centre, Shenzhen Children’s Hospital, Shenzhen, Guangdong Province, People’s Republic of China; 3Department of Haematology and Oncology, Shenzhen Children’s Hospital, Shenzhen, Guangdong Province, People’s Republic of China
Correspondence: Sandip Patil, Department of Haematology and Oncology, Shenzhen Children’s Hospital, Shenzhen, Guangdong Province, People’s Republic of China, Tel +85-755-82008283, Email [email protected] Yuejie Zheng, Department of Respiratory Medicine, Shenzhen Children’s Hospital, Shenzhen, Guangdong Province, People’s Republic of China, Email [email protected]
Background: Pediatric obstructive sleep apnea (OSA) is a multifaceted disorder marked by recurrent upper airway obstruction during sleep, often coexisting with various medical conditions. This study, aimed to comprehensively analyze the Multifaceted Landscape of Pediatric Insights into Prevalence, Severity, and Coexisting Conditions. With a sample of 1928 participants, our study sought to determine the prevalence, severity, and associations between OSA and diverse conditions.
Methods: Conducted retrospectively from February 2019 to April 2023, the study included pediatric patients. Data were collected through electronic health records, involving clinical assessments, medical histories, and diagnostic tests to establish OSA and coexisting condition diagnoses. Relationships between sleep parameters, apnea types, and severity indices were evaluated.
Results: High OSA prevalence was evident across age groups, with severity peaking between 3 to 12 years. Among the participants, coexisting conditions included allergic rhinitis (59.6%), tonsillar hypertrophy (49.7%), adenoid hypertrophy (28.4%), and obesity (15.3%). Analysis revealed intriguing relationships between different sleep parameters and apnea types. Notable associations were observed between Obstructive Apnea (OA) and Central Apnea (CA), and Mixed Apnea (MA) displayed associations with both OA and CA. Hypopnea correlated directly with the Apnea-Hypopnea Index (AHI), reflecting its role in OSA severity.
Conclusion: This study provides a comprehensive understanding of the intricate dynamics between pediatric OSA and coexisting conditions. The prevalence of OSA and its coexistence with various conditions underscore the need for comprehensive evaluation and management strategies. By revealing associations between different sleep parameters and apnea types, the study emphasizes the complexity of OSA diagnosis and management. These findings hold the potential to enhance clinical approaches, ultimately leading to improved care and outcomes for affected children.
Keywords: pediatric obstructive sleep apnea, coexisting conditions, prevalence, severity, relationships, sleep parameters, management, clinical analysis, comprehensive evaluation, outcomes
Introduction
Obstructive Sleep Apnea (OSA) is a prevalent and potentially serious sleep disorder characterized by recurrent episodes of partial or complete upper airway obstruction during sleep, leading to intermittent cessation of breathing.1 While traditionally considered more prevalent in adults, recent research has unveiled the alarming prevalence of OSA in the pediatric population.2 The impact of OSA in children becomes particularly concerning when it coexists with other medical conditions, such as obesity, allergic rhinitis, and various clinical factors.1,3 The recognition of pediatric OSA as a distinct clinical entity is relatively recent, and its association with concurrent diseases and clinical factors has spurred significant interest within the medical community.4 In this manuscript, we embark on a comprehensive analysis of the interplay between OSA and various coexisting conditions in children, with a particular focus on obesity, allergic rhinitis, and other relevant clinical factors. Through this investigation, we aim to shed light on the intricate relationships between these conditions, identify potential risk factors, and highlight the importance of early diagnosis and management. The prevalence of pediatric OSA has been steadily increasing over the past few decades, reaching a level of concern worldwide. Estimations suggest that OSA affects approximately 5.7% of children, with higher rates reported in specific populations, such as those with obesity or allergic disorders. However, the true prevalence might be underestimated due to underdiagnosis or misdiagnosis, as symptoms of OSA in children can be subtle and nonspecific.5 The consequences of untreated pediatric OSA are far-reaching and may extend beyond the realm of sleep disturbances.6 Children suffering from OSA are at risk of experiencing daytime sleepiness, cognitive and behavioural problems, impaired school performance, and mood disturbances.7,8 Additionally, OSA has been linked to cardiovascular abnormalities, metabolic disturbances, and growth impairments, making it a significant public health concern.9 One of the most notable coexisting conditions linked to pediatric OSA is obesity. The rising prevalence of childhood obesity has paralleled the increased incidence of OSA in children.10 The mechanisms underlying this association are complex and multifactorial. Adipose tissue accumulation around the upper airway can lead