Back to Journals » Journal of Pain Research » Volume 16
Exploring Outcome Priorities and Real-Life Management of Chemotherapy-Induced Peripheral Neurotoxicity: A Survey of the Italian Association for the Study of Pain members
Authors Sardo S , Varrassi G , Scartozzi M, Pace MC, Schweiger V, Tamburin S , Musu M, Finco G
Received 27 March 2023
Accepted for publication 18 September 2023
Published 25 September 2023 Volume 2023:16 Pages 3227—3238
DOI https://doi.org/10.2147/JPR.S414389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Salvatore Sardo,1 Giustino Varrassi,2 Mario Scartozzi,3 Maria Caterina Pace,4 Vittorio Schweiger,5 Stefano Tamburin,6 Mario Musu,1 Gabriele Finco1
1Department of Medical Sciences and Public Health, University of Cagliari, Monserrato, Italy; 2Paolo Procacci Foundation, Rome, Italy; 3Medical Oncology Unit, University Hospital and University of Cagliari, Monserrato, Italy; 4Department of Anesthesia and Pain Medicine, University of Napoli, Napoli, Italy; 5Department of Anesthesia and Pain Medicine, University of Verona, Verona, Italy; 6Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy
Correspondence: Salvatore Sardo, Department of Medical Sciences and Public Health, University of Cagliari, Monserrato, Italy, Email [email protected]
Introduction: Chemotherapy-induced peripheral neurotoxicity (CIPN) affects nearly 70% of cancer patients after chemotherapy, causing sensory, motor, autonomic dysfunction, and neuropathic pain. The Desirability of Outcome Ranking (DOOR) framework is proposed as a better way to assess preventive or therapeutic interventions for CIPN.
Methods: A survey was conducted among Italian healthcare professionals and researchers affiliated to the Italian Chapter of the International Association for the Study of Pain (AISD) to identify the most important outcomes in clinical management and research.
Results: Among the 73 respondents, 61 qualified for the survey, with an overall response rate of 1.2%. The vast majority were physicians (77%), most of whom were anesthesiologists (47.5%). The results showed that pain, survival, sensory impairment, motor impairment, and quality of life were consistently ranked as the most important outcomes, but there was significant disagreement in the outcomes relative ranking, making it difficult to develop a DOOR algorithm. The study also revealed that clinicians commonly use structured interviews to evaluate patients with CIPN, and the most prescribed drugs or supplements were palmitoylethanolamide, pregabalin, gabapentin and alpha lipoic acid as preventive agents and pregabalin, palmitoylethanolamide, duloxetine, gabapentin, and amitriptyline as therapeutic agents. However, many of these drugs have not been clinically proven to be effective for CIPN.
Discussion: This study suggests that the implementation of a DOOR framework for CIPN using healthcare professionals is more difficult than expected, given the significant disagreement in our respondents’ ranking of outcomes. Our work provides interesting topics for future research in CIPN, but its limitations include a small sample size, a low response rate, and a possible selection bias.
Keywords: neuropathic pain, peripheral neuropathy, cancer, taxane, Desirability of Outcome Ranking, survey, chemotherapy induced peripheral neuropathy
Introduction
Modern oncology has undergone a significant evolution with numerous drugs being introduced and many years of life saved for millions of patients. Unfortunately, for many of them and for the clinicians who struggle with cancer through antineoplastic drugs, chemotherapy-induced peripheral neurotoxicity (CIPN) represents a sobering and hurtful situation. CIPN is defined as peripheral neuropathy with sensory, motor, autonomic dysfunction, and neuropathic pain arising from the administration of different classes of antineoplastic drugs, especially taxanes, platinum derivatives, and vinca alkaloids. At the end of the chemotherapy administration cycle, it is estimated that almost 70% of patients are affected by CIPN and approximately 30% will be symptomatic 6 months after chemotherapy completion.1
Despite being an iatrogenic condition that occurs quite frequently during chemotherapy treatments with individual differences regarding onset and duration that might be related to individual risk and the class and dosage of drugs, there is still discussion on the exact incidence of CIPN and the best tools to quantify its characteristics, its severity, and its burden on quality of life.
