Back to Journals » Drug Design, Development and Therapy » Volume 17
Evaluation of the Effect of New Multimodal Analgesia Regimen for Cardiac Surgery: A Prospective, Randomized Controlled, Single-Center Clinical Study
Authors Jin L, Liang Y, Yu Y, Miao P, Huang Y, Xu L, Wang H, Wang C, Huang J, Guo K
Received 24 February 2023
Accepted for publication 23 May 2023
Published 7 June 2023 Volume 2023:17 Pages 1665—1677
DOI https://doi.org/10.2147/DDDT.S406929
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Lin Jin,1 Yafen Liang,2 Ying Yu,1 Peng Miao,1 Yihao Huang,1 Liying Xu,1 Huilin Wang,1 Chunsheng Wang,3 Jiapeng Huang,4 Kefang Guo1
1Department of Anesthesia, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Department of Anesthesiology, University of Texas Health Center at Houston, Houston, TX, USA; 3Department of Cardiac Surgery, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 4Department of Anesthesiology & Perioperative Medicine University of Louisville, Louisville, KY, USA
Correspondence: Kefang Guo, Department of Anesthesia, Zhongshan Hospital, Fudan University, No. 180, Fenglin Road, Xuhui District, Shanghai, People’s Republic of China, Email [email protected] Jiapeng Huang, Department of Anesthesiology & Perioperative Medicine University of Louisville, Louisville, KY, 40202, USA, Email [email protected]
Objective: To investigate the feasibility of multimodal regimen by paracetamol, gabapentin, ketamine, lidocaine, dexmedetomidine and sufentanil among cardiac surgery patients, and compare the analgesia efficacy with conventional sufentanil-based regimen.
Design: A single-center, prospective, randomized, controlled clinical trial.
Setting: One participating center, the cardiovascular center of the major integrated teaching hospital.
Participants: A total of 115 patients were assessed for eligibility: 108 patients were randomized, 7 cases were excluded.
Interventions: The control group (group T) received conventional anesthesia management. Interventions in the multimodal group (group M) were as follows in addition to the standard of care: gabapentin and acetaminophen 1 hour before surgery; ketamine for induction and to maintain anesthesia with lidocaine and dexmedetomide. Ketamine, lidocaine, and dexmedetomidine were added to routine sedatives postoperatively in group M.
Measurements and Main Results: The incidence of moderate-to-severe pain on coughing made no significant difference (68.5% vs 64.8%, P=0.683). Group M had significantly less sufentanil use (135.72μg vs 94.85μg, P=0.000) and lower rescue analgesia rate (31.5% vs 57.4%, P=0.007). There was no significant difference in the incidence of chronic pain, PONV, dizziness, inflammation index, mechanical ventilation time, length of stay, and complications between the two groups.
Conclusion: Our multimodal regimen in cardiac surgery is feasible, but was not superior to traditional sufentanil-based regimen in the aspects of analgesia effects; however, it did reduce perioperative opioid consumption along with rescue analgesia rate. Moreover, it showed the same length of stay and the incidences of postoperative complications.
Keywords: cardiac surgery, multimodal analgesia, prognosis
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Chen has been published for this article.
Introduction
More than 1.5 million patients underwent cardiac surgery each year all over the world. In 2019, the number of cardiac surgeries in China reached to 253,867.1 Postoperative pain is still an unresolved clinical problem, which can cause stress, delirium, respiratory complications, and prolong length of hospital stay.2 About 11–56% of patients suffer from chronic pain, which can affect sleep, reduce quality of life and increase medical costs even one year after surgery.3,4 Thus, pain intensity postoperatively is considered for inclusion as one of the six defined standard endpoints assessing patient comfort after surgery.5
Opioids are the essential medication in the anesthesia and analgesia of cardiac surgery. However, opioids have many unpreventable side effects, including respiratory depression, nausea and vomiting, etc., which could affect the postoperative recovery.6 With the development of Enhanced Recovery After Cardiac Surgery (ERACS), clinical evidence showed that the combination of different analgesics can reduce the dosage of opioids and also achieve better pain management.7 Nevertheless, the level of evidence recommended by the ERACS guidelines for multimodal analgesia is B-NR (nonrandomized studies). It is necessary to design randomized controlled trials to verify the efficacy of multimodal analgesia.