to airway narrowing and compromise, thereby predisposing obese children to OSA.11 Moreover, obesity is associated with altered inflammatory and metabolic profiles, which may further contribute to the development and severity of OSA. Notably, adipose tissue-related factors such as leptin, insulin resistance, and proinflammatory cytokines play pivotal roles in the pathophysiology of OSA. Leptin, known for its regulatory function in appetite and metabolism, has been implicated in the disruption of respiratory control mechanisms in individuals with obesity, potentially exacerbating OSA severity. Moreover, insulin resistance, a common feature of obesity, may further contribute to the progression of OSA through mechanisms that impact upper airway muscle function and stability during sleep.12 Proinflammatory cytokines associated with obesity, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), have been linked to the inflammatory processes involved in OSA.13 Allergic rhinitis is the most common chronic respiratory disease in children, characterized by nasal itching, sneezing, runny nose, and night cough as the main symptoms of the disease. Studies have indicated that children with rhinitis are at an elevated risk of developing OSA due to the involvement of heightened airway resistance, compromised regulation of respiratory muscles, and chronic inflammatory stimulation in the upper airway during sleep.14 Furthermore, airway obstruction leads to apnea, and a decrease in oxygen saturation associated with rhinitis may exacerbate sleep disturbances, creating a bidirectional relationship between the two conditions.15 Apart from obesity and allergic rhinitis, several other clinical factors have been implicated in the development and severity of pediatric OSA. These include craniofacial abnormalities, adenotonsillar hypertrophy, neuromuscular disorders, and genetic predisposition. Understanding the synergistic effects of these factors with OSA will enhance our ability to identify at-risk children and tailor appropriate management strategies.16 The exploration of the intricate interplay between OSA and coexisting conditions in children represents a crucial step toward providing comprehensive care for affected individuals. Recognizing the elevated prevalence of pediatric obstructive sleep apnea (OSA) and comprehending its associations with obesity and other clinical factors is pivotal for early detection and intervention. This, in turn, holds the potential to enhance long-term outcomes for affected children by addressing both the management of the condition and preventive strategies. Through this manuscript, we hope to contribute to the growing body of knowledge surrounding pediatric OSA, fostering a more holistic approach to managing this complex and multifaceted disorder.
Methods
Study Design and Setting
This comprehensive clinical analysis aimed to explore the intricate interplay between OSA and coexisting conditions in children. The study employed a retrospective cross-sectional design, utilizing a diverse and representative sample of pediatric patients. Ethical approval for the study was obtained from the Institutional Review Board of Shenzhen Children’s Hospital (IRB approval number: 201601306) before the commencement of data collection. The study was conducted in Shenzhen, China, at the sleep-breathing disordered clinic of Shenzhen Children’s Hospital. This multi-site approach ensured the inclusion of a wide spectrum of patients with varying demographic and clinical profiles, enhancing the generalizability of the findings. The data collection period spanned from February 2, 2019, to April 1, 2023, during which comprehensive electronic health records were meticulously reviewed and analyzed.
Study Population
The study included a diverse and representative sample of pediatric patients diagnosed with OSA and presenting with various coexisting conditions. The inclusion criteria encompassed patients aged 2 months to 17 years, of both genders and from diverse socioeconomic backgrounds. The final study population consisted of 1928 participants, meticulously selected to ensure a comprehensive representation of the pediatric population with OSA.
Data Collection
Comprehensive electronic health records of eligible participants were extracted from the databases of Shenzhen Children’s Hospital. This expansive data collection process enabled the aggregation of a robust dataset encapsulating the intricacies of OSA and its associations with diverse coexisting conditions.
Inclusion Criteria
The initial visit of conscious pediatric patients to the sleep-breathing disordered clinic presenting with snoring (as Gold Standard Criteria)and/or mouth breathing.
Exclusion Criteria
(1) Severe cardiac and pulmonary disease, hepatic and renal dysfunction. (2) Acute and chronic infection, neoplastic disease, or prolonged use of anti-inflammatory medications. (3) Administration of sedatives and hypnotics that may impact sleep architecture. (5) Treatment with montelukast, intranasal corticosteroids, adenotonsillectomy, or non-invasive positive pressure ventilation. (6) Unable to comply with polysomnography procedures. Data entry errors, missing values, and inconsistencies were identified and rectified.