The risk of developing CIPN and its onset are quite variable, with microtubule-targeting agents and proteasome inhibitors being more associated with a higher risk than platinum and thalidomide agents.2
As confirmed by current guidelines and reviews, we have not enough evidence to support a strategy of pharmacologic primary prevention, while the effective drugs for managing established CIPN are still few and have disappointing results.3,4
As stated in the “Analgesic, Anesthetic and Addiction Clinical Trial Translations, Innovations, Opportunities and Networks (ACTTION)–Consortium on Clinical Endpoints and Procedures for Peripheral Neuropathy Trials”, a unidimensional perspective on CIPN is not appropriate to evaluate the effects of preventive or therapeutic interventions effects.5 Combination of multiple outcomes in a Desirability of Outcome Ranking (DOOR) framework is a relatively new way of assessing the effects of interventions. DOOR is based on the creation of multiple overall clinical outcomes composed of different unidimensional measures. The ranking of these outcomes is based on the expected desirability of clinicians and participants from the highest and best to the lowest and worst.6
This approach could better describe a complex clinical situation where a harm/benefit balance cannot be reliably measured by a single outcome. For example, chemotherapy interruption or dose reduction may cause an improvement in CIPN severity, but it could result in a reduced survival of patients. To the best of our knowledge, the DOOR methodology was never used in this setting.
Considering this premise, to investigate CIPN in clinical trials under this methodological framework, we should assess which outcomes are most important to stakeholders, namely patients, healthcare professionals, researchers.
Materials and Methods
The authors designed an electronic survey form on a secure web-based electronic data capture system (Redcap, Vanderbilt University) hosted at Cagliari State University (Supplementary Figures S1–S10).7
Our survey main aims were:
1) assess the relative importance or priorities allocated to the main outcomes in CIPN patients management;
2) measure the consensus in the outcomes ranking in order to assess the feasibility of a Desirability of Outcome Ranking (DOOR) framework;
3) establish the current clinical practice in the prevention or treatment of CIPN;
4) investigate the use of structured questionnaires or tools.
This survey was structured to screen out healthcare practitioners or researchers who were not actively involved with CIPN patients.
This strict filter was introduced to collect data that could better represent the actual scenario of CIPN management.
The first section was designed to describe the role, experience, educational background, and clinical setting of the respondents using closed-ended questions with a single answer.
The second section investigated the value attributed to widely studied and relevant outcomes: survival, sensory impairment, motor impairment, autonomic impairment, severity of pain, CIPN- related quality of life, chemotherapy completion rate, safety of treatment. This assessment was aimed to measure both the absolute and the relative values in guiding clinical management or research planning. The absolute value was measured through a 100 mm Visual Analogue Scale (VAS), while the relative value was evaluated through a ranked matrix of fields to force the attribution of a single ank to each single outcome.
Every respondent could report their use of non-pharmacologic treatment, while, according to the self-reported professional title, only physicians and clinical researchers could express their preferences about pharmacologic prophylaxis or therapy. This section was designed using multiple-choice questions.
The invitation to the survey was disseminated via mailing list to the members of the Italian Chapter of the International Association for the Study of Pain (“Associazione Italiana per lo Studio del Dolore”, AISD). No personal data or any type of identifier were collected.
Data were analyzed with descriptive statistics, using mean and standard deviation (sd) or median and interquartile range (IQR), as appropriate. Pie and count bar plots were generated with Matplotlib package version 3.6.2 in Python 3.10.8 Outcomes ranking was analyzed using branch-and-bound and heuristic algorithms to find consensus (median) ranking according to the Kemeny’s axiomatic approach, as implemented in the R Package ConsRank version 2.1.2.9–13
This method of ranking analysis was preferred over Kendall’s tau to better address ties and missing data.14
Results
Survey Results
Respondents
The survey link was distributed to every AISD member via mailing list, and the response rate was 1.2%. Out of 73 respondents, 61 were actively involved in CIPN care or research, six did not provide an answer to this screening question, and twelve were not involved in CIPN care or research.
The authors analyzed only the responses of the 61 respondents who reported to be involved in CIPN management or research.
Among respondents, the vast majority were physicians (47) and nurses (13) in different stages of their careers.
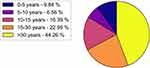
The entire cohort was based in Italy. The main characteristics of respondents’ cohort were described in Figures 1–3.
Outcome Importance and Ranking
The VAS scores were quite similar with a remarkably skewed distribution towards higher values (Figure 4). The highest median scores were attributed to CIPN-related quality of life (median 92 mm, interquartile range IQR 81.5–87.7 mm) and pain intensity (median 91 mm, IQR 83–99 mm), the lowest score to chemotherapy completion rate (median 72 mm, IQR 50–85 mm).
