Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Exploring Real-World HER2-Low Data in Early-Stage Triple-Negative Breast Cancer: Insights and Implications
Authors da Silva JL , Carvalho GDS, Zanetti de Albuquerque L, Rodrigues FR, Fernandes PV , Kischinhevsky D, de Melo AC
Received 19 March 2023
Accepted for publication 30 April 2023
Published 8 May 2023 Volume 2023:15 Pages 337—347
DOI https://doi.org/10.2147/BCTT.S408743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Jesse Lopes da Silva,1 Giselle de Souza Carvalho,1 Lucas Zanetti de Albuquerque,1 Fabiana Resende Rodrigues,2 Priscila Valverde Fernandes,2 Daniel Kischinhevsky,1 Andreia Cristina de Melo1
1Division of Clinical Research and Technological Development, Brazilian National Cancer Institute, Rio de Janeiro, Brazil; 2Division of Pathology, Brazilian National Cancer Institute, Rio de Janeiro, Brazil
Correspondence: Jesse Lopes da Silva, Brazilian National Cancer Institute (INCA), Clinical Research Division, 37 André Cavalcanti Street, 5th Floor, Annex Building, Rio de Janeiro, 20231-050, Brazil, Tel/Fax +55 21 32076585, Email [email protected]
Purpose: This study aimed to compare the clinical behavior, clinicopathological and sociodemographic characteristics of patients with early-stage triple-negative breast cancer (TNBC) who belong to the HER2-low and HER2-zero subgroups.
Patients and Methods: This study involved a thorough search in the internal database of a single Brazilian institution to identify women with TNBC who underwent neoadjuvant chemotherapy (NACT) followed by curative surgery within the period from January 2010 to December 2014. HER2 analysis through immunohistochemistry (IHC) and, if required, amplification by in situ hybridization, was conducted using core biopsy samples. The study assesses outcomes of residual cancer burden (RCB), event-free survival (EFS), and overall survival (OS).
Results: A total of 170 cases were analyzed, with a mean age of 51.4 years (standard deviation, SD 11.2). The HER2 status was categorized as IHC 0, 1+, or 2+ in 80 (47.1%), 73 (42.9%), and 17 (10%) patients, respectively. No significant differences were observed in the prevalence of clinical pathological characteristics among the subgroups. The absence of significant results for clinicopathological and demographic features hindered the multivariate analysis of HER2 subgroups. Similarly, no significant differences were found in the RCB, EFS, and OS outcomes between HER2 subgroups.
Conclusion: The findings of this study suggest that, in early-stage TNBC, the clinical behavior and survival outcomes of the HER2-low subgroup may not differ significantly from those of the HER2-zero subgroup.
Keywords: HER2 status, HER2-low, biomarker, triple-negative breast cancer
Introduction
Boundaries of breast cancer subtypes definition based on Human Epidermal Growth Factor Receptor 2 (HER2) status have never been so intensely debated so far. The immunohistochemistry classification of breast cancer, which has traditionally been used for systemic treatment sequencing and surgical treatment strategies, still relies on the status of hormone receptors and HER2.1,2 HER2 status has conventionally been classified dichotomously, based on the expression of immunohistochemistry (IHC) and amplification by in situ hybridization (ISH), as either positive or negative.3 Triple-negative breast cancer (TNBC) is a subtype of breast cancer that accounts for approximately 15% of cases and is characterized by a lack of expression of the estrogen receptor (ER), progesterone receptor (PR) and HER2. This subtype is associated with aggressive clinical behavior and a high risk of early visceral recurrence.4