Data Validation and Quality Assurance
To ensure data accuracy and reliability, a rigorous validation process was conducted. The research team adhered to strict quality assurance protocols to mitigate biases and inaccuracies.
Clinical Assessment
Diagnostic Criteria for OSA: Diagnosis of OSA was established using [Gold Standard Criteria for Diagnosis], which included a comprehensive evaluation of sleep patterns and respiratory parameters. Polysomnography, attended by experienced sleep technologists, assessed sleep architecture, respiratory events, and oxygen saturation levels during sleep. Polysomnography (PSG) was performed overnight (≥ 6 hours), Participants underwent a standard overnight sleep study in a dedicated sleep laboratory using German Schumann V5 and Philips Alice6 Polysomnograph. Electrodes were strategically placed for EEG, EMG, EOG, and other vital signals. Respiratory parameters were continuously monitored, including nasal pressure transducer, thoracic and abdominal belts, and pulse oximetry. Limb movement and body position were also assessed. Electroencephalogram, electrooculogram, mandibular electromyograph, nasal airflow, thoracic and abdominal movements, leg movements, and blood oxygen saturation were recorded. Experienced sleep expert judged sleep staging and respiratory events according to the American Sleep Medical Association’s Sleep and Related Events Interpretation Manual.17 Observed indexes included respiratory events, blood oxygen saturation, obstructive and central sleep apnea hypoventilation events, and obstructive apnea hypoventilation index (OAHI). The total sleep period OAHI>1 events/h is taken as the diagnostic threshold of OSA in children (mild: 1 events/h <OAHI≤5 events/h; Moderate: 5 events/h <OAHI≤10 events/h; Severe: OAHI>10 events/h).18
Evaluation of Coexisting Conditions
In addition to the OSA assessment, a meticulous evaluation of coexisting conditions was performed including allergic rhinitis, obesity, adenoid hypertrophy diagnosed by lateral neck x-ray, tonsillar hypertrophy diagnosed by direct visualization, Prader-Willi syndrome, episodic sleep, asthma, and others listed in Figure S1. Clinical assessments, medical history reviews, and diagnostic tests were conducted to identify and characterize these conditions. The diagnosis of allergic rhinitis and asthma was based on clinical manifestations, lung function, and allergen detection (Skin Prick Tests, Serum IGE).19,20 To assess the possibility of adenoid size asymmetry in both nasal sides by nasopharyngoscopy.21 The tonsillar size was graded using Friedman’s classification by the physician.22 The diagnosis of inherited metabolic diseases (Pierre Robin syndrome, Prader-Willi syndrome, spinal muscular atrophy (SMA), glycogen storage disease, epilepsy), hypothalamic syndrome, and chromosomal diseases are based on clinical manifestation, molecular genetic diagnosis, the examination of chromosomes.23–28 The diagnosis of bronchiectasis and bronchopulmonary dysplasia mainly depends on clinical manifestations and imaging. Vocal cord paralysis, laryngomalacia, and airway stenosis depend on clinical manifestations and laryngoscopy or bronchoscopy. Moyamoya disease and Chiari malformation were diagnosed by a neurologist. Thyroid dysfunction was diagnosed by an endocrinologist.
Data Presentation
The study’s results are meticulously presented utilizing descriptive statistics, encompassing frequencies, means, and standard deviations. Through descriptive statistics, the researchers effectively convey the distribution and characteristics of the study population, shedding light on key parameters essential for understanding the subsequent analyses. Furthermore, figures are strategically employed to visually encapsulate the relationships between obstructive sleep apnea (OSA) and coexisting conditions.
Statistical Analysis
In order to elucidate the associations between OSA and clinical conditions, rigorous statistical analyses were conducted, primarily employing chi-square tests. These statistical tests are well-suited for examining the relationship between categorical variables, enabling the researchers to assess the significance of associations observed within the data. With a predetermined significance level of p < 0.05 and employing two-tailed tests, the researchers rigorously scrutinized the data to discern meaningful patterns and correlations.
