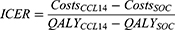Back to Journals » ClinicoEconomics and Outcomes Research » Volume 16
Exploring the Cost-Utility of a Biomarker Predicting Persistent Severe Acute Kidney Injury: The Case of C-C Motif Chemokine Ligand 14 (CCL14)
Authors Echeverri J, Martins R, Harenski K, Kampf JP, McPherson P, Textoris J, Koyner JL
Received 6 September 2023
Accepted for publication 12 December 2023
Published 12 January 2024 Volume 2024:16 Pages 1—12
DOI https://doi.org/10.2147/CEOR.S434971
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Jorge Echeverri,1 Rui Martins,2,3 Kai Harenski,4 J Patrick Kampf,5 Paul McPherson,5 Julien Textoris,6,7 Jay L Koyner8
1Global Medical Affairs, Baxter Healthcare Corporation, Deerfield, IL, USA; 2University Medical Center Groningen, University of Groningen, Groningen, the Netherlands; 3Health Economics; Global Market Access Solutions, Saint Prex, Switzerland; 4Global Medical Affairs, Baxter Deutschland GmbH, Unterschleissheim, Germany; 5Biomarker Research, Astute Medical Inc. (a bioMerieux Company), San Diego, CA, USA; 6Medical Affairs; bioMerieux, SA, Lyon, France; 7Service d´Anesthésie et de Réanimation; Hospices Civils de Lyon, Lyon, France; 8Section of Nephrology, Department of Medicine, University of Chicago, Chicago, IL, USA
Correspondence: Rui Martins, University Medical Center Groningen, University of Groningen, Groningen, 9713 GZ, the Netherlands, Email [email protected]
Background: Approximately 24% of hospitalized stage 2– 3 acute kidney injury (AKI) patients will develop persistent severe AKI (PS-AKI), defined as KDIGO stage 3 AKI lasting ≥ 3 days or with death in ≤ 3 days or stage 2 or 3 AKI with dialysis in ≤ 3 days, leading to worse outcomes and higher costs. There is currently no consensus on an intervention that effectively reverts the course of AKI and prevents PS-AKI in the population with stage 2– 3 AKI. This study explores the cost-utility of biomarkers predicting PS-AKI, under the assumption that such intervention exists by comparing C-C motif chemokine ligand 14 (CCL14) to hospital standard of care (SOC) alone.
Methods: The analysis combined a 90-day decision tree using CCL14 operating characteristics to predict PS-AKI and clinical outcomes in 66-year-old patients, and a Markov cohort estimating lifetime costs and quality-adjusted life years (QALYs). Cost and QALYs from admission, 30-day readmission, intensive care, dialysis, and death were compared. Clinical and cost inputs were informed by a large retrospective cohort of US hospitals in the PINC AI Healthcare Database. Inputs and assumptions were challenged in deterministic and probabilistic sensitivity analyses. Two-way analyses were used to explore the efficacy and costs of an intervention preventing PS-AKI.
Results: Depending on selected costs and early intervention efficacy, CCL14-directed care led to lower costs and more QALYs (dominating) or was cost-effective at the $50,000/QALY threshold. Assuming the intervention would avoid 10% of PS-AKI complications in AKI stage 2– 3 patients identified as true positive resulted in 0.066 additional QALYs and $486 reduced costs. Results were robust to substantial parameter variation.
Conclusion: The analysis suggests that in the presence of an efficacious intervention preventing PS-AKI, identifying people at risk using CCL14 in addition to SOC is likely to represent a cost-effective use of resources.
Keywords: acute kidney injury, dialysis, biomarkers, nephrology, cost effectiveness
Introduction
Acute kidney injury (AKI) affects 7.2% to 18.3%1,2 of all US hospitalized patients with up to 40.0% presenting as moderate to severe,1,3,4 Kidney Disease Improving Global Outcomes (KDIGO) stages 2 to 3.5 Some patients recover within 2 to 3 days of AKI onset but in others, kidney dysfunction persists for up to 7 days and may progress to acute kidney disease (7–90 days) or chronic kidney disease (CKD, >90 days).5 Among those with stage 2–3 AKI, 24.4% are likely to develop persistent severe AKI (PS-AKI), defined as stage 3 AKI lasting ≥3 days or with death in ≤3 days or stage 2 or 3 AKI with dialysis in ≤3 days.6 Duration of AKI and PS-AKI specifically has been linked to higher intensive care requirements, length of hospital stay, readmission rates, dialysis use, and death;4,6–8 nonetheless, testing for PS-AKI is not routinely done in clinical practice.