Recent emerging findings from the DESTINY-Breast04 trial have led the oncology community to crack the rigid dichotomous HER2 classification, boosting a great shift toward the introduction into clinical practice of the HER2-low concept, a subgroup opportunely defined by IHC 0 or 1+, or even IHC 2+ with a negative ISH assay.5 Notably, the study demonstrated significant clinical benefits of trastuzumab deruxtecan (T-DxD), an HER2-directed antibody-drug conjugate (ADC), in patients with heavily treated HER2-low metastatic breast cancer, including those with hormone receptor-negative disease (traditionally known as triple-negative breast cancer).5 The efficacy of T-DxD can be attributed to its high cytotoxic payload and potent bystander-killing effect, leading to the release of cytotoxic payload from dead antigen-positive cells and subsequent elimination of surrounding antigen-negative tumor cells, resulting in a strong paracrine effect.6 These findings have paved the way for new treatment options for this previously overlooked group of patients.7
Currently, scarce data in the literature that reliably describe the demographic and clinical characteristics of patients with different levels of HER2 expression to better personalize the treatment of these patients is available.8,9 Tumor heterogeneity is a major contributor to the divergent clinical outcomes observed in TNBC, leading to varied responses to neoadjuvant chemotherapy and survival outcomes.10 Retrospective studies in early breast cancer have shown conflicting results regarding the clinical behavior of HER2-low compared to HER2-zero, as determined through genomic expression profiling tests.11–13 Therefore, the evaluation of the HER2-low subgroup in TNBC is of paramount importance to determine whether it represents a distinct subtype. This would enable the design of new clinical trials with targeted treatment options and the development of more accurate assays for HER2 status diagnosis.
The present study aimed to assess the demographic and clinicopathological features, along with chemosensitivity and survival outcomes, of women with locally advanced TNBC who received neoadjuvant chemotherapy (NACT) followed by curative surgery, according to the distinct levels of HER2 expression.
Materials and Methods
Study Design and Ethical Considerations
This investigation is a retrospective cohort study that adhered strictly to the guidelines of the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE). It was approved by the local Institutional Review Board and conducted according to the principles of Good Clinical Practice guidelines.
Patient Selection
To be eligible for inclusion in this study, patients were required to meet specific criteria, which were as follows: a) being female and over 18 years of age; b) confirmation of the histopathological diagnosis of TNBC by the Division of Pathology of the Brazilian National Cancer Institute (DIPAT-INCA) based on the criteria outlined in the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines:2,14 tumors with an ER and PR score of less than 1%, as well as a HER2 score of 0/1+ or 2+ with negative ISH; c) having stage II–III breast cancer as defined by the 8th American Joint Committee on Cancer (AJCC);15 and d) receiving NACT with anthracycline-taxane-based regimens followed by surgery with curative intent at the Brazilian National Cancer Institute (INCA). Patients who had previously received antineoplastic agents or had second primary or unresectable breast tumors after neoadjuvant treatment were excluded from the study.
Immunohistochemistry
The entire tissue sections of the core biopsy samples were subjected to analysis, with immunostaining for ER (clone EP1, Dako, prediluted), PR (clone PgR636, Dako, prediluted), and HER2 (clone SP3, Cell Marque, diluted 1:500).16 Negative immunostaining scores were validated per the guidelines established by the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP). In cases where the HER2 immunostaining score was indeterminate (score 2+), ISH was performed with results that were defined as the ratio of HER2 gene amplification to the chromosome 17 enumeration probe (CEP17). Therefore, negative ISH was identified when the HER2/CEP17 ratio was < 2.0, and the HER2 copy number signals/cell was < 4. An HER2 immunostaining score of 0 corresponded to a HER2 IHC-negative score, whereas a HER2 immunostaining score of 1 + or 2+ with negative ISH corresponded to a HER2-low score. Ki67 was evaluated using nuclear staining with a mouse monoclonal antibody (SP6 clone, Cell Marque) at a dilution of 1:500.