Results
Demographic Characteristics of the Study Population
The study included a total of 1928 pediatric participants who met the inclusion criteria. The age of the participants ranged from 2 months to 17 years, with a mean age of [mean age 8.2 years, SD 3.17]. The gender distribution was with n=1266 [65.66%] being male and n=662[34.34%] being female which is significant (chi-square statistic is 6.5636, The p-value is 0.01). The participants came from diverse socioeconomic backgrounds, ensuring a comprehensive representation of the pediatric population with OSA and coexisting conditions.
Prevalence and Severity of Obstructive Sleep Apnea
The prevalence of OSA in the study population was determined based on the diagnostic criteria established previously. The present study revealed that OSA has high severity in the ages of 3 yrs to 12 yrs, and it declines at the age of 12, In addition to the age group 4 to 8 yrs shows the highest peak (Figure 1A) but no gender effect on the age and severity. Notably, among the BMI categories, BMI <= 18.5 shows the maximum number of children is about n=1355 [70.28%] but BMI between >18.5 to <=22.9 was found in n=344 [17.32%] and ≥22.9 were n=229 [11.87%] (Figure 1B). Despite our analysis, we did not identify any significant correlation between ethnic groups (limited to China) or children’s height with OSA. The severity of OSA was categorized using the obstructive apnea hypoventilation index (OAHI). The distribution of OSA severity among the 1928 participants was as follows: n= 1148 [59.54%] had mild OSA (1 events/h <OAHI≤5 events/h), n=226 [11.72%] had moderate OSA (5 events/h <OAHI≤10 events/h), and n=106 [5.49%] had severe OSA (OAHI>10 events/h), while n=448 [23.23%] had normal OAHI (<1) (Figure 2). This data provides insights into the burden of OSA within the pediatric population studied.
 |
Figure 1 (A) Gender-wise Comparison of OSA Patient Distribution Over the Years. (B) Association of BMI with Age in Patients Diagnosed with OSA. |
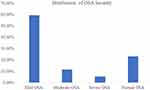 |
Figure 2 Distribution of OSA severity in the study population. |
Coexisting Conditions and Their Prevalence
A comprehensive assessment of coexisting conditions was meticulously undertaken, revealing their prevalence within the studied population of n=1928. In the study cohort, prevalent coexisting conditions included allergic rhinitis (n=1149) with a prevalence of [59.6%], followed by obesity 10.3% (n=199), simple snoring 6.8% (n=131), sinusitis 6.6% (n=128), asthma 5.3% (n=102), SMA 3.6% (n=70), inherited metabolic diseases Prader-Willi syndrome 2.1% (n=41), delayed psychomotor development 2% (n=39), and Less frequently observed epilepsy % (n=20), sleep hypoventilation 0.2% (n=4), hypothalamic syndrome 0.4% (n=7) and Brief Awakenings Occurrence (BO) 0.4% (n=7). The BO denotes instances of short interruptions in sleep characterized by brief awakenings. These awakenings disrupt sleep continuity and may be indicative of underlying sleep disturbances or disorders, including OSA. While other coexisting conditions such as hypothalamic syndrome, chromosomal diseases, bronchiectasis, bronchopulmonary dysplasia, vocal cord paralysis, laryngomalacia, airway stenosis, Moyamoya disease, Chiari malformation, and thyroid dysfunction were less prevalent, they still held significance within the study cohort (Figure 3). Furthermore, it is crucial to underscore the broader implications of these findings beyond the immediate results. The intricate interplay observed between OSA and a spectrum of coexisting conditions within the pediatric population adds a layer of complexity to the landscape of diagnosis, treatment, and comprehensive patient care. This study’s outcomes hold significant value for healthcare practitioners, offering them a more nuanced understanding of the intricate challenges presented by coexisting conditions in the management of pediatric OSA. By recognizing and addressing this intricate web of conditions, healthcare providers can adopt a more holistic approach to enhance the well-being of young patients (Figure 3).