Of the 27.7 million annual US adult hospital admissions,9 AKI is likely to affect 2 to 5 million people, with PS-AKI developing in 0.7% to 1.7% of the entire adult hospitalized population. In the absence of an intervention, effectively reverting AKI progression,10 early identification and prevention represent opportunities to improve patient outcomes and health systems’ cost-effectiveness.
Currently, staging AKI severity relies on serum creatinine and urinary output thresholds, imperfect markers of acute changes in renal function.11 The multifactorial AKI etiology and incident population’s heterogeneity pose significant challenges to promptly implementing renal protective therapies, particularly at the onset of severe AKI.12,13 Not identifying individuals at greatest risk for disease progression may lead to inadequate clinical interventions, unnecessary adverse effects, and confound the results of scientific research.14 All the above contribute to inefficient resource utilization, in health systems operating under pressing budgets.
To better predict outcomes in AKI, a range of diagnostic tests are being explored to provide predictive capacity to inform treatment choices and direct outcomes. The RUBY study identified urinary C-C motif chemokine ligand 14 (CCL14) as the most predictive of PS-AKI among several biomarkers evaluated,15 raising interest on its role to guide clinical decision-making and its cost-effectiveness. A large retrospective study including about one-fifth of the US hospitalized population reported clinical outcomes, healthcare resource utilization, and costs associated with PS-AKI development among stage 2–3 AKI patients.6,16 This information, together with the performance characteristics of a standardized CCL14 test17 enable further investigation of CCL14 cost-effectiveness. This study aims to assess the cost-effectiveness of adding CCL14 testing to the standard of care (SOC) for patients with stage 2–3 AKI in a US hospital setting, using a third-party payer perspective. Whilst the focus of this analysis is specific to CCL14, the framework described can inform future diagnostic tests in development and costs in relation to preventing AKI disease progression.
Methods
Overview
The modelled population consisted of 66-year-old hospitalized individuals (49.7% females) with KDIGO stage 2–3 AKI from any cause as informed by a large retrospective study representative of the real-world patient population with the full range of the standard comorbidities including CKD.6
In the model, individuals were either tested for CCL14 levels in addition to SOC or received SOC alone. Since no biomarker is currently used to identify individuals at higher risk of PS-AKI, SOC was considered the appropriate comparator.
Model Structure
The analysis was conducted in Microsoft Excel18 and combined a decision tree capturing 90-day clinical outcomes with a Markov model estimating lifetime costs and quality-adjusted life years (QALYs). The modelling approach is aligned with previous cost-effectiveness studies19,20 (Figure 1).
Decision Tree
The decision tree modelled the likelihood of intensive care unit (ICU) admission, dialysis requirements, discharge from hospital, 30-day readmission, outpatient follow-up, and death, conditionally to PS-AKI status during index hospital admission. The pathway of the cohort tested with CCL14 was parameterized using the test receiver operating characteristics with the rate of true positives (TP) being informed by CCL14 sensitivity 0.91 (0.84 to 0.96) and true negatives (TN) by the test specificity 0.51 (0.44 to 0.57) at the 1.3 ng/mL CCL14 concentration cut-off.17 The rates of false positive (FP) and false negative (FN) tests were calculated as 1-sensitivity and 1-specificity, respectively.
Testing for PS-AKI risk is not part of current practice, and AKI treatments are mostly supportive, leading to substantial uncertainty about the efficacy of early interventions at reversing AKI. Consequently, all individuals were assumed to receive renal protective interventions, implemented earlier in TP individuals. The early intervention was assumed to result in the complete avoidance of PS-AKI consequences in 10% of TP patients, having no impact on FP patients. Ten percent was considered conservative because current research aims for higher efficacy to determine if an intervention is clinically meaningful.21 Despite this, testing cost-effectiveness at a range of efficacy effects was mapped in sensitivity analyses. In the base case, adverse events and costs of the intervention were assumed not to differ between cohorts. People identified as TN and FN were assumed to incur similar risks and cost as non-persistent severe AKI (NPS-AKI) and PS-AKI patients receiving SOC only, respectively.