Our study aims to investigate the feasibility of multimodal regimen by paracetamol, gabapentin, ketamine, lidocaine, dexmedetomidine and sufentanil among cardiac surgery, to compare their analgesia efficacy with a conventional sufentanil-based regimen.
Materials and Methods
Study Population
This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (Ethics approval number: B2018-039R, Chairperson Xinyu Qin, approved on 17 April 2018). This trial was registered prior patients’ enrollment at Chinese Clinical Trial Registry (ChiCTR2000038585, Principal investigator: Lin Jin, Date of registration: 24 September 2020). All methods were performed in accordance with the relevant guidelines and regulations. This study was conducted during October 2020 to September 2022. The conduction of this study complies with the Declaration of Helsinki. Our study protocol was evaluation of the effect of new multimodal analgesia regimen for cardiac surgery: a prospective, randomized controlled, single-center clinical study (English Version 1.0). The protocol has been provided as Supplementary Material.
This study was designed as a single-center, prospective, randomized, controlled clinical trial. The inclusion criteria were: (1) Patients aged 18 to 80 years who underwent elective sternotomy with cardiopulmonary bypass cardiac surgery; (2) ASA Grades II–III; (3) Body mass index (BMI) 18–31; (4) Voluntary participation and signed informed consent. The exclusion criteria were: (1) Unwilling to join the study or unable to communicate; (2) Minimally invasive cardiac surgery; (3) People with liver and kidney dysfunction, defined as the values of serum alanine transaminase (ALT), aspartate transaminase (AST), urea nitrogen (BUN) or creatinine (Scr) more than twice the upper limit of the normal range (ULN); (4) People with uncontrolled diabetes, defined as fasting blood glucose higher than 7mmol/l after drug treatment; (5) People with sleep apnea; (6) Known history of alcohol, drugs or narcotics abuse; (7) Use of opioids or other analgesics within 3 months before surgery; (8) Chronic pain. Of 115 patients assessed for eligibility, 108 were randomized and 7 were excluded. A flowchart of the study process is shown in Figure 1.
 |
Figure 1 Flowchart of current study. |
All subjects provided written informed consent to participate in this clinical trial. The enrolled patients were randomly divided into 2 groups (1:1) using sequential numbered opaque sealed envelopes, created by a statistics expert using a computer-generated random sequence of numbers: traditional group (group T) and multimodal analgesia group (group M). All patients in both groups followed standard NPO guidelines before surgery. After the patients entered the operating room, the right internal jugular vein and left radial artery were catheterized. The electrocardiogram (ECG), pulse oxygen saturation (SpO2) and radial artery pressure were monitored.
Anesthesia Care
The anesthesia induction and maintenance plan for group T included: no preoperative medication, anesthesia was induced with midazolam 2mg, propofol target effect-site concentration 1–3μg/mL, sufentanil 0.2–0.5μg/kg, and rocuronium 0.9–1.2mg/kg. After tracheal intubation, sevoflurane 0.6–1 MAC and propofol target effect-site concentration 1–3μg/mL were used to maintain anesthesia. During the surgery, the attending anesthesiologist determined the dose and timing of sufentanil 0.1–0.2μg/kg and rocuronium 0.2–0.4mg/kg as necessary, based on the surgical procedure, hemodynamic parameters, muscle relaxation, and adjust the dosage of sevoflurane and propofol to maintain BIS at 40–60.