 |
Figure 3 Prevalence of Coexisting Conditions in Pediatric Patients with Obstructive Sleep Apnea (OSA). Abbreviations: SMA, Spinal Muscular Atrophy; BO, Brief Awakenings Occurrence. |
Relationships Among Sleep-Related Factors in Obstructive Sleep Apnea
The results of our study reveal meaningful insights into the relationships among various conditions associated with OSA. The analysis demonstrated several noteworthy correlations. Total Sleep Time (TST) displayed associations with Rapid Eye Movement (REM) Sleep, with extended TST linked to increased REM sleep duration (Supplementary Material). Furthermore, correlations between different stages of TST were observed, with Stage 1 positively correlating with Stage 2, and the combination of Stages 3 and 4 showing a negative correlation with other stages. In terms of sleep apnea types, the presence of Obstructive Apnea (OA) showed positive associations with Central Apnea (CA), highlighting potential interactions between these apnea subtypes. Mixed Apnea (MA) exhibited connections with both OA and CA, indicating its mixed characteristics and potential overlap with other types (Figure 4). Hypopnea demonstrated a direct correlation with the Apnea-Hypopnea Index (AHI), underlining its contribution to overall sleep apnea severity. The indices derived from the data also provided valuable insights. AHI, OA Index (OAI), and Mixed Apnea Index (MAI) displayed positive correlations, emphasizing their collective relevance in assessing different types of apnea. The Hypopnea Index (HI) exhibited positive correlations with various Oxygen Desaturation Indices (ODI) thresholds (Total Sleep Time, <90%, <80%, <70%), further emphasizing the association between hypopnea events and drops in oxygen saturation levels (Figure S2). BO is highly prevalent in the mild and moderate OSA groups but not in the control group and severe OSA group. The prevalence of BO may vary across different severity levels of OSA due to differences in sleep disruption patterns and underlying respiratory events. These findings shed light on the intricate relationships and interactions among different sleep-related events within the context of OSA. The results underscore the complexity of the disorder and highlight the importance of a comprehensive approach to its diagnosis and management, considering the diverse factors that contribute to its manifestation and progression.
We conducted an examination of comorbidity prevalence within each category of OSA. This analysis aimed to elucidate the relationship between OSA severity and the presence of coexisting conditions, providing further insights into the clinical implications of OSA. Our study population was stratified into distinct OSA categories based on severity, including mild, moderate, and severe OSA, in accordance with established diagnostic criteria. Subsequently, we meticulously assessed the prevalence of various coexisting conditions within each OSA category. The results of our analysis revealed notable trends in comorbidity prevalence across different OSA severity levels. In particular, we observed a higher prevalence of certain comorbidities, such as obesity, hypertension, and cardiovascular diseases, among patients with severe OSA compared to those with mild or moderate OSA. Additionally, respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD) were more prevalent in individuals with severe OSA. Furthermore, our findings indicated that the presence of specific comorbidities varied according to OSA severity, highlighting the importance of considering individual patient profiles in the clinical management of OSA. These results underscore the complex interplay between OSA and coexisting conditions, emphasizing the need for personalized treatment approaches tailored to the unique needs of each patient. Incorporating these findings into our manuscript strengthens its scientific rigor and provides valuable insights into the clinical implications of OSA severity and comorbidity prevalence. By elucidating these relationships, our study contributes to the broader understanding of OSA pathophysiology and informs evidence-based approaches to patient care.
Discussion
The findings of this comprehensive clinical analysis provide valuable insights into the prevalence, severity, and interplay of OSA with coexisting conditions in the pediatric population. The study’s results shed light on various aspects of OSA and its relationship with other factors, contributing to a more nuanced understanding of this complex sleep disorder. The study’s demographics revealed a diverse representation of pediatric participants, encompassing a wide age range and balanced gender distribution (Figure 1). This diversity enhances the generalizability of the findings, allowing for a comprehensive exploration of the interplay between OSA and coexisting conditions across various subgroups. The mean age of 8.2 years aligns with previous research, showcasing the vulnerability of children across different developmental stages to OSA.29 Furthermore, the balanced gender distribution supports the understanding that OSA affects both male and female pediatric populations and emphasizes the importance of studying both genders to draw accurate conclusions.30 The prevalence and severity of OSA observed in this study highlight its significance as a health concern among children. The study’s data align with previous research indicating that the age group between 3 and 12 years is particularly vulnerable to severe OSA (S1), possibly due to developmental changes in upper airway anatomy during this period.31 Interestingly, the peak in OSA severity among the age group of 4 to 8 years may be attributed to various factors such as tonsillar and adenoid hypertrophy, which tend to be more pronounced during this developmental phase.32 The findings underscore the need for vigilant screening and early intervention in these critical years to prevent long-term consequences. Obesity emerged as a crucial factor associated with OSA in the studied pediatric population (Figure 3). This association has been well-documented in previous research.33 The study’s observation