Markov Model
Lifetime costs and consequences of 90-day survivors were modelled using a 3-state Markov process. As 90-day dialysis requirements were not available from the PINC AI Healthcare Database, 30-day dialysis was used as a proxy for lifetime dialysis dependence (ESRD). Individuals not requiring dialysis at this point were assumed to recover completely from AKI (non-ESRD). Dialysis dependence was challenged in sensitivity analysis. People dying from ESRD comorbidities or general causes transitioned to the dead absorbing state. This simplified structure was preferred to modelling all CKD severity stages due to the lack of data informing the landing distribution and progression from PS-AKI to CKD. This was considered conservative because delaying renal protective measures would likely lead to worse clinical and cost outcomes. No further transitions were allowed between the non-ESRD and ESRD states. The Markov model used a 90-day cycle matching the decision tree duration, allowing continuous track of time and discounting. Half-cycle correction was applied to the first cycle of the Markov process.
Model Inputs
Clinical Inputs
Clinical inputs informing ICU requirements, 30-day readmission, outpatient appointment, dialysis dependence, and 30-day mortality in people with and without PS-AKI were sourced from the analyses of a large retrospective cohort. The cohort included adults discharged between 01/01/2017 and 31/12/2019 from 1000 US hospitals, captured in the PINC AI Healthcare database, formerly the Premier Healthcare database.6,16 Publications by Koyner and colleagues suggested that developing PS-AKI during index admission was associated with worse clinical outcomes and higher costs.6,16 A comprehensive description of all model inputs and its implementation is included in Supplemental Material (Section 1).
Mortality
Transition to dead was possible from any health state. During the 90-day period following index hospital admission, mortality rates were informed by the analysis of PINC AI Healthcare Data.6 People with recovered kidney function (non-ESRD) were modelled to have approximately the risk of death of the age and gender-matched US population.22 For those with ESRD, mortality was calculated as 1 minus the probability of survival for US individuals with ESRD aged 65–74 years (Table S2).23 Survival was extrapolated beyond the available 10-year period using an exponential distribution.
Cost Inputs
The duration and costs of in-hospital stay and outpatient costs were sourced from the analysis of PINC AI Healthcare data.16 For simplicity and to be conservative, the model base case considers only the excess costs of dialysis.
Dialysis annual costs used the Centers for Medicare & Medicaid Services base rate for renal dialysis services ($266)24 assuming three weekly sessions over 52 weeks ($41,496 annually). Since there is no current market price for CCL14, testing-related costs were assumed to be zero in the base case, and were extensively varied in sensitivity analyses, allowing for an intuitive exploration of the test cost-effectiveness at multiple testing costs.
Utilities
Utilities summarize quality of life associated with specific health states, ranging from zero (death) to one (perfect health). Utilities assigned to the model health states were multiplied by life years to produce QALYs. Utility inputs were sourced from peer-reviewed publications. Further details about the identification and implementation of utility inputs were included as Supplemental Material (Section 1.3).
Model Outcomes
Incremental cost and QALYs accrued over index admission, 30-day readmission, outpatient, and long-term follow-up in the CCL14 + SOC and SOC arms were calculated and synthesized as incremental cost-effectiveness ratios (ICER) (Equation 1).
Costs were reported in US dollars 2022. Costs and QALYs were discounted at 3% annually.25 Cost-effectiveness was assessed at $50,000 to $100,000 per QALY willingness to pay (WTP).25
Sensitivity Analyses
One-way sensitivity analyses (OWSA) were implemented by replacing each base case parameter at a time, using the lower and upper value of their 95% confidence interval (CI). Results were summarized in a tornado diagram, depicting the effect of varying the most influential inputs on the base case ICER.
Two-way sensitivity analyses (TWSA) were implemented by varying two base case inputs simultaneously, namely the efficacy and costs of an early intervention, and CCL14 costs. The ICERs originated from TWSA were synthesized in visualization grids.
