Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Exploring the Diagnostic Value of High-Frequency Ultrasound Technology for Subcutaneous Lipohypertrophy in Diabetes Patients Receiving Insulin Injections
Received 21 October 2023
Accepted for publication 19 February 2024
Published 20 March 2024 Volume 2024:17 Pages 1359—1366
DOI https://doi.org/10.2147/DMSO.S443737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Zhan-He Sun,* Cheng-Hong Yu,* Xin Wang
Department of Ultrasound, Tongxiang First People’s Hospital, Tongxiang, Zhejiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xin Wang, Email [email protected]
Objective: This study aims to investigate the clinical application value of high-frequency ultrasound technology in diagnosing subcutaneous lipohypertrophy at insulin injection sites in diabetes patients.
Methods: All diabetes patients treated in our hospital from January 2022 to January 2023 were selected as the study subjects. The incidence of subcutaneous lipohypertrophy was calculated at the end of the study period. All patients were screened, and those meeting the inclusion criteria were registered, and basic data were collected. Patients were screened for subcutaneous lipohypertrophy using conventional clinical examination (control group) and high-frequency ultrasound technology (study group).
Results: The study found that the incidence of subcutaneous lipohypertrophy in diabetes patients receiving insulin injections in our hospital from January 2022 to January 2023 was 80.99%. The average longitudinal diameter of subcutaneous lipohypertrophy in these patients was 11.66 (7.56, 21.44) mm, the transverse diameter was 12.04 (8.96, 18.29) mm, depth was (5.62± 2.17) mm, and the area was 188.79 (76.85, 331.78) mm². The clinical detection rate in the study group was higher than that in the control group (P< 0.05). The quantity of detected sites was greater in the study group compared to the control group (P< 0.05).
Conclusion: The incidence of subcutaneous lipohypertrophy in diabetes patients receiving insulin injections is relatively high clinically, and high-frequency ultrasound technology demonstrates significant potential in diagnosis. By providing high-resolution imaging and quantitative data, it effectively improves the clinical detection rate and clarifies symptoms. This technology is likely to become an important auxiliary tool in future diabetes treatment, providing more precise treatment plans for patients.
Keywords: high-frequency ultrasound technology, diabetes, insulin injection, subcutaneous lipohypertrophy, diagnostic value
Introduction
Diabetes is a chronic metabolic disorder characterized by abnormal elevation of blood glucose levels due to insufficient insulin secretion or impaired insulin effectiveness.1 Insulin, a hormone secreted by the pancreas, plays a crucial role in aiding cells in the body to absorb glucose, thereby maintaining normal blood glucose levels.2 Insulin injection is a primary method of diabetes treatment, but subcutaneous lipohypertrophy has become a common yet challenging phenomenon during insulin injection therapy.3 This hypertrophy not only affects the absorption and efficacy of insulin but may also lead to issues such as pain and bruising at the injection site, impacting the effectiveness of treatment.4
High-frequency ultrasound technology not only provides visual imaging but also enables quantitative analysis. It utilizes the propagation characteristics of high-frequency sound waves in tissues to offer clear and real-time imaging of subcutaneous tissues.5 Its advantages, including radiation-free and non-invasive features, allow for more frequent monitoring, providing timely feedback for treatment. Through high-resolution imaging of injection sites, healthcare professionals can visually observe the hypertrophy of the fat layer, offering a basis for treatment adjustments.6 Given its potential value in detecting subcutaneous lipohypertrophy in diabetes patients, this article explores the possible significance and future applications of high-frequency ultrasound technology in diagnosing subcutaneous lipohypertrophy in diabetes patients receiving insulin injections. Real-time, high-definition imaging of injection sites holds the promise of a more comprehensive and accurate understanding of dynamic changes in subcutaneous tissues, providing robust support for the development of personalized treatment plans. In the face of the complex challenges in diabetes management, high-frequency ultrasound technology may emerge as a groundbreaking diagnostic tool, contributing positively to enhancing treatment outcomes and improving the quality of life for patients.
This study aims to investigate the clinical application value of high-frequency ultrasound technology in diagnosing subcutaneous lipohypertrophy at insulin injection sites in diabetes patients. The primary endpoint of this study is to assess the incidence of subcutaneous lipohypertrophy in diabetes patients receiving insulin injections. The secondary endpoints include evaluating the size and characteristics of lipohypertrophic lesions, comparing the clinical detection rates between high-frequency ultrasound technology and conventional clinical examination, and exploring the potential of high-frequency ultrasound technology as an auxiliary tool in diabetes treatment.