Other Pathological and Clinical Variables
Data derived from electronic medical records and clinical charts were used in the analyses. The following variables were obtained: age at diagnosis, ethnicity categorized as either Caucasian or non-Caucasian as per the Brazilian Institute of Geography and Statistics (IBGE),17 body mass index (BMI), clinical stage (II–III), response to neoadjuvant chemotherapy (NACT) as indicated by residual cancer burden (RCB), histological type, Elston histological grade (1: low grade; 2: moderate grade; 3: high grade), lymphovascular invasion (LVI), perineural invasion (PI), type of NACT (5-fluorouracil + doxorubicin + cyclophosphamide followed by paclitaxel or docetaxel [FAC-T] or doxorubicin + cyclophosphamide followed by paclitaxel or docetaxel [AC-T]) and type of surgery (radical or conservative, type of axillary approach). By established protocols, RCB scores were stratified into a standard 4-level categorical variable denoted as RCB “classes” 0, 1, 2, and 3, with an incremental increase in score corresponding to a greater residual disease burden.18
Statistical Analysis
Event-free survival (EFS) was defined as the time interval from the date of diagnosis to the earliest occurrence of disease progression, all-cause mortality, or discontinuation of treatment for initiation of complementary therapy due to inadequate response to standard neoadjuvant chemotherapy (NACT). Overall survival (OS) was calculated from the date of diagnosis until death from any cause, or until the last day of data collection. Categorical data were presented as absolute counts (n) and proportions (%), while continuous data were expressed as means with standard deviation (SD) or medians with interquartile range (IQR), following the Shapiro–Wilk test for normality. Logistic regression analysis was employed to evaluate the association between HER2 level and each variable evaluated. The Kaplan-Meier method was utilized to estimate EFS and OS for the HER2 subgroups, and compared using the Log rank test. Crude hazard ratios (HRs) were calculated for each factor using Cox proportional hazards model. The multivariate analysis would be performed by including all variables associated with response and survival outcomes at a p-value < 0.20 and selecting the most appropriate model using the Akaike Information Criterion. A p-value < 0.05 was considered statistically significant, and missing data were excluded from the analysis. Statistical analyzes were conducted using the R environment version 4.2.1.
Results
A total of 235 patients were selected from the institutional database. Following the exclusion of patients with essential missing data, primarily due to scarce or unavailable tumor samples, 170 cases of women with locally advanced TNBC met the inclusion criteria (Figure 1). The main characteristics of the patients are presented in Table 1. The HER2 status was determined by immunohistochemistry (IHC) and was identified as IHC 0 in 80 patients (47.1%), IHC 1+ in 73 patients (42.9%), and IHC 2 + in 17 patients (10%) (Figure 2). The mean age (standard deviation, SD) was 51.4 (11.2) years, with minimal variation observed between the groups.
 |
Table 1 Demographic and Clinical-Pathological Characteristics by HER2 Status |
 |
Figure 1 Study design. |
Upon evaluating the clinicopathological variables, no noteworthy differences in the frequency distribution were discerned among subgroups (please refer to Table 1). Remarkably, individuals classified as obese, that is, those with a BMI surpassing 30, were more commonly represented in the HER2 IHC 0 group (37.5%). Furthermore, this trend was also observed in the HER2 IHC 0 group in relation to Caucasian ethnicity (50%), grade 3 tumors (73.8%), and tumors with metaplastic histology (8.8%). Concerning ethnicity, the prevalence of non-Caucasian women was slightly higher in patients with HER2 IHC 2+ (58.8%), in which there was also a higher frequency of LVI (23.5%), PNI (11.8%), and mean Ki67 expression (51.2%, SD 36.3). Regrettably, the absence of significant associations between any of the clinicopathological and demographic characteristics evaluated with the different levels of HER2 has rendered multivariate analysis unfeasible.