Parameter uncertainty was assessed in probabilistic sensitivity analysis (PSA). Each model input was assigned a probabilistic distribution.26 Alternative inputs were sampled from these distributions according to base case mean and standard error and utilized to run 10,000 Monte Carlo simulations. Probabilistic outputs were summarized as means and 95% credible intervals (CrI) of all simulations. The distribution of individual iterations was presented graphically on a cost-effectiveness plane. The probability of CCL14 being cost-effective over a range of WTP values was presented in a cost-effectiveness acceptability curve (CEAC). The inputs for the early hypothetical intervention efficacy and cost and that for testing cost were not varied probabilistically.
Scenario Analyses
Uncertainty left unexplored after OWSA, and TWSA was investigated in scenario analyses varying the (1) time horizon, (2) simultaneously varying intervention efficacy and intervention and testing costs, (3) costs of ESRD on dialysis, and (4) proportion of those requiring acute dialysis developing lifetime dialysis dependence.
Additional steps followed to support model validation were reported as Supplemental Material (Section 2).
Results
Base Case
The strategy using CCL14 was associated with reduced ICU usage, reduced mortality during index hospital admission, and reduced dialysis requirements (Table 1). Testing with CCL14 was associated with a slightly higher proportion of readmissions and outpatient visits, which could be explained by a higher survival after index admission.
 |
Table 1 Base Case Clinical Outcomes Given a 10% Efficacy of the Early Intervention |
The mean cost of index hospitalization for each patient with stage 2–3 AKI was $34,090.37 in the CCL14 cohort versus $34,508.65 for SOC alone, representing savings of $418.27 per person (Table 2). The CCL14 cohort was also associated with a $67.94 reduction in lifetime costs, mostly from reduced lifetime costs of dialysis dependence ($69.89). Assuming a 10% efficacy of an early hypothetical intervention, the CCL14 cohort was associated with an excess of 0.078 life-years and 0.066 QALY gained. Under base case assumptions, CCL14 was dominant being associated with less costs and higher efficacy than SOC alone.
 |
Table 2 Base Case Deterministic Incremental Cost-Effectiveness Results |
One-Way Sensitivity Analysis
Figure 2 summarizes the impact of the 10 most influential inputs considered in OWSA. The utility of being alive with renal recovery had the strongest impact on the absolute value of the ICER, causing a 9% decrease and 7% increase at the lower and upper bound of the 95% CI, respectively. The second most influential input was the odds ratio (OR) of ICU death in people developing PS-AKI, causing a 3% variation in the absolute value of the ICER. All other inputs caused variations of less than 2% from the base case ICER. Overall, the model results were robust to OWSA.
Two-Way Sensitivity Analysis
Figure 3A represents the value of the ICER as the efficacy of an early intervention is varied from 10% to 100% and the cost of the intervention is varied from $0 to $10,000. Using CCL14 was dominant in over 50% of the scenarios and was mostly cost-effective at a $50,000/QALY WTP. For excess intervention cost values above $6500 and 10% efficacy, CCL14 would be cost-effective at higher WTP of $50,000 to $100,000/QALY.
Figure 3B represents values of the ICER when varying efficacy of the early intervention over testing costs. The strategy using CCL14 was mostly dominant and was always cost-effective at $50,000/QALY.
Figure 4 represents ICER values resulting from varying efficacy and cost for an early intervention at testing costs of $200, $400, $600, and $800 per person. The strategy using CCL14 remained dominant or cost-effective at less than $50,000/QALY in about 95% of scenarios.
Probabilistic Sensitivity Analysis
Base case PSA results (Supplemental Materials Section 3) were very similar to the deterministic results with CCL14-led care dominating SOC (mean ICER $-7425.87/QALY, 95% CrI -$8115.89 to -$6906.27) (Table S3) and being associated with a 100% probability of being cost-effective (Figures S1 and S2). Probabilistic results were also produced for the scenario, using testing costs of $500 and treatment costs of $1000 at efficacy levels of 5% and 10% (Figure 5). At efficacy values of 5% and 10%, using CCL14 in addition to SOC was associated with ICERs of $25,858.62/QALY (95% CrI $22,994.54 to $29,299.73) and $9109.38/QALY (95% CrI $7884.58 to $10,575.21). The distribution of ICERs resulting from 10,000 Monte Carlo simulations for the base case and scenario 2 is reported in Figure S3. Overall, the PSA suggested that model results were robust to parameter uncertainty.
Scenario Analyses
Deterministic and additional probabilistic results for scenario analyses were reported as Supplemental Material (Figure S4, and Tables S1, S4-S7).