Materials and Methods
Ethical Statement
The protocol was approved by the ethics committee of Tongxiang First people’s Hospital, No.19075917. Informed consent was obtained from all study participants. All the methods were carried out in accordance with the Declaration of Helsinki.
Study Design and Sample Size
This retrospective and single-center study aimed to investigate lipohypertrophy (LH) in diabetes patients. A calculated sample size of 200 patients was determined, considering a power of 80% and a significance level of 0.05. The study included patients who received treatment at our hospital between January 2022 and January 2023. A comprehensive examination was conducted for all patients, and those who met the predefined criteria were included in the study, resulting in a total of 100 eligible cases after excluding individuals who did not meet the inclusion criteria. Patients were screened for subcutaneous lipohypertrophy using conventional clinical examination (control group) and high-frequency ultrasound technology (study group). All patients and their guardians were informed about the study and signed written consent forms.
Inclusion and Exclusion Criteria
Inclusion Criteria: Complete clinical data, no gender restrictions; Clinical diagnosis fully consistent with diabetes; Undergoing insulin injection therapy in our hospital.
Exclusion Criteria: Allergic reactions to medications used in this study; Language expression or cognitive communication disorders; Presence of autoimmune system disorders, etc.
- Type of diabetes: The study included patients with both type 1 and type 2 diabetes to assess the prevalence of lipohypertrophy in both populations.
- Number of injections per day: Patients who received multiple daily injections (MDI) of insulin were included in the study. The number of injections per day ranged from 3 to 5, based on individual insulin requirements and treatment plans.
- Duration of insulin therapy: Included patients had a minimum duration of insulin therapy of at least 1 year to evaluate the long-term effects on lipohypertrophy development.
- Doses of insulin per day: The daily doses of insulin administered to the patients ranged from 0.5 to 1.2 units per kilogram of body weight, as determined by their healthcare providers.
- Compliance with prevention rules: Patients were selected based on their adherence to prevention rules, including consistent injection site rotation with each injection and the avoidance of needle or syringe reuse. Patients who reported non-compliance were excluded from the study.
- Needle length: Insulin injections were performed using 8mm needles to ensure proper subcutaneous delivery and minimize the risk of lipohypertrophy.
- Use of ice-cold insulin: Ice-cold insulin was not used during injections, as it has been associated with an increased risk of lipohypertrophy formation. Patients were instructed to store insulin at recommended temperatures and allow it to reach room temperature before administration.
Methods
After receiving standardized training, research personnel were divided into two groups: one group conducted visual inspection and palpation, while the other group performed ultrasound examinations. Physicians and nurses executed blind methods during the procedures, and screening results were collectively analyzed and determined by medical staff. In case of disagreements, a third researcher participated in discussions and joint decisions.
Conventional Clinical Examination: This included visual inspection and palpation, with diabetes specialist nurses conducting examinations after professional training. Patients were instructed to assume appropriate positions, relax, and expose the injection site. The use of adjustable light sources was recommended, with an angle of 30°-45° directed towards the skin surface for visual inspection of the injection site. If subcutaneous lipohypertrophy was observed, the center point was marked with a pen, and then ultrasound gel or clinically used water-soluble lubricant was applied to the injection area. Subsequent gentle palpation using fingertip massage was performed to examine the injection site. When touching the hypertrophied fat area, the boundaries were marked for identification. High-Frequency Ultrasound Technology: An ultrasound machine with an 8–18 MHz probe was used for examinations, ensuring uniform equipment usage and parameter settings throughout the study. To diagnose positive subcutaneous lipohypertrophy, three or more of the following ultrasound features were required: subcutaneous layer with clearly defined hypoechoic or hyperechoic nodular lesions; distorted surrounding connective tissue; absence of vessels and capsule; uneven echo texture compared to surrounding tissues.
Diabetes patients used intermediate-acting insulin. The frequency of insulin injections varied depending on individual treatment plans, which consider factors like insulin requirements and blood glucose levels. Needle lengths of 8 mm were used for insulin injections. Multiple Daily Injections (MDI) of insulin were performed using a syringe. Lipohypertrophy commonly occurs at injection sites with poor rotation and repeated use, such as upper arms are common injection sites for insulin, which can be prone to lipohypertrophy if proper rotation and site selection are not practiced.