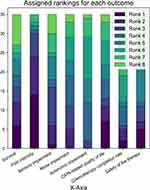
Outcomes rankings were available for 61 participants, of whom only 35 provided a complete ranking and three missed a single rank value (Figure 5). The median ranking was pain intensity as the most important outcome, followed by survival, CIPN-related quality of life, sensory impairment, motor impairment, autonomic impairment, safety of therapy, chemotherapy completion rate. The overall agreement was low (averaged TauX rank correlation coefficient 0.19).
Considering only full ranking reports (35/61), two consensus rankings emerged with the same first two rankings (pain 1st rank, survival 2nd rank) and last rankings (safety 6th rank, autonomic impairment 7th rank, chemotherapy completion 8th rank) and a different order for the outcomes sensory impairment (5th vs 3rd rank), motor impairment (3rd vs 4th rank), and CIPN-related quality of life (4th vs 5th rank), with a marginally higher agreement than full data analysis-derived consensus ranking (averaged TauX rank correlation coefficient 0.29).
Figure 5 shows the cumulative frequency of rankings attributed to each outcome.
Drugs or Supplements for CIPN
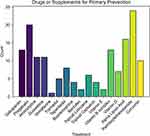
Among the 47 physicians, 35 respondents reported the prescription of drugs or supplements for the primary prevention of CIPN (Figure 6). Based on 35 respondents, the most prescribed drugs were: palmitoylethanolamide (PEA, 24, 68.6%), pregabalin (20, 57.1%), alpha lipoic acid (16, 45.7%), and gabapentin (13, 37.1.%).
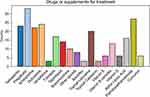
Forty-two respondents reported the prescription of drugs or supplements to treat CIPN (Figure 7). The most common choices among these respondents were pregabalin (33, 78.6%), PEA (27,77.1%), duloxetine (24, 57.1%), gabapentin (23, 54.8%), and amitriptyline (22, 52.4%).
Nonpharmacological Treatment of CIPN
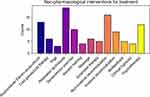
Thirty-eight respondents reported the use of nonpharmacological treatments (Figure 8). The most reported techniques were relaxation techniques (19, 50%), non-invasive neuromodulation (16,42.1%), and psychotherapy (12, 31.6%).
Use of Structured Interviews or Tools to Assess CIPN
Severity or CIPN-Related Quality of Life
About half of respondents reported using tools or structured interviews to assess their CIPN patients (28, 46%). The most used tools and scales were: Brief Pain Inventory (BPI) and Douleur Neuropathique 4 (DN4, 13, 46.4%), McGill Pain Questionnaire (10, 35.7%). Full data are displayed in Figure 9.
Discussion
This survey provides an insight into the current clinical practice among a multidisciplinary group of Italian healthcare professionals and researchers affiliated to the AISD.
All participants found the task of providing an absolute measure of outcome importance to be straightforward. However, the ratings were highly skewed toward the upper limit of the VAS range, which made it challenging to differentiate between the priority of one outcome over another.
A significant proportion of participants, about one-half of the sample, left the ranking section incomplete. This result highlights the perceived difficulty or uneasiness of applying a rigid prioritization of outcomes. We perceived this issue by comparing the number of missing responses between the absolute measure through VAS score and the ranking field of our survey: the two sections were completed by 97% and 57% of responders, respectively.
Furthermore, the analysis of the available ranking values demonstrated a significant disagreement between raters.
Our findings suggest that participants were uncertain about how to prioritize outcomes in the context of managing CIPN. Moreover, the extent of disagreement among participants in ranking the proposed outcomes was high, precluding the use of these measures to support the creation of a DOOR algorithm.