The anesthesia induction and maintenance plan for group M included: gabapentin 300mg (reduced to 100mg for subjects >65 years old) and acetaminophen 500mg oral administration (per os, p.o) 1 hour before surgery; anesthesia was induced with midazolam 2mg, propofol target effect-site concentration 1–3μg/mL, sufentanil 0.2–0.5μg/kg, ketamine 0.5mg/kg, rocuronium 0.9–1.2mg/kg. After tracheal intubation, sevoflurane 0.6–1 MAC, propofol 1–3μg/mL, ketamine 5μg/kg/min (maximum total dose 3mg/kg), lidocaine 2mg/min, and dexmedetomidine 0.4μg/kg/h were used to maintain anesthesia. During the operation, the attending anesthesiologist will determine the dose and timing of sufentanil 0.1–0.2μg/kg and rocuronium 0.2–0.4mg/kg as necessary, based on the surgical procedure, hemodynamic parameters and muscle relaxation, and adjust the dosage of sevoflurane and propofol to maintain BIS at 40–60.
All patients received intravenous tropisetron 6mg and dexamethasone 4mg 30 minutes prior to the end of the surgery to prevent postoperative nausea and vomiting (PONV). After surgery, all patients were transferred to ICU for recovery. Patients in group T received continuous infusion of normal saline (as a placebo blind control) and propofol in ICU until initiation of the extubation procedure. Patients in group M received ketamine 5μg/kg/min (maximum total dose 3mg/kg), lidocaine 2mg/min, dexmedetomidine 0.4μg/kg/h and continuous infusion of propofol in ICU until the initiation of the extubation procedure. Criteria for extubation included: sufficiently awake with intact airway reflexes and adequate spontaneous breathing rate and tidal volume, stable hemodynamics, normal blood gas level and blood electrolyte, normal body temperature, less drainage and surgically controllable bleeding. If extubation failed, infusion of propofol was continued in ICU.
Gabapentin 300mg (reduced to 100mg for those >65 years old) and acetaminophen 500mg p.o three times a day starting from tracheal extubation till discharge for group M. A patient-controlled intravenous analgesia (PCIA) pump (1μg/mL sufentanil, background 0mL/h, bolus 4mL, lock-in time 10 min) was used for all patients after tracheal extubation until 72h after surgery. Training on using PCIA pumps was provided during preoperative visit and after tracheal extubation. If pain cannot be relieved after 3 consecutive bolus, or the patient cannot tolerate the side effects of the PCIA, rescue analgesia was provided by the on-call physicians.
Implementation of Blind Method
This trial was a single center, prospective, randomized, controlled clinical trial.
Attending anesthesiologists participating in the preoperative and intraoperative care were not blinded; they prepared the pumped drug ketamine, lidocaine, dexmedetomidine or 3 syringes of placebo saline for use in the ICU, with syringes labeled trial drug 1, trial drug 2, and trial drug 3, along with the rate of infusion. However, the staff responsible for follow-up and evaluation of study outcomes were blinded. The patients did not know the randomization allocations. Other participants did not know the random results.
Assessments
The level of pain was determined using a 100-millimeter visual analog scale (VAS). Patients were followed up twice a day (7–9am and 7–9pm) to record the VAS at rest and on coughing until discharge. The maximum VAS within the certain time period was recorded. VAS ≥4 is used as a criterion for poor analgesia effect and defined as moderate-to-severe pain. The incidences of daily moderate-to-severe pain after surgery were defined as the proportion of patients with a daily VAS score ≥4 at least once.
The dosage of sufentanil was recorded intraoperatively and daily until the third day after surgery. Postoperative dosage of sufentanil was defined as the total dosage of sufentanil for the first three days after surgery. The total dosage was defined as the total intraoperative dosage and postoperative dosage. It does not include opioids, which are used for rescue analgesia.
Rescue analgesia was defined as the once or more occurrence of any sort of analgesics provided by on-call physicians if pain cannot be relieved after 3 PCIA consecutive bolus or the patient cannot tolerate the side effects during the hospitalization, which were recorded for both groups every day until discharge.