Observation Indicators
- Subcutaneous Fat Hypertrophy/Lipohypertrophy Incidence Rate: The incidence rate of subcutaneous lipohypertrophy is calculated by taking the number of patients with the occurrence of subcutaneous lipohypertrophy and dividing it by the total number of diabetes patients receiving insulin injection treatment in the hospital during the specified period (patients with subcutaneous fat hypertrophy/lipohypertrophy occurrence divided by all patients × 100%).
- Basic Information: Basic information is collected for all enrolled patients, including age, gender, duration of illness, and other relevant details. This data is typically collected through patient interviews or medical records.
- Symptom Measurement Indicators: High-frequency ultrasound examinations are used to measure lipohypertrophy. Specifically, the maximum cross-sectional length, width, depth, and area (length x width) of abnormal echo regions are measured using ultrasound imaging techniques.
- Clinical Detection Rate: The positive patients detected in both the control group (conventional clinical examination) and the study group (high-frequency ultrasound technology) are recorded. The detection rate is calculated by dividing the number of positive patients by the total number of patients in each group, and then comparing the detection rates between the two groups.
- Quantity of Detected Sites: The number of positive subcutaneous lipohypertrophy sites detected in both the control group and the study group is recorded. The quantity of detected sites is compared between the two groups to assess any differences in the occurrence of subcutaneous lipohypertrophy.
Statistical Analysis
GraphPad Prism 8 was used for image processing in this study. Data were organized and analyzed using SPSS 22.0. Quantitative data were expressed as mean value (± standard deviation), and intergroup comparisons were conducted using the t-test. Non-normally distributed quantitative data were represented as M (Q1, Q3). Count data were presented as [n (%)], and intergroup comparisons were performed using the chi-square test. Differences with P<0.05 were considered statistically significant.
Results
Incidence Rate of Subcutaneous Lipohypertrophy
The incidence rate of subcutaneous lipohypertrophy in diabetes patients receiving insulin injections in our hospital from January 2022 to January 2023 was 80.99%. See Figure 1.
 |
Figure 1 Incidence rate of subcutaneous lipohypertrophy in diabetes patients receiving insulin injections over one year. |
Basic Information of Enrolled Subjects
This study included 100 patients, comprising 54 males and 46 females, with ages ranging from 28 to 65 years (mean age: 45.21±8.77 years). The duration of illness ranged from 5 to 18 years (mean duration: 12.11±3.72 years), and the duration of insulin treatment ranged from 3 to 10 years (mean duration: 7.96±1.26 years). There were 26 cases of type 1 diabetes and 74 cases of type 2 diabetes See. Table 1.
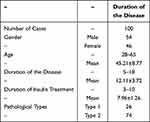 |
Table 1 Basic Information of Included Subjects. Study Subjects |
Symptom Measurement Indicators
The average longitudinal diameter of subcutaneous lipohypertrophy in diabetes patients receiving insulin injections was 11.66 (7.56, 21.44) mm, the width was 12.04 (8.96, 18.29) mm, the depth was (5.62±2.17) mm, and the area was 188.79 (76.85, 331.78) mm^2. See Table 2.
 |
Table 2 Subcutaneous Lipohypertrophy Symptom Measurement Indices |
Clinical Detection Rate
The clinical detection rate of the study group patients was higher than that of the control group patients (P<0.05). See Table 3.
 |
Table 3 Comparison of Clinical Detection Rates Between Two Groups |
Quantity of Detected Sites
The quantity of detected sites was higher than that of the control group patients (P<0.05). See Table 4.
 |
Table 4 Comparison of Detected Sites Quantity Between Two Groups |
Discussion
Currently, the occurrence of subcutaneous lipohypertrophy is quite common in diabetes patients.7 Patients may not be very aware of this hypertrophy in the early stages of treatment, but with the passage of treatment time, subcutaneous lipohypertrophy may gradually become apparent. In clinical management, healthcare professionals need to closely monitor the injection sites of diabetes patients, promptly detect and assess the degree of subcutaneous lipohypertrophy.8 The present study investigated the prevalence of subcutaneous lipohypertrophy (LH) in diabetes patients receiving insulin injections at our hospital between January 2022 and January 2023. The study revealed that the incidence rate of LH was 80.99% among these patients. These findings are consistent with previous research conducted by Stefano et al,9 which emphasized the overlooked aspect of skin involvement in the management of pediatric patients with type 1 diabetes. Dermatological side effects, such as insulin-induced lipodystrophies and allergic contact dermatitis caused by insulin pumps or glycemic sensors, were frequently observed among diabetic children and adolescents. In a study by Deng et al,10 it was concluded that the type of diabetes mellitus had a significant influence on the development of LH. The pooled prevalence of LH was found to be higher among patients with type 2 diabetes mellitus compared to those with type 1 diabetes mellitus (49%, 95% CI 23–74% vs 34%, 95% CI 19–49%). Studies involving a mixed type of diabetes mellitus reported a pooled prevalence of LH of 37% (95% CI 25–48%, I2 = 98.3%). Additionally, Deeb et al11 suggested an association between LH and rotation frequency in children (P = 0.026). LH was found to be the most common skin complication in the multiple daily injection (MDI) group, while nodules, allergy marks, and hyperpigmentation were observed in pump users.