The hurdles to implementing the DOOR framework on this topic could be related to multiple issues. The Antibacterial Resistance Leadership Group successfully applied the DOOR framework in their randomized controlled clinical trial, “Short- vs Standard-Course Outpatient Antibiotic Therapy for Community-Acquired Pneumonia in Children: The SCOUT- CAP Randomized Clinical Trial.”15 This clinical trial investigated the effect of two different regimens of antibiotics, taking into account the adequate clinical response, the resolution of pneumonia symptoms, and the maximal severity of drug-induced adverse effects. The nature of these endpoints contributing to the DOOR ranking is quite objective, and they could be more readily recognized and agreed upon by clinicians. Most of our outcomes are patient-reported and are variably interconnected. For example, the burden of the different positive or negative symptoms of CIPN could affect patients in a variable way according to many biopsychosocial factors. We expect that these factors could be important mediators of both the perceived quality of life and the scores of our assessments. If we acknowledge the low agreement rate in a small sample of healthcare professionals sharing the same cultural and, for most of them, educational background, we could presume even lower agreement if we performed the same survey on a wider and more heterogeneous sample of professionals. A possible solution could be to identify some patterns of preferences expressed by the patients and present the results of new trials incorporating the most common systems of belief as different rankings instead of adopting a single desirability of outcome ranking. This approach could lead to a personal belief phenotype that could put the patients at the center of management not only by identifying the main features and pathophysiological mechanisms of their neuropathy but also their choices and values about treatments and priorities for outcomes.
Currently, there is no solid evidence to suggest a pharmacological intervention for CIPN primary prevention. Moderate quality evidence suggests that exercise during chemotherapy may improve sensory symptoms, prevent strength loss, improve balance, reduce falls and related injuries and stabilize the functional capacity of patients16 of interest, most of the respondents preferred nutraceutical and supplements to pharmacological treatments, probably because of the limited efficacy of the currently recommended drugs for neuropathic pain related to CIPN.
According to our results, the most reported supplement (67.6%) was PEA, an endogenous endocannabinoid mediator marketed as a nutraceutical product. The rationale for this choice might be related to the role of endocannabinoids as regulatory system in microglial neuroinflammation.17,18 Furthermore, as many other neuropathic conditions, neuroinflammation and microglial phenotypic change are fundamental mechanisms in the pathogenesis of CIPN.19,20 PEA modulates the cannabinoid receptors 2 (CB2) expression and potentiates the activity of other endocannabinoid mediators, and its role as a preventive or therapeutic has been demonstrated in animal models of CIPN induced by oxaliplatin and taxanes.20–22 Furthermore, in a small clinical study, PEA administration to patients affected by painful CIPN has shown a beneficial effect on myelinated nerve fibers function and pain.23 As confirmed by the results of this survey, most respondents consider PEA as a first line agent for CIPN management, despite no robust evidence is available and this nutraceutical is not mentioned in CIPN guidelines.4,24
Among nutraceutical treatments, 48.6% of respondents reported the prescription of Alpha lipoic acid (ALA), that is an endogenous antioxidant and anti-inflammatory molecule.25 It directly neutralizes reactive oxygen species and indirectly regenerates other antioxidant agents, such as glutathione. It also chelates heavy metals and toxins.25 In a recent small, randomized placebo-controlled clinical trial, ALA improved the neurotoxicity CTCTAE grading of breast cancer patients undergoing taxane chemotherapy.26 Indeed, moderate grade evidence supports the use of ALA for CIPN prevention.24
Considering pharmacologic treatments, most respondents reported following current therapeutical guidelines for neuropathic pain management.27 Most of the respondents use gabapentinoids and antidepressant drugs as therapeutic agents for the establish CIPN, as supported by current clinical guidelines and evidence.27 On the contrary, the use of gabapentinoids and antidepressant drugs was also reported as preventive treatment, despite they are not currently approved for this use. Gabapentinoids, gabapentin and pregabalin, are selective antagonists of neuronal voltage-dependent calcium channels and their mechanism of action is critically dependent on the binding of subunit α2δ-1.28,29
Despite the pharmacological choices of most respondent are in accordance with the therapeutical guidelines for neuropathic pain, they do not conform to treatment guidelines on CIPN that stipulate duloxetine as the only agent that has appropriate evidence to support its use for patients with established painful CIPN and suggest avoiding gabapentinoids in this condition.4,24,27 Relaxation techniques, mindfulness meditation, yoga and biofeedback have been reported to show some beneficial effects on pain and CIPN-related quality of life and may be considered according to moderate evidence.4,24,30,31
About half of those surveyed reported the use of nonpharmacologic strategies to manage CIPN. This demonstrates how current drug therapy is still not enough to address all components of the CIPN experience, and it highlights the extent to which the biopsychosocial paradigm supported by our leading scientific societies has been internalized by practitioners.