Discussion
To our knowledge, this is the first cost-utility analysis (CUA) exploring the value of a biomarker predicting PS-AKI in hospitalized individuals with stage 2–3 AKI. Whilst the results described here are specific to CCL14, the intention of this work was to outline a framework for informing the development of biomarkers in this disease area and investigate acceptable testing prices at different thresholds for efficacy and costs of an intervention preventing AKI progression. There is a clear link between PS-AKI and increased dialysis, ICU care requirements, and in-hospital mortality, compared to hospitalized patients with mild or no AKI. Given the higher incidence of complications and potential cost offsets, the cost-effectiveness of biomarker use is likely to be higher in this cohort compared to those at lower risk.27 Furthermore, this cohort represents up to 40% of hospitalized patients with AKI,1,3,4 an important population in which to determine the value of early detection, risk stratification, and treatment to prevent progression to PS-AKI.28 As identified by previous economic evaluations, it is challenging to prove the value of biomarkers for predicting PS-AKI without evidence of an intervention preventing disease progression.20,27 Nevertheless, exploring how biomarker for early detection and risk assessment might impact costs (whether positively or negatively) can contribute to designing pragmatic clinical trials and defining biomarker use-cases by healthcare providers and payors. Our analysis suggests that in the presence of an effective early intervention such as the prompt implementation of the KDIGO care bundle,29 preventing progression to PS-AKI in only 10% of patients with a TP test for increased risk of PS-AKI, could translate into improved patient outcomes and reduced health system costs.
Strengths and Limitations
There are several strengths to this economic evaluation. The analysis structure is aligned with previous models for AKI diagnostics, complies with current modeling standards, and is reported in a clear and reproducible format. The model draws on published evidence from a large US dataset, capturing approximately one-fifth of all hospital admissions, likely representative of the US hospital population. This allows capturing real-world outcomes of hospitalized individuals with stage 2–3 AKI and progressed to PS-AKI. Finally, base case assumptions were extensively explored in sensitivity and scenario analyses, allowing scrutiny of the results. In particular, the TWSA provided compelling evidence of the likely value of biomarker-directed clinical practice.
Importantly, the model incorporated published operating characteristics of a standardized CCL14 test in a heterogeneous population of stage 2–3 AKI patients from a multicenter clinical study,17 thus representing the realistic use and performance of an available biomarker test.
Several assumptions and limitations underpin the development of this CUA. The model did not capture 30-day readmissions and costs outside the PINC AI Healthcare database hospital network. Nonetheless, the sample informing the primary analyses6,16 was considered large enough to remain representative.
Conservatively, it was assumed that all PS-AKI cases would eventually be diagnosed in the SOC arm, only later, relative to the intervention cohort.
The base case considered a 10% efficacy of an early hypothetical intervention in avoiding PS-AKI in TP patients, preventing all downstream consequences. Early identifying PS-AKI does not constitute clinical practice, and there is limited consensus about an intervention or bundle of interventions that should be used in this patient population. The 10% input was considered conservative as ongoing research established a 20% efficacy threshold to be clinically meaningful.21 The efficacy of an early intervention was explored in TWSA.
It was assumed that the efficacy and costs of an intervention preventing PS-AKI early (TP) or at a later stage (SOC, or FN) would be identical. It is clinically likely that delayed treatment would be associated with higher costs and poorer health outcomes. Also, adverse events of a hypothetical intervention were assumed to be identical between comparators, regardless of intervention timing. These assumptions were challenged by raising early intervention costs, which did not change the conclusions.
In the absence of CCL14 acquisition costs, base case testing-related costs were assumed to be zero. This assumption favors the intervention arm and was therefore extensively challenged in TWSA.
Following index AKI, people were modelled to completely recover or to develop dialysis-dependent ESRD. Progressing AKI would likely lead to different degrees of CKD, with arguably worse disease in the SOC arm. Modeling CKD progression implied more granular data, which we believe does not currently exist. We suggest that future research investigates the medium to long-term progression of PS-AKI to the various CKD Stages and ESRD.
Conservatively, renal transplant was not modelled. The higher prevalence of ESRD in the SOC would lead to higher transplant-related costs. In addition, waiting time for a transplant reduces the weight of not modelling this intervention.