Furthermore, Peng et al12 highlighted that the prevalence of LH varies based on gender and differs among different types of diabetes mellitus.
Probably, there is no gender differences in the LH prevalence in adult patients with T1DM, but it has a gender difference between male and female in T2DM. The lesions had an average longitudinal diameter of 11.66 (7.56, 21.44) mm, a width of 12.04 (8.96, 18.29) mm, a depth of (5.62±2.17) mm, and an area of 188.79 (76.85, 331.78) mm^2, consistent with the results of similar investigations into repeated insulin injections leading to subcutaneous lipohypertrophy. Currently, the pathophysiological mechanisms of subcutaneous lipohypertrophy are not clear. The widely accepted reasons mainly involve the insulin injected into subcutaneous tissues exerting a pro-synthetic effect on local fat tissue, which may be related to the damage and repair of subcutaneous tissue caused by repeated injections.13,14 In addition, the proper use of insulin on a daily basis may also be a contributing factor. For example, storing insulin in the refrigerator may exacerbate mechanical trauma to local subcutaneous fat tissue due to cold stimulation. Furthermore, the biological activity of refrigerated insulin may decrease, preventing it from being fully and effectively absorbed intothe subcutaneous fat layer within the prescribed time, leading to prolonged action of insulin on local fat tissue. The latter can be improved through proper use, but the former currently lacks effective solutions, highlighting the importance of timely diagnosis and treatment.
In recent years, the application of imaging diagnostic methods such as high-frequency ultrasound has provided a new avenue for the timely and accurate assessment of subcutaneous lipohypertrophy, contributing to the formulation of personalized treatment plans. The results of this study indicate that the clinical detection rate in the research group was higher than that in the control group, and the number of detected sites was greater than in the control group (all P<0.05). Firstly, the non-invasiveness of high-frequency ultrasound technology, in comparison to conventional clinical diagnosis, represents a safer means of examination. Unlike traditional visual and tactile examinations, patients undergoing high-frequency ultrasound examinations do not require extensive positional adjustments and exposure of injection sites, reducing discomfort during the examination.5,15 This is crucial for improving patient acceptance and monitoring frequency, especially for patients requiring long-term insulin therapy. Secondly, high-frequency ultrasound technology provides more accurate and detailed imaging information. By observing indicators such as the longitudinal diameter, width, and depth of the subcutaneous fat layer, doctors can have a more comprehensive understanding of lipohypertrophy.16 This quantitative data provides a more scientific basis for formulating individualized treatment plans, assisting in timely adjustments of insulin injection sites, and reducing the risk of complications. When comparing the detection rates and the number of detected sites between the two groups, the research group demonstrated a more pronounced advantage. The application of high-frequency ultrasound technology enables more reliable early diagnosis of lipohypertrophy, thereby increasing the detection rate of positive patients. Furthermore, through the comparison of the number of detected sites, we can clearly see the outstanding performance of high-frequency ultrasound technology in discovering more lipohypertrophy areas. However, there is currently a lack of reference standards for imaging features. In this study, the ultrasound standards we formulated used adjacent fat tissue as a reference for ultrasound examination, providing a detailed description of hyperechoic regions, including the nature of echoes and the manifestations of hypoechoic areas. Combined with clinical inquiries, visual examinations, and tactile examinations, we can more accurately detect subcutaneous lipohypertrophy, enabling early intervention to prevent its progression. Similar domestic studies have also reached similar conclusions, indicating that high-frequency ultrasound can more accurately assess the extent of damage to subcutaneous fat tissue, including size, distribution, and elasticity. Local complications associated with insulin therapy are frequently observed in young patients with type 1 diabetes (T1D). Among these complications, lipohypertrophy (LH) has a significant impact on metabolic control and has been found to affect nearly 12% of patients.17 Lipodystrophy remains a prevalent complication among individuals undergoing insulin therapy, and our study highlights its detrimental effect on glycemic variability. This underscores the significance of implementing preventive strategies to minimize the occurrence of this dermatological complication, as it can potentially interfere with the clinical management and progression of the disease.18 It can also measure the thickness of subcutaneous fat and the proportion of hypertrophic tissue, facilitating graded interventions based on the degree of damage.19–21 It is worth noting that relying solely on visual and tactile examinations for screening may lead to a high rate of missed diagnoses and imprecise diagnostic locations. Patients continuing to inject at positive sites may further exacerbate fat tissue hypertrophy, leading to serious consequences such as adverse blood glucose events, worsening blood glucose control, increased insulin requirements, and increased medical costs, consistent with the results of this study. Therefore, the comprehensive assessment using ultrasound technology combined with various clinical information is of great significance for improving diagnostic accuracy and formulating more precise treatment plans.