Psychotherapy addresses the cognitive and behavioral aspects of the biopsychosocial model of chronic pain, and it may target specific neural networks involved in the pain experience processing.32 Cognitive and behavioral features are significantly related to quality of life, disability, social isolation, and overall suffering.33
About 42% of our respondents reported the use of non-invasive neuromodulation to treat neuropathic pain related to CIPN. Specifically, transcutaneous electrical nerve stimulation (TENS) was the most reported, while Scrambler therapy was less represented in our results. Scrambler therapy has some support by current evidence, as shown by a crossover randomized trial that resulted in an improvement of different patient-reported outcomes.34
Spinal Cord Stimulation has been described only in small case series or case reports, so, despite the improvements reported, the evidence is insufficient to recommend it for CIPN patients.12,13
Most healthcare professionals of our cohort reported the use of a scoring system to assess CIPN severity. The most used tools were not CIPN-specific scales, but neuropathic pain scoring systems, such as BPI, DN4, McGill Pain Questionnaire. The Brief Pain Inventory was designed by the Pain Research Group of the WHO Collaborating Center for Symptom Evaluation in Cancer Care.35 It includes both a body picture to localize pain sites and the core questions about the pain intensity at different times, drugs and their effects, and interference with activities, mood, and sleep.35 It has been validated for clinical and research purposes.36 The Douleur Neuropathique DN4 has been designed to identify probable neuropathic pain and it is composed of an assessment of qualitative features of pain and objective signs evoked by the clinician.37 The McGill Pain Questionnaire was designed by Melzack to provide a comprehensive inventory of pain verbal descriptors and a body picture to indicate the anatomical location.38,39
CIPN-specific tools were used by a minority of respondents, especially the most comprehensive and reliable scales, such as European Organization for Research and Treatment of Cancer (EORTC QLQ – CIPN20) and Functional Assessment of Cancer Therapy/Gynecologic Oncology Group—Neurotoxicity (FACT/GOG-Ntx).40–42 This finding may depend on the sample not adequately representing oncologists. The main limitations of this survey lie in the size and the features of our sample. It should be noted that all participants were based in Italy and affiliated with the Italian Association for the Study of Pain (AISD), which may limit the generalizability of the results to other countries.
Since this survey was aimed at assessing the feasibility of a DOOR ranking assessing the beliefs of healthcare practitioners instead of setting a standard ranking as a future reference for CIPN research, we did not perform a formal a priori estimation of the required sample size. Finally, we acknowledge that a significant proportion of patients with CIPN are managed by oncologists in our country, and in our sample only 5 respondents reported Oncology as their primary specialization. Furthermore, it is possible that our survey participants’ affiliation with AISD may have led to an overestimation of the importance of certain treatments or structured interviews.
Conclusions
The results of this survey highlight the intrinsic hurdles for the implementation of a DOOR framework in pain medicine research, specifically for the topic of chemotherapy-induced peripheral neurotoxicity. Despite the quite small size and relative homogeneity of our respondents’ sample, no significant consensus emerged to rank clinical outcomes to define a Desirability of Outcome Ranking (DOOR) algorithm. We believe that our results could be replicated in a wider survey involving a more heterogeneous sample, and this poses a serious risk to the implementation of DOOR as suggested by the research experts of the ACTTION panel. Nevertheless, we do not believe that our findings should discourage further attempts at implementation of the DOOR framework in the CIPN trials, but they could suggest diversifying DOOR rankings based on patient studies on the priority of outcomes to address the expected differences in beliefs and choices. Our findings show that healthcare professionals commonly use structured interviews to assess patients with chemotherapy-induced peripheral neurotoxicity (CIPN), and frequently prescribe drugs or supplements whose effectiveness has not yet been supported by current evidence. While the survey provides interesting insights into potential areas for future research in CIPN, its findings are limited by the small sample size and a potential selection bias.
Institutional Review Board Statement
The Institutional Research Board (IRB) of the University of Cagliari considered this research to be exempt from formal evaluation because it was an anonymous online survey collecting the opinions of healthcare professionals without sensitive personally identifiable information.
Data Sharing Statement
Raw data without any identifier were uploaded to a public repository (Open Science Framework repository) under a CC-By Attribution 4.0 International license. They are freely accessible at the following website https://osf.io/hxdc5/.