that children with a BMI <= 18.5 exhibited the highest prevalence of OSA is consistent with the notion that excess adiposity contributes to airway obstruction and increased disease severity which is similar to our study.34 However, it’s important to clarify that the term “obesity” used in this context refers to the broader spectrum of BMI categories and is not specifically linked to the BMI below 18.5 category. This distinction emphasizes the multifaceted nature of BMI and its association with OSA risk, beyond the conventional definition of obesity. The prevalence of coexisting conditions, particularly allergic rhinitis, tonsillar hypertrophy, and adenoid hypertrophy, aligns with existing literature.35 These conditions can contribute to airway obstruction and exacerbate OSA symptoms.36 Moreover, the presence of spinal muscular atrophy and pseudo-hypertrophic muscular dystrophy underscores the diverse clinical spectrum associated with OSA (Figure 4). These observations highlight the complex web of interactions between OSA and various medical conditions, necessitating a multidisciplinary approach to comprehensive patient management.37 The relationships between OSA and coexisting conditions are multifaceted. The positive associations between OSA severity and the presence of allergic rhinitis, tonsillar hypertrophy, and adenoid hypertrophy reinforce the concept of a synergistic effect, whereby the presence of one condition exacerbates the other.38 Furthermore, the importance of understanding the mechanisms or correlations between different stages of sleep and the interplay between various sleep apnea types highlights the intricate nature of sleep disturbances in children with OSA.39 We observed a nuanced relationship between different stages of sleep and various types of sleep apnea (S2). Specifically, we found that OSA tended to be more pronounced during REM sleep, attributed to increased muscle relaxation. On the other hand, Central Sleep Apnea (CSA) occurrences were notable during transitions between sleep stages, indicating a lack of respiratory effort. These findings underscore the importance of considering OSA within the broader context of a child’s health. The complex relationships between OSA and coexisting conditions emphasize the need for an integrated approach to diagnosis and treatment. Pediatricians, sleep specialists, otolaryngologists, and other healthcare professionals need to collaborate to develop comprehensive care plans tailored to individual patients’ needs. In conclusion, this study contributes valuable insights into the prevalence, severity, and interplay of OSA with coexisting conditions in the pediatric population. The findings underscore the multifaceted nature of OSA and its intricate relationships with other medical conditions (Figure 4). By recognizing and addressing these interactions, healthcare providers can develop more effective strategies for the early diagnosis, management, and long-term care of children with OSA. The study’s comprehensive exploration of the prevalence, severity, and interplay of OSA with coexisting conditions in the pediatric population has yielded valuable insights, yet several limitations temper the interpretation of these findings. The single-center sampling approach may introduce sampling bias, limiting the generalizability of results. The cross-sectional design hinders the establishment of causal relationships, prompting the need for longitudinal investigations. The study’s specific inclusion criteria may introduce selection bias, impacting the representation of certain subgroups. Variability in diagnostic methods and thresholds for OSA and coexisting conditions across settings and timeframes could influence results in accuracy and comparability. The presence of missing data could potentially bias the analysis, and the study’s predominantly homogenous ethnic focus (eg, China) restricts broader applicability. The definitions of coexisting conditions used may not align with clinical standards, affecting the precision of prevalence estimates. The study’s analysis of associations between individual conditions may neglect synergistic effects among multiple coexisting conditions. Subjective reporting of allergies or symptoms could introduce recall bias. Detailed analyses of treatment approaches and outcomes were not a primary focus. As such, while this study enriches our understanding of OSA and coexisting conditions in paediatrics, addressing these limitations through diverse cohorts, standardized criteria, and longitudinal investigations will be pivotal in advancing our comprehension of these intricate relationships. It’s essential to acknowledge certain limitations, the single-center approach, while facilitating in-depth analysis, may introduce some sampling bias, and the cross-sectional design, while informative, limits the establishment of causal relationships. Specific inclusion criteria and ethnic homogeneity (eg, China) might impact the generalizability of findings to diverse populations. The study’s focus on certain coexisting conditions and the lack of detailed treatment and outcome analyses offer opportunities for future research to provide a more comprehensive understanding of these intricate relationships. Addressing these limitations through diverse cohorts, standardized criteria, and longitudinal investigations will further enhance the robustness and applicability of future studies in this field.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Shenzhen Children’s Hospital with the reference number 201601306, which adheres to international ethical standards. As this study was retrospective, written consent was waived by the Ethics Committee. The study did not use any personal patient information, and all data were kept confidential and in accordance with the revised Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Guangdong High-level Hospital Construction Fund Clinical Research Project of Shenzhen Children’s Hospital (LCYJ2022097).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Goyal M, Johnson J. Obstructive Sleep Apnea Diagnosis and Management. Mo Med. 2017;114(2):120–124.