Despite the lack of novelty, this study makes valuable contributions in several aspects. For instance, it replicates and validates previous findings, which is essential for ensuring the reliability and generalizability of research results. Additionally, the study provides relevant and contextual information within a specific hospital or healthcare setting, offering insights that can benefit healthcare professionals in similar environments. Furthermore, the longitudinal analysis conducted over the course of a year allows for the examination of the incidence rate and detection of subcutaneous fat hypertrophy/lipohypertrophy, providing valuable insights into the progression of the condition. The comprehensive data collection, including basic patient information, facilitates further analysis and understanding of the factors associated with subcutaneous fat hypertrophy/lipohypertrophy. Moreover, the utilization of high-frequency ultrasound examinations and quantitative measurements enhances the accuracy and reliability of the study’s findings. Lastly, the comparative analysis between the control and study groups allows for evaluating the effectiveness of different detection methods.
However, it is important to acknowledge the limitations of this study. These limitations include the lack of innovation, limited generalizability due to the specific setting, potential selection bias in participant recruitment, inadequate methodological details, reliance on subjective measurements, and the absence of control for potential confounding factors. These factors should be taken into consideration when interpreting the study’s findings and applying them to other contexts or populations.
Conclusion
For diabetic patients, prolonged insulin injections in the same area may lead to gradual hypertrophy of the subcutaneous fat layer in that region. The clinical occurrence rate of subcutaneous lipohypertrophy is relatively high, and high-frequency ultrasound technology demonstrates significant potential in diagnosis. By providing high-resolution images and quantitative data, it effectively enhances clinical detection rates, enabling clearer symptom identification. This technology holds promise as a crucial auxiliary tool in future diabetes treatment, offering more precise treatment plans for patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guo H, Wu H, Li Z. The pathogenesis of diabetes. Int J Mol Sci. 2023;24(8):6978. PMID: 37108143; PMCID: PMC10139109. doi:10.3390/ijms24086978.
2. Drugs and Lactation Database (LactMed®) [Internet]. 2006-Insulin. Bethesda (MD): National Institute of Child Health and Human Development; 2023. PMID: 30000050.
3. Ruze R, Liu T, Zou X, et al. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol. 2023;14:1161521. PMID: 37152942; PMCID: PMC10161731. doi:10.3389/fendo.2023.1161521
4. Walsh J, Roberts R, Bailey TS, Heinemann L. Insulin titration guidelines for patients with type 1 diabetes: it is about time! J Diabetes Sci Technol. 2023;17(4):1066–1076. PMID: 35369773; PMCID: PMC10348003. doi:10.1177/19322968221087261
5. Lee JE, Jeon HJ, Lee OJ, Lim HG. Diagnosis of diabetes mellitus using high frequency ultrasound and convolutional neural network. Ultrasonics. 2024;136:107167. PMID: 37757513. doi:10.1016/j.ultras.2023.107167
6. Yu J, Wang H, Zhou M, et al. A hypothesis on the progression of insulin-induced lipohypertrophy: an integrated result of high-frequency ultrasound imaging and blood glucose control of patients. Diagnostics. 2023;13(9):1515. PMID: 37174907; PMCID: PMC10177501. doi:10.3390/diagnostics13091515
7. Barlas T, Yalcin MM, Coskun M, et al. Evaluation of lipohypertrophy in patients with type 1 diabetes mellitus on multiple daily insulin injections or continuous subcutaneous insulin infusion. Endocr Pract. 2023;29(2):119–126. PMID: 36423861. doi:10.1016/j.eprac.2022.11.008.