Acknowledgments
This survey was endorsed by the Italian Chapter of the International Association for the Study of Pain AISD (Associazione Italiana per lo Studio del Dolore). The authors are particularly grateful to Lorenza Saini, AISD Secretary, for her valuable support in conducting our survey. We thank our colleagues for sharing with us details on their clinical or research practice and their personal views on outcomes priority.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
Prof. Dr. Mario Scartozzi reports personal fees from MSD, MERCK, AMGEN, and GSK, outside the submitted work. The authors declare no other conflicts of interest in this work.
References
1. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi:10.1016/j.pain.2014.09.020
2. Kishimoto S, Oshima N, Rinker M, et al. Identification of high-risk drugs related to chemotherapy- induced peripheral neuropathy in cancer therapy evaluation program-sponsored Phase I trials. Eur J Cancer. 2019;115:111–119. doi:10.1016/j.ejca.2019.04.023
3. Mezzanotte JN, Grimm M, Shinde NV, et al. Updates in the treatment of chemotherapy- induced peripheral neuropathy. Updates in the treatment of chemotherapy-induced peripheral neuropathy. Curr Treat Options in Oncol. 2022;23:29–42. doi:10.1007/s11864-021-00926-0
4. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020;38(28):3325–3348. doi:10.1200/JCO.20.01399
5. Gewandter JS, Brell J, Cavaletti G, et al. Trial designs for chemotherapy-induced peripheral neuropathy prevention: ACTTION recommendations neurology. Neurology. 2018;91(9):403–413. doi:10.1212/WNL.0000000000006083
6. Evans SR, Rubin D, Follmann D, et al. Desirability of Outcome Ranking (DOOR) and Response Adjusted for Duration of Antibiotic Risk (RADAR). Clin Infect Dis. 2015;61(5):800–806. doi:10.1093/cid/civ495
7. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)-A metadata- driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi:10.1016/j.jbi.2008.08.010
8. Hunter JD. Matplotlib: a 2D graphics environment. Comput Sci Eng. 2007;9:90–95. doi:10.1109/MCSE.2007.55
9. R Core Team. R: a language and environment for statistical computing; 2014.
10. D’ambrosio A, Amodio S, Mazzeo G. ConsRank: compute the median ranking(s) according to the Kemeny’s axiomatic approach; 2021. Available from: https://cran.r-project.org/web/packages/ConsRank/ConsRank.pdf.
11. Amodio S, Ambrosio A, Siciliano R. Accurate algorithms for identifying the median ranking when dealing with weak and partial rankings under the Kemeny axiomatic approach. Eur J Oper Res. 2016;249(2):667–676. doi:10.1016/j.ejor.2015.08.048
12. Abd-Elsayed A, Gyorfi M, Hughes M. Spinal cord stimulator for treating chemotherapy-induced peripheral neuropathy. Pain Med Case Rep. 2021;5:4.
13. Kamdar MM, Mccall LW, Saba AM, et al. Improvement in neuropathic pain, proprioception, and gait stability after spinal cord stimulator implantation for chemotherapy-induced peripheral neuropathy. Pain Med Case Rep. 2021;5(6):6. doi:10.36076/pmcr.2021.5.291
14. Emond EJ, Mason DW. A new rank correlation coefficient with application to the consensus ranking problem. J Multi-Criteria Decis Anal. 2002;11(1):17–28. doi:10.1002/mcda.313
15. Williams DJ, Creech CB, Walter EB, et al. Short- vs Standard-Course Outpatient Antibiotic Therapy for Community-Acquired Pneumonia in Children: the SCOUT-CAP randomized clinical trial. JAMA Pediatr. 2022;176(3):253–261. doi:10.1001/jamapediatrics.2021.5547
16. Tamburin S, Park SB, Schenone A, et al. Rehabilitation, exercise, and related non- pharmacological interventions for chemotherapy-induced peripheral neurotoxicity: systematic review and evidence-based recommendations. Crit Rev Oncol Hematol. 2022;171:103575. doi:10.1016/j.critrevonc.2021.103575
17. Chen G, Zhang YQ, Qadri YJ, et al. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100(6):1292–1311. doi:10.1016/j.neuron.2018.11.009
18. Zajączkowska R, Kocot-Kępska M, Leppert W, et al. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 2019;20(6):1451.