If modelled, CKD-related productivity losses in patients and caregivers would possibly be aggravated by delayed treatment.
Two UK health technology appraisals19,20 conducted original analyses to inform the biomarker decision space and concluded that determining the cost-effectiveness of biomarkers is currently limited by the lack of evidence on efficacious interventions addressing AKI progression. The controversy resides specifically in clinically defining these interventions given the multiplicity of AKI etiologies. Similar limitations apply to our analysis and future interventional trials are required to identify suitable interventions and their efficacy for preventing progression to PS-AKI in patients with stage 2–3 AKI.
Conclusion
The in-hospital AKI population is not typically monitored using biomarkers of kidney damage. This analysis explores the potential value of CCL14 in the early detection of risk for PS-AKI. The CUA estimated that given an intervention preventing progression to PS-AKI in as few as 10% of patients testing as TP, using CCL14 is very likely to be cost-effective compared to SOC alone. Testing informing risk stratification of PS-AKI may prompt the implementation of clinical guidelines in everyday practice and steer future research on AKI interventions.
Data Sharing Statement
The data supporting the economic evaluation have been cited in the body of the manuscript and Supplemental Materials.
The present economic model has not been registered on clinical.trials.gov.
Ethics Approval and Informed Consent
Ethics committee approval and patient informed consent were not required for this analysis. The analysis was conducted using patient characteristics and treatment effects from published studies, for which ethics approval had previously been obtained. No direct patient contact or primary collection of individual patient data were required.
Acknowledgments
We thank Dr. Michael Blackowicz for his contributions during the early design phase of the economic model.
The results presented in this paper have not been published previously in whole or in part. The present economic analysis was funded by Baxter Healthcare Corporation. This publication was subject to review by internal employees from Baxter Healthcare Corporation prior to submission for protection of Confidential Information. However, the Authors retain full responsibility for the content of this publication.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The study was sponsored by Baxter Healthcare Corporation.
Disclosure
RM is an employee for Global Market Access Solutions and has received consulting fees from Baxter Healthcare Corporation for developing the present economic model.
JE and KH are full-time employees of Baxter International with ownership interest.
JLK has received consulting fees from Baxter and bioMerieux during the conduct of the current study; personal fees from SeaStar Medicine, Mallinckrodt, Guard Therapeutics, Novartis, and from Alexion, outside the submitted work
JPK and PM were full-time employees of Astute Medical (a bioMerieux company) which manufactures in vitro diagnostics, including the NEPHROCLEAR CCL14 Test when this work was conducted. They have a patent PCT/US2015/056462 issued to Astute Medical/bioMerieux, a patent PCT/US2018/013561 issued to Astute Medical/bioMerieux, a patent PCT/US2020/034400 pending to Astute Medical/bioMerieux, a patent PCT/US2021/059897 pending to Astute Medical/bioMerieux, a patent PCT/US23/63524 pending to Astute Medical/bioMerieux.
JT is a full-time employee of bioMerieux, Inc. The authors report no other conflicts of interest in this work.
References
1. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12–20. doi:10.2215/CJN.02730313
2. Pavkov HJ, Burrows NR, Trends in Hospitalizations for Acute Kidney Injury - United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67:289–293. doi:10.15585/mmwr.mm6710a2
3. Sawhney S, Marks A, Fluck N, Levin A, Prescott G, Black C. Intermediate and Long-term Outcomes of Survivors of Acute Kidney Injury Episodes: a Large Population-Based Cohort Study. Am J Kidney Dis. 2017;69(1):18–28. doi:10.1053/j.ajkd.2016.05.018
4. Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–1493. doi:10.2215/CJN.00710113
5. International Society of Nephrology. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Injury. Kidney Int. Suppl. Web site. Available from: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf.
6. Koyner JL, Mackey RH, Rosenthal NA, et al. Clinical outcomes of persistent severe acute kidney injury among patients with KDIGO stage 2 or 3 AKI. Am J Nephrol. 2022;53(11–12):816–825. doi:10.1159/000528158
7. Mehta S, Chauhan K, Patel A, et al. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):91. doi:10.1186/s12882-018-0876-7
8. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207. doi:10.1038/nrneph.2013.282
9. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project: number of Discharges, United States, 2000 to 2020; 2022. Available from: http://datatools.ahrq.gov/hcupnet?type=subtab&tab=hcnis&count=11.
10. Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders H-J. Acute kidney injury. Nature Reviews Disease Primers. 2021;7(1):52. doi:10.1038/s41572-021-00284-z
11. Kellum JA. Diagnostic Criteria for Acute Kidney Injury: present and Future. Crit Care Clin. 2015;31(4):621–632. doi:10.1016/j.ccc.2015.06.001
12. Moledina DG, Belliveau O, Yamamoto Y, et al. Variation in Best Practice Measures in Patients With Severe Hospital-Acquired Acute Kidney Injury: a Multicenter Study. Am J Kidney Dis. 2021;77(4):547–549. doi:10.1053/j.ajkd.2020.08.013
13. Küllmar M, Weiß R, Ostermann M, et al. A Multinational Observational Study Exploring Adherence With the Kidney Disease: improving Global Outcomes Recommendations for Prevention of Acute Kidney Injury After Cardiac Surgery. Anesth Analg. 2020;130(4):910–916. doi:10.1213/ANE.0000000000004642
14. Vaara ST, Bhatraju PK, Stanski NL, et al. Subphenotypes in acute kidney injury: a narrative review. Critical Care. 2022;26(1):251. doi:10.1186/s13054-022-04121-x
15. Hoste E, Bihorac A, Al-Khafaji A, et al. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. Intensive Care Med. 2020;46(5):943–953. doi:10.1007/s00134-019-05919-0
16. Koyner JL, Mackey RH, Rosenthal NA, et al. Healthcare resource utilization and costs of persistent severe AKI among hospitalized Stage 2/3 AKI patients. Kidney360. 2022.
17. Koyner JL, Chawla LS, Bihorac A, et al. Performance of a Standardized Clinical Assay for Urinary C-C Motif Chemokine Ligand 14 (CCL14) for Persistent Severe Acute Kidney Injury. Kidney360. 2022;3(7):1158–1168. doi:10.34067/KID.0008002021
18. Microsoft Excel for Microsoft 365, [computer program]. Version Version 2304 Build 16.0.16327.20200 - 64 bit2022.
19. Brazzelli M, Aucott L, Aceves-Martins M, et al. Biomarkers for assessing acute kidney injury for people who are being considered for admission to critical care: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2022;26(7):1–286. doi:10.3310/UGEZ4120
20. Hall PS, Mitchell ED, Smith AF, et al. The future for diagnostic tests of acute kidney injury in critical care: evidence synthesis, care pathway analysis and research prioritisation. Health Technol Assess. 2018;22(32):1–274. doi:10.3310/hta22320
21. ClinicalTrials.gov. Effect of an Intervention to Prevent Acute Kidney Injury Versus Standard Care in High-risk Patients After Major Surgery (PrevProgAKI) - NCT05275218. Available from: https://clinicaltrials.gov/ct2/show/NCT05275218.
22. Arias E, Xu J. United States Life Tables, 2020. National Vital Stat Rep. 2022;71:1–64.
23. United States Renal Data System. USRDS Annual Data Report: epidemiology of kidney disease in the United States. Available from: https://adr.usrds.org/2022.
24. CMS. Calendar Year 2023 End-Stage Renal Disease (ESRD) Prospective Payment System (PPS) Final Rule (CMS-1768-F). Available from: https://www.cms.gov/newsroom/fact-sheets/calendar-year-2023-end-stage-renal-disease-esrd-prospective-payment-system-pps-final-rule-cms-1768-f.
25. ICER. 2020-2023 Value Assessment Framework. Available from: https://icer-review.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf.
26. Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford University Pres; 2006.
27. Jacobsen E, Sawhney S, Brazzelli M, et al. Cost-effectiveness and value of information analysis of NephroCheck and NGAL tests compared to standard care for the diagnosis of acute kidney injury. BMC Nephrol. 2021;22(1):399. doi:10.1186/s12882-021-02610-9
28. Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS. Recovery after Acute Kidney Injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi:10.1164/rccm.201604-0799OC
29. Zarbock A, Küllmar M, Ostermann M, et al. Prevention of Cardiac Surgery-Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: the PrevAKI-Multicenter Randomized Controlled Trial. Anesth Analg. 2021;133(2):292–302. doi:10.1213/ANE.0000000000005458
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.