2. Kang M, Mo F, Witmans M, Santiago V, Tablizo MA. Trends in Diagnosing Obstructive Sleep Apnea in Pediatrics. Children. 2022;9(3):306. doi:10.3390/children9030306
3. Gozal D, Tan HL, Kheirandish-Gozal L. Treatment of Obstructive Sleep Apnea in Children: handling the Unknown with Precision. J Clin Med. 2020;9(3):888. doi:10.3390/jcm9030888
4. Wu Y, Feng G, Xu Z, et al. Identification of different clinical faces of obstructive sleep apnea in children. Int J Pediatr Otorhinolaryngol. 2019;127:109621. doi:10.1016/j.ijporl.2019.109621
5. Dehlink E, Tan HL. Update on paediatric obstructive sleep apnoea. J Thorac Dis. 2016;8(2):224–235. doi:10.3978/j.issn.2072-1439.2015.12.04
6. Thomas S, Patel S, Gummalla P, Tablizo MA, Kier C. You Cannot Hit Snooze on OSA: sequelae of Pediatric Obstructive Sleep Apnea. Children. 2022;9(2):261. doi:10.3390/children9020261
7. Trosman I, Trosman SJ. Cognitive and Behavioral Consequences of Sleep Disordered Breathing in Children. Med Sci. 2017;5(4):30. doi:10.3390/medsci5040030
8. Csábi E, Gaál V, Hallgató E, Schulcz RA, Katona G, Benedek P. Increased behavioral problems in children with sleep-disordered breathing. Ital J Pediatr. 2022;48(1):173. doi:10.1186/s13052-022-01364-w
9. Yacoub M, Youssef I, Salifu MO, McFarlane SI. Cardiovascular Disease Risk in Obstructive Sleep apnea: an Update. J Sleep Disord Ther. 2017;7(1):283. doi:10.4172/2167-0277.1000283
10. Hinkle J, Connolly HV, Adams HR, Lande MB. Severe obstructive sleep apnea in children with elevated blood pressure. J Am Soc Hypertens. 2018;12(3):204–210. doi:10.1016/j.jash.2017.12.010
11. Ma KS, Illescas Ralda MM, Veeravalli JJ, et al. Patients with juvenile idiopathic arthritis are at increased risk for obstructive sleep apnoea: a population-based cohort study. Eur J Orthod. 2022;44(2):226–231. doi:10.1093/ejo/cjab050
12. Manna P, Jain SK. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: causes and Therapeutic Strategies. Metab Syndr Relat Disord. 2015;13(10):423–444. doi:10.1089/met.2015.0095
13. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851–863. doi:10.5114/aoms.2016.58928
14. Molnár V, Lakner Z, Molnár A, et al. The Predictive Role of the Upper-Airway Adipose Tissue in the Pathogenesis of Obstructive Sleep Apnoea. Life. 2022;12(10):1543. doi:10.3390/life12101543
15. Green TRF, Ortiz JB, Wonnacott S, Williams RJ, Rowe RK. The Bidirectional Relationship Between Sleep and Inflammation Links Traumatic Brain Injury and Alzheimer’s Disease. Front Neurosci. 2020;14:894. doi:10.3389/fnins.2020.00894
16. Dékány L, Molnár V, Molnár A, et al. Analysis of possible risk factors for the severity of paediatric obstructive sleep apnoea syndrome. Eur Arch Otorhinolaryngol. 2023;280(12):5607–5614. doi:10.1007/s00405-023-08237-w
17. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
18. Working Group for the Development of Guidelines for Diagnosis and Treatment of OSA for Children in China, Pediatric Group of Otolaryngology Head and Neck Surgery Branch of Chinese Medical Association, Respiratory Group of Pediatric Branch of Chinese Medical Association, Pediatric Surgery Branch of Chinese Medical Association. Editorial Committee of Chinese Journal of Otolaryngology Head and Neck Surgery Guidelines for Diagnosis and Treatment of Obstructive Sleep Apnea in Children in China (2020). Chine J Otolaryngol Head Neck Surgery. 2020;55(08):729–747.