19. Naguib M, Xu JJ, Diaz P, et al. Prevention of paclitaxel-induced neuropathy through activation of the central cannabinoid Type 2 receptor system. Anesth Analg. 2012;114(5):1104–1120. doi:10.1213/ANE.0b013e31824b0191
20. Cristiano C, Avagliano C, Cuozzo M, et al. The beneficial effects of ultramicronized palmitoylethanolamide in the management of neuropathic pain and associated mood disorders induced by paclitaxel in mice. Biomolecules. 2022;12(8):1155. doi:10.3390/biom12081155
21. Mannelli LDC, Pacini A, Corti F, et al. Antineuropathic profile of N-palmitoylethanolamine in a rat model of oxaliplatin-induced neurotoxicity. PLoS One. 2015;10:128080.
22. Donvito G, Wilkerson JL, Damaj MI, et al. Palmitoylethanolamide reverses paclitaxel-induced allodynia in mice. J Pharmacol Exp Ther. 2016;359(2):310–318. doi:10.1124/jpet.116.236182
23. Truini A, Biasiotta A, Stefano GD, et al. Palmitoylethanolamide restores myelinated-fibre function in patients with chemotherapy-induced painful neuropathy. CNS Neurol Disord Drug Targets. 2011;10(8):916–920. doi:10.2174/187152711799219307
24. Jordan B, Margulies A, Cardoso F, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020;31(10):1306–1319. doi:10.1016/j.annonc.2020.07.003
25. Dinicola S, Fuso A, Cucina A, et al. Natural products - alpha-lipoic acid and acetyl-L-carnitine - in the treatment of chemotherapy-induced peripheral neuropathy. Eur Rev Med Pharmacol Sci. 2018;22(14):4739–4754. doi:10.26355/eurrev_201807_15534
26. Werida RH, Elshafiey RA, Ghoneim A, et al. Role of alpha-lipoic acid in counteracting paclitaxel- and doxorubicin-induced toxicities: a randomized controlled trial in breast cancer patients. Support Care Cancer. 2022;30(9):7281–7292. doi:10.1007/s00520-022-07124-0
27. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
28. Patel R, Dickenson AH. Mechanisms of the gabapentinoids and α 2 δ −1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect. 2016;4(2):e00205. doi:10.1002/prp2.205
29. Doan L. Voltage-gated calcium channels and pain. Reg Anesth Pain Manag. 2010;14:42–47. doi:10.1053/j.trap.2010.03.003
30. Knoerl R, Smith EML, Barton DL, et al. Self-guided online cognitive behavioral strategies for chemotherapy-induced peripheral neuropathy: a multicenter, pilot, randomized, wait-list controlled trial. J Pain. 2018;19:382–394. doi:10.1016/j.jpain.2017.11.009
31. Knoerl R, Giobbie-Hurder A, Berfield J, et al. Yoga for chronic chemotherapy-induced peripheral neuropathy pain: a pilot, randomized controlled trial. J Cancer Surviv Res Pract. 2022;16:882–891.
32. Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neurosci Biobehav Rev. 2014;39:61–78. doi:10.1016/j.neubiorev.2013.12.006
33. Sturgeon J. Psychological therapies for the management of chronic pain. Psychol Res Behav Manag. 2014;115. doi:10.2147/PRBM.S44762
34. Loprinzi C, Le-rademacher JG, Majithia N, et al. Scrambler therapy for chemotherapy neuropathy: a randomized Phase II pilot trial. Support Care Cancer. 2020;28:1183–1197. doi:10.1007/s00520-019-04881-3
35. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med. 1994;23:129–138.
36. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
37. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29–36. doi:10.1016/j.pain.2004.12.010
38. Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi:10.1016/0304-3959(75)90044-5
39. Melzack R, Raja S. The McGill pain questionnaire: from description to measurement. Anesthesiology. 2005;103:199–202. doi:10.1097/00000542-200507000-00028
40. Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC Quality of Life Questionnaire to assess Chemotherapy-Induced Peripheral Neuropathy: the QLQ-CIPN20. Eur J Cancer Oxf Engl. 1990;41:1135–1139. doi:10.1016/j.ejca.2005.02.012
41. Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13:741–748.
42. Li T, Park SB, Battaglini E, et al. Assessing chemotherapy-induced peripheral neuropathy with patient reported outcome measures: a systematic review of measurement properties and considerations for future use. Qual Life Res. 2022;31(11):3091–3107. doi:10.1007/s11136-022-03154-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.