As presented in Table 2, there was no statistically significant difference in the treatment modality administered to patients across the three HER2 subgroups. Concerning systemic neoadjuvant therapy, the majority of patients in this study (67.6%, 115 cases) received the AC-T regimen, whereas 24.7% (42 cases) received FAC-T. The majority of patients underwent radical surgery (96.5%, 164 cases) and axillary dissection (85.9%, 146 cases).
 |
Table 2 Treatment Strategies by HER2 Status |
In terms of the response to NACT, there was no significant difference observed among the HER2 subgroups. However, Residual Cancer Burden (RCB) of 0/1 showed a trend toward higher frequency in HER2 IHC 2+ (47.1%) in comparison to HER2 IHC 1+ (31.5%) and HER2 IHC 0 (25%), as demonstrated in Table 1. The median follow-up period for this study was 62.5 months (95% Confidence Interval (CI) 60.2–67.9). Univariate analysis for Event-Free Survival (EFS) revealed no significant difference among the subgroups, with the 3-year EFS estimate for HER2 IHC 0 being 64.2% (95% CI 54.3–75.8%), for HER2 IHC 1+ being 60.7% (95% CI 50.3–73.2%), and for HER2 IHC 2+ being 58.23% (95% CI 38.7–87.5%), as shown in Figure 3. Similarly, the univariate analysis for 3-year Overall Survival (OS) did not indicate any meaningful differences among the HER2 subgroups, with the 3-year OS estimate for HER2 IHC 0 being 66.5% (95% CI 56.5–78.2%), for HER2 IHC 1+ being 73.2% (95% CI 63.6–84.3%), and for HER2 IHC 2+ being 69.0% (95% CI 49.7–95.8%), as depicted in Figure 4.
 |
Figure 3 Kaplan-Meier estimates of event-free survival stratified by HER2 immunohistochemistry subgroups. |
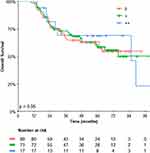 |
Figure 4 Kaplan-Meier estimates of overall survival stratified by HER2 immunohistochemistry subgroups. |
Discussion
The categorization of the HER2 biomarker into a binary status has been subject to significant scrutiny in light of recent clinical trials, which have demonstrated promising response rates and improved survival outcomes in patients with HER2-low tumors treated with anti-HER2 ADCs.19 This emerging evidence has led proponents to advocate for a more nuanced understanding of HER2 expression as a continuous spectrum biomarker.20,21 However, there remains an unmet need to comprehensively characterize the extensive tumor heterogeneity observed in the context of TNBC.22 The primary findings of the current study indicate that sociodemographic and clinicopathological factors, response rates to neoadjuvant chemotherapy and survival outcomes do not differ significantly across TNBC subgroups stratified by varying levels of HER2 expression.
The HER2 subgroups displayed a close proportional distribution, with a predominant representation of younger, non-white women, and those with poorly differentiated stage III disease. These patients also exhibited a high prevalence rate of obesity, higher Ki67 score, as well as LVI and PNI, all of which are traditional prognostic factors for poorer outcomes in breast cancer patients.23,24 Additionally, RCB, an outcome used to determine the volume of residual disease after NACT with well-known strong impact on patient survival, displayed similar proportions among HER2 subgroups.25 That said, the pattern of response to NACT in patients with TNBC might be independent of HER2 expression status by IHC. Notably, the systemic and surgical interventions provided to the patients demonstrated a remarkable degree of consistency across the HER2 subgroups, with the majority receiving the NACT regimen AC-T, as well as a radical mastectomy and axillary dissection.
In a large cohort study of 15,054 patients diagnosed with metastatic breast cancer without HER2 overexpression, Calbiac et al26 found that patients with TNBC had a smaller proportion of the HER2-low subgroup (21%) as compared to the group, suggesting a slightly better overall survival (OS) compared to patients with HER2-zero tumors. However, there was no significant difference in progression-free survival (PFS) between these subgroups. In another pooled analysis of prospective individual patient data on 2310 women with early breast cancer, Denkert et al11 reported that among patients with TNBC, those who were HER2-low (36% of patients) had no significant difference in pathologic complete response when compared to their HER2-zero counterparts. However, the HER2-low subgroup exhibited significantly higher 3-year disease-free survival and OS. Furthermore, Agostinetto et al12 reported in their study on 74 patients with TNBC and HER2-low status, where intrinsic PAM50 subtypes were characterized, that HER2-enriched tumors were more prevalent, accounting for 13.7% of cases.