8. Ucieklak D, Mrozińska S, Wojnarska A, Małecki MT, Klupa T, Matejko B. Type 1 diabetes mellitus and lipohypertrophy - impact of the intervention on glycemic control via patient’s examination and retraining on change of infusion set. Endocr Pract. 2023;29(3):174–178. PMID: 36581082. doi:10.1016/j.eprac.2022.12.015
9. Passanisi S, Salzano G, Lombardo F. Skin involvement in paediatric patients with type 1 diabetes. Curr Diabetes Rev. 2022;18(4):e030921196145. PMID: 34477525. doi:10.2174/1573399817666210903153837
10. Deng N, Zhang X, Zhao F, Wang Y, He H. Prevalence of lipohypertrophy in insulin-treated diabetes patients: a systematic review and meta-analysis. J Diabetes Investig. 2017;9(3):536–543. PMID: 28862814; PMCID: PMC5934253. doi:10.1111/jdi.12742
11. Deeb A, Abdelrahman L, Tomy M, et al. Impact of insulin injection and infusion routines on lipohypertrophy and glycemic control in children and adults with diabetes. Diabetes Ther. 2019;10(1):259–267. PMID: 30617932; PMCID: PMC6349294. doi:10.1007/s13300-018-0561-7
12. Peng S, Xu M, Zhao H, et al. Gender differences in prevalence and clinical correlates of lipohypertrophy in insulin-exposed patients with diabetes mellitus. Diabetes Metab Syndr Obes. 2022;15:3871–3887. PMID: 36540349; PMCID: PMC9760066. doi:10.2147/DMSO.S392324
13. Ikeda T, Tani N, Hirokawa T, et al. Biodistribution of insulin following massive insulin subcutaneous injection. Intern Med. 2022;61(13):1999–2006. PMID: 35283372; PMCID: PMC9334236. doi:10.2169/internalmedicine.7364-21
14. Sato G, Uchino H, Shimizu Y, Tatebe J, Morita T, Hirose T. Quantitative evaluation of insulin-induced abdominal subcutaneous dystrophic tissue using shear wave elastography. J Diabetes Investig. 2022;13(6):1004–1010. Epub 2022 Feb 11. PMID: 35100500; PMCID: PMC9153836. doi:10.1111/jdi.13762
15. Omolade OK, Stephenson J Best rating scale design theory: implications for developing questionnaires in nursing and Health Sciences. J Mod Nurs Pract Res. 2023;3(4):25. doi:10.53964/jmnpr.2023025
16. Huang H, Tang C, Li M, Huang J, Li Y, Wu S. Sensitivity and specificity of high frequency ultrasound score (DCEC) in diabetic peripheral neuropathy. J Diabetes Metab Disord. 2022;21(2):1459–1467. PMID: 36404810; PMCID: PMC9672188. doi:10.1007/s40200-022-01080-6
17. Demir G, Er E, Atik Altınok Y, Özen S, Darcan Ş, Gökşen D. Local complications of insulin administration sites and effect on diabetes management. J Clin Nurs. 2022;31(17–18):2530–2538. PMID: 34622517. doi:10.1111/jocn.16071
18. Lombardo F, Bombaci B, Alibrandi A, Visalli G, Salzano G, Passanisi S. The impact of insulin-induced lipodystrophy on glycemic variability in pediatric patients with type 1 diabetes. Children. 2022;9(7):1087. doi:10.3390/children9071087
19. Tomaszewicz V, Bach AM, Tafil-Klawe M, Klawe JJ. Non-invasive evaluation techniques to efficacy of anti-cellulite treatment: the high frequency (HF) ultrasound as a useful imaging technique of the skin and subcutaneous tissue. J Cosmet Laser Ther. 2021;23(3–4):72–80. PMID: 34376107. doi:10.1080/14764172.2021.1964537
20. Gnyawali SC, Sinha M, El Masry MS, et al. High resolution ultrasound imaging for repeated measure of wound tissue morphometry, biomechanics and hemodynamics under fetal, adult and diabetic conditions. PLoS One. 2020;15(11):e0241831. doi:10.1371/journal.pone.0241831
21. Lusher J, Henton I, Banbury S. Assessment and feedback in Post-pandemic Healthcare Provider Education: a meta-synthesis. J Mod Nurs Pract Res. 2023;3(3):13. doi:10.53964/jmnpr.2023013
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