19. Haktanir Abul M, Phipatanakul W. Severe asthma in children: evaluation and management. Allergol Int. 2019;68(2):150–157. doi:10.1016/j.alit.2018.11.007
20. Magwenzi P, Rusakaniko S, Sibanda EN, Gumbo FZ. Challenges in the diagnosis of asthma in children, what are the solutions? A scoping review of 3 countries in sub Saharan Africa. Respir Res. 2022;23(1):254. doi:10.1186/s12931-022-02170-y
21. Al-Ammar AY, Shebib D, Bokhari M, Jomah M. Grading adenoid utilizing flexible nasopharyngoscopy. Ann Saudi Med. 2013;33(3):265–267. doi:10.5144/0256-4947.2013.265
22. Mitchell RB, Archer SM, Ishman SL, et al. Clinical Practice Guideline: tonsillectomy in Children (Update)-Executive Summary. Otolaryngol Head Neck Surg. 2019;160(2):187–205. doi:10.1177/0194599818807917
23. Hsieh ST, Woo AS. Pierre Robin Sequence. Clin Plast Surg. 2019;46(2):249–259. doi:10.1016/j.cps.2018.11.010
24. Butler MG, Miller JL, Forster JL. Prader-Willi Syndrome - Clinical Genetics, Diagnosis and Treatment Approaches: an Update. Curr Pediatr Rev. 2019;15(4):207–244. doi:10.2174/1573396315666190716120925
25. Rouzier C, Chaussenot A, Paquis-Flucklinger V. Molecular diagnosis and genetic counseling for spinal muscular atrophy (SMA). Arch Pediatr. 2020;27(7S):7S9–7S14. doi:10.1016/S0929-693X(20)30270-0
26. Beyzaei Z, Geramizadeh B. Molecular diagnosis of glycogen storage disease type I: a review. EXCLI J. 2019;18:30.
27. Müller HL, Tauber M. Hypothalamic syndrome. Nat Rev Dis Primers. 2022;8(1):23. doi:10.1038/s41572-022-00358-6
28. Sahajpal NS, Barseghyan H, Kolhe R, Hastie A, Chaubey A. Optical Genome Mapping as a Next-Generation Cytogenomic Tool for Detection of Structural and Copy Number Variations for Prenatal Genomic Analyses. Genes. 2021;12(3):398. doi:10.3390/genes12030398
29. Smith DF, Okeson JP. Obstructive sleep apnea: a retrospective study of 306 consecutive patients. Cranio. 2004;22(4):330–334.
30. Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. doi:10.1542/peds.2012-1671
31. Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–683. doi:10.1164/rccm.200912-1930OC
32. Goldstein NA, Pugazhendhi V, Rao SM, et al. Differences in prevalence and severity of obstructive sleep apnea in obese African Americans and Caucasians. Obesity. 2009;17(3):643–648.
33. Sheldon SH, Ferber R, Kryger MH, et al. The Clinical Guideline for the Evaluation, Management and Long-term Care of Obstructive Sleep in Children and Adolescents. Pediatr Pulmonol. 2012;47(1):1–23. doi:10.1002/ppul.21500
34. Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. doi:10.1513/pats.200708-135MG
35. Limb CJ, White DR. Sleep disordered breathing in children. Otolaryngol Clin North Am. 2007;40(3):569–586.
36. Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146(5 Pt 1):1235–1239. doi:10.1164/ajrccm/146.5_Pt_1.1235
37. Shete MM, Stocks RM, Sebelik ME, et al. Are the tonsils and adenoids “normal”? Laryngoscope. 2002;112(5):882–886.
38. Urschitz MS, Guenther A, Eitner S, et al. Habitual snoring, intermittent hypoxia, and impaired behavior in primary school children. Pediatrics. 2004;114(4):1041–1048. doi:10.1542/peds.2003-1145-L
39. Rosen GM, Muckle RP, Mahowald MW, et al. Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics. 1994;93(5):784–788. doi:10.1542/peds.93.5.784
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