In a cohort study by Schettini et al13 that analyzed molecular subtypes using PAM50 in patients with metastatic breast cancer, it was found that 36% of TNBC cases were HER2-low. The study also emphasized that there were no significant differences in OS and distribution of intrinsic molecular subtypes among the HER2 subgroups and highlighted the suboptimal reproducibility of HER2-low among pathologists. Moreover, a Chinese cohort study of 1433 patients with non-overexpressed HER2 metastatic breast cancer reported a prevalence of 43.1% for the HER2-low subtype, which was associated with significantly longer survival compared to patients with HER2-zero tumors.8 Additionally, results from the Austrian AGMT_MBC-Registry on metastatic breast cancer also indicated that low HER2 expression did not significantly affect OS as compared to HER2-zero expression.9
An intriguing proposal to expand the concept of HER2 status in breast cancer as a continuum spectrum has emerged, leading to the possibility of HER2 targeting in HER2 “ultra-low” subgroups (ie tumors with a score of 0, exhibiting incomplete and faint staining in ≤10% of tumor cells) due to increasing knowledge about intra-tumor heterogeneity phenomena.21 This unique phenotype could be the reason for some favorable evidence on treatment response in HER2-zero breast cancer.11 Given the limitations of IHC for HER2 testing, more precise and reliable methods based on artificial intelligence (AI) have been evaluated.27,28 Gustavson et al29 proposed a novel HER2 Quantitative Continuous Score using deep learning-based image analysis, which has the potential to improve the prediction of patient outcomes with T-Dxd.
Recently, the HER2Complete® program, a product of AI-based software, has gained popularity in the digital pathology community for its ability to detect HER2 expression levels in HER2-zero and HER2-low cases in an objective and reproducible manner. Despite not yet being approved for diagnostic procedures, it has received United Kingdom Conformity Assessed (UKCA) and In Vitro Diagnostic (IVD) CE marking.30 Additionally, Moutafi et al31 developed a quantitative immunofluorescence coupled with a standardized mass spectrometry HER2 array that measures absolute amounts of HER2 protein on conventional histology sections. Kennedy et al32 reported promising results with immunoaffinity enrichment paired with multiple reaction monitoring-mass spectrometry (immuno-MRM-MS) as it showed higher concordance with predicate assays, especially at low HER2 expression levels. Xu et al33 suggested that molecular methods like mRNA could be useful in accurately defining HER2-low cancer for treatment decision-making due to its wider dynamic range.
The robustness of this study primarily rests on its meticulous patient selection process, which facilitated a precise evaluation of distinct HER2 subgroups in the early-stage TNBC scenario. Specifically, only patients who received NACT followed by primary surgery were eligible for inclusion. Additionally, the analysis of HER2 status was centralized within the Department of Pathology of INCA, which conferred greater reliability to the results. To enhance the rigor of the study, experienced pathologists, who were unaware of the clinical data, performed a blinded double-check of the core biopsies.
The present study is subject to certain limitations, most notably its retrospective nature, which could result in some confounding factors not being taken into account and hence weakening the analysis. Furthermore, the single-center design of the study raises the possibility of regional differences and specific patient characteristics in the cohort that may have impacted the results. Additionally, due to the unavailability of adequate samples, gene expression profiling was not conducted. Finally, the assessment of core biopsy analysis might have been influenced by intratumoral heterogeneity.
Conclusion
By restricting the patient cohort to a defined subset of individuals diagnosed with early-stage TNBC and analyzing the HER2 subtypes based on IHC, the current study humbly proposes that the HER2-low subgroup does not exhibit distinct clinical, sociodemographic, or clinical pathological features compared to the HER2-zero subgroup. Nevertheless, additional research using AI-based methods capable of providing objective and quantitative assessment to improve the identification of HER2-low status should be given high priority, given the potential for significant benefit from novel anti-HER2 ADCs in this subgroup.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval
The current investigation was subjected to ethical scrutiny and received approval from the Ethics in Human Research Committee of INCA, Rio de Janeiro, Brazil, with registration number CAAE 61675516.9.0000.5274, adhering to the principles of Good Clinical Practice guidelines.
Informed Consent
Considering the retrospective nature of this observational study, which involved the use of anonymized data for analysis, the Institutional Review Board (IRB), also known as the Ethics in Human Research Committee of the National Cancer Institute (Comitê de Ética em Pesquisa do Instituto Nacional de Câncer; CEP-INCA), deemed it appropriate to waive the requirement for informed consent from participants.
Acknowledgments
The authors express their profound gratitude to all the patients and their families who demonstrated trust and willingness to participate in this study and provided invaluable biological samples for research purposes. They extend their thanks to Mrs. Isabele Avila Small for providing technical assistance with statistical analysis and to Andréa Rodrigues Cordovil Pires for her contribution in selecting images that represent the HER2 status.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Smolarz B, Nowak AZ, Romanowicz H. Breast cancer-epidemiology, classification, pathogenesis and treatment (review of literature). Cancers. 2022;14(10):2569. doi:10.3390/cancers14102569
2. Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134(7):e48–72. doi:10.1043/1543-2165-134.7.e48
3. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. JCO. 2018;36(20):2105–2122. doi:10.1200/JCO.2018.77.8738
4. Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20(7):1071–1082. doi:10.1007/s10552-009-9331-1
5. Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi:10.1056/NEJMoa2203690
6. Nguyen X, Hooper M, Borlagdan JP, Palumbo A. A review of fam-trastuzumab deruxtecan-nxki in HER2-positive breast cancer. Ann Pharmacother. 2021;55(11):1410–1418. doi:10.1177/1060028021998320
7. Koster KL, Huober J, Joerger M. New antibody-drug conjugates (ADCs) in breast cancer—an overview of ADCs recently approved and in later stages of development. Explor Target Antitumor Ther. 2022;3(1):27–36. doi:10.37349/etat.2022.00069
8. Li Y, Abudureheiyimu N, Mo H, et al. In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the national cancer center, China. Front Oncol. 2021;11:774577. doi:10.3389/fonc.2021.774577
9. Gampenrieder SP, Rinnerthaler G, Tinchon C, et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021;23(1):112. doi:10.1186/s13058-021-01492-x
10. Chiu AM, Mitra M, Boymoushakian L, Coller HA. Integrative analysis of the inter-tumoral heterogeneity of triple-negative breast cancer. Sci Rep. 2018;8(1):11807. doi:10.1038/s41598-018-29992-5
11. Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. doi:10.1016/S1470-2045(21)00301-6
12. Agostinetto E, Rediti M, Fimereli D, et al. HER2-low breast cancer: molecular characteristics and prognosis. Cancers. 2021;13(11):2824. doi:10.3390/cancers13112824
13. Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. Npj Breast Cancer. 2021;7(1):1–13. doi:10.1038/s41523-020-00208-2
14. Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi:10.1200/JCO.2013.50.9984
15. Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25(7):1783–1785. doi:10.1245/s10434-018-6486-6
16. Irvin WJ, Carey LA. What is triple-negative breast cancer? Eur J Cancer. 2008;44(18):2799–2805. doi:10.1016/j.ejca.2008.09.034
17. IBGE. Pesquisa das Características Étnico—Raciais da População—PCERP [Research on the Ethnic-Racial Characteristics of the Population—Raciais da População—PCERP]; 2008. Available from: https://www.ibge.gov.br/estatisticas/sociais/populacao/9372-caracteristicasetnico-raciais-da-populacao.html?=&t=o-que-e.
18. Nahleh Z, Sivasubramaniam D, Dhaliwal S, Sundarajan V, Komrokji R. Residual cancer burden in locally advanced breast cancer: a superior tool. Curr Oncol. 2008;15(6):271–278. doi:10.3747/co.v15i6.242
19. Hurvitz SA. DESTINY-changing results for advanced breast cancer. N Engl J Med. 2022;387(1):75–76. doi:10.1056/NEJMe2206661
20. Polónia A, Canelas C, Caramelo A. The spectrum of HER2 expression in breast cancer: linking immunohistochemistry quantification with in situ hybridization assay. Virchows Arch. 2022;480(6):1171–1179. doi:10.1007/s00428-022-03290-y
21. Venetis K, Crimini E, Sajjadi E, et al. HER2 low, ultra-low, and novel complementary biomarkers: expanding the spectrum of HER2 positivity in breast cancer. Front Mol Biosci. 2022;9. doi:10.3389/fmolb.2022.834651
22. Asleh K, Riaz N, Nielsen TO. Heterogeneity of triple negative breast cancer: current advances in subtyping and treatment implications. J Exp Clin Cancer Res. 2022;41(1):265. doi:10.1186/s13046-022-02476-1
23. da Silva JL, de Paula BHR, Small IA, Thuler LCS, de Melo AC. Sociodemographic, clinical, and pathological factors influencing outcomes in locally advanced triple negative breast cancer: a Brazilian Cohort. Breast Cancer (Auckl). 2020;14:1178223420962488. doi:10.1177/1178223420962488
24. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi:10.1158/1078-0432.CCR-06-3045
25. Peintinger F, Sinn B, Hatzis C, et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod Pathol. 2015;28(7):913–920. doi:10.1038/modpathol.2015.53
26. de Calbiac O, Lusque A, Mailliez A, et al. Comparison of management and outcomes in ERBB2-Low vs ERBB2-Zero metastatic breast cancer in France. JAMA Netw Open. 2022;5(9):e2231170. doi:10.1001/jamanetworkopen.2022.31170
27. Qaiser T, Mukherjee A, Reddy PC, et al. HER2 challenge contest: a detailed assessment of automated HER2 scoring algorithms in whole slide images of breast cancer tissues. Histopathology. 2018;72(2):227–238. doi:10.1111/his.13333
28. Farahmand S, Fernandez AI, Ahmed FS, et al. Deep learning trained on hematoxylin and eosin tumor region of Interest predicts HER2 status and trastuzumab treatment response in HER2+ breast cancer. Mod Pathol. 2022;35(1):44–51. doi:10.1038/s41379-021-00911-w
29. Gustavson M, Haneder S, Spitzmueller A, et al. Abstract PD6-01: novel approach to HER2 quantification: digital pathology coupled with AI-based image and data analysis delivers objective and quantitative HER2 expression analysis for enrichment of responders to trastuzumab deruxtecan (T-DXd; DS-8201), specifically in HER2-low patients. Cancer Res. 2021;81(4_Supplement):PD6–01. doi:10.1158/1538-7445.SABCS20-PD6-01
30. Businesswire. Paige answers call to better identify breast cancer patients with low expression of HER2; 2022. Available from: https://www.businesswire.com/news/home/20220623005253/en/Paige-Answers-Call-to-Better-Identify-Breast-Cancer-Patients-with-Low-Expression-of-HER2.
31. Moutafi M, Robbins CJ, Yaghoobi V, et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab Invest. 2022;102(10):1101–1108. doi:10.1038/s41374-022-00804-9
32. Kennedy JJ, Whiteaker JR, Kennedy LC, et al. Quantification of human epidermal growth factor receptor 2 by immunopeptide enrichment and targeted mass spectrometry in formalin-fixed paraffin-embedded and frozen breast cancer tissues. Clin Chem. 2021;67(7):1008–1018. doi:10.1093/clinchem/hvab047
33. Xu K, Bayani J, Mallon E, et al. Discordance between Immunohistochemistry and Erb-B2 Receptor Tyrosine Kinase 2 mRNA to determine human epidermal growth factor receptor 2 low status for breast cancer. J Mol Diagn. 2022;24(7):775–783. doi:10.1016/j.jmoldx.2022.04.002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