The most common adverse reactions of opioid analgesia after cardiac surgery are PONV and dizziness. Nausea was defined as a subjective distasteful sensation connected with awareness of the urge to vomit, while vomiting was defined as the vigorous expulsion of gastric contents from the mouth. PONV positive was recorded whenever the patient experienced nausea or vomiting during the observation period, as was dizziness.
Venous blood samples were collected preoperatively, on the first, second and third morning after surgery to measure blood glucose and hematology. Systemic inflammatory indexes (including neutrophil/lymphocyte ratio, NLR; platelet/lymphocyte ratio, PLR; lymphocyte/monocyte ratio, LMR; and systemic immune-inflammation index, SII) were calculated according to the hematology result.
The mechanical ventilation time, length of ICU stay, and length of hospital stay were also collected.
Stroke, acute myocardial infarction, acute kidney injury, pulmonary complications and other complications were recorded for both groups until the end of follow-up, which were the most common complication after cardiac surgery. Cerebral complications were recorded as stroke, which was defined as a rapidly developing clinical syndrome of focal disturbance of cerebral function lasting more than 24 hours or leading to death, with radiological examination confirmed.
Transient ischaemic attacks (TIAs) with symptoms lasting less than 24 hours were not included in cerebral infarction calculation. Acute myocardial infarction was defined as CKMB over 80 units/L and/or ECG changes with chest pain, confirmed by coronary angiography. Acute kidney injury was defined as initiation of continuous renal replacement therapy determined by the attending cardiac surgeon and anesthesiologist. Pulmonary complications include four respiratory pathologies – atelectasis, pneumonia, acute respiratory distress syndrome, and pulmonary aspiration – which were confirmed by clinical signs, blood gas analysis and radiological evidence. All remaining adverse events mentioned in the medical history were recorded as other complications.
The patients were followed up by telephone at 3 months and 1 year after surgery, and the Bruggrmann Comfort Scale (BCS) was used to evaluate the postoperative chronic pain. The same investigator performed all the follow ups. Postoperative chronic pain was defined as surgical pain or discomfort (BCS ≤3) that lasted for at least 3 months after the surgery. The pain has to be localized to the original surgical or drainage tube incision which was well-healed, when all other causes of pain were excluded.
Primary Outcome
Primary outcome was the incidence of moderate-to-severe pain on coughing during hospitalization, which was defined as the proportion of patients with VAS score on coughing ≥4 at least once during hospitalization in each group.
Secondary Outcomes
Secondary outcomes included the VAS scores and the incidences of daily moderate-to-severe pain at rest and on coughing at the first, second and third postoperative day, and at discharged. The amounts of intraoperative, postoperative and total dosage of sufentanil; the occurrence of rescue analgesia during hospitalization; the incidence of daily PONV and dizziness for the first three days after surgery; blood glucose and NLR, PLR, LMR and SII on the first, second and third day after surgery; the mechanical ventilation time, length of ICU stay and length of hospital stay; in-hospital adverse events, including stroke, acute myocardial infarction, acute kidney injury, pulmonary complications; and the incidences of postoperative chronic pain at 3 months and 1 year after operation.
Statistical Analysis
Sample size was calculated based on our previous study with the same protocol, which showed the incidence of moderate-to-severe pain in group T at 64%, and group M at 35%. With a power of 80% and significance level of 5%, each group should recruit 43 patients. Therefore, 54 patients in each group was sufficient to meet the primary endpoint, with consideration of drop out.
Statistical analysis was performed by SPSS 20.0. Count data was expressed as the frequency (percentage), and the chi-square test or Fisher's exact test was used for comparisons between groups, and the two-sided significance value was evaluated. Measurement data were expressed as the mean (95% confidence interval) for continuous variables and the median (interquartile range) for discrete variables. Two sets of sample t-tests were used for the comparison of normally distributed data, the Wilcoxon rank-sum test was used for non-normally distributed data, and repeated measures analysis of variance/multiple level model was used for repeated measurement data. P<0.05 was defined as statistically significant difference.
Results
There were no significant difference in the demographic characteristics and baseline conditions between the two groups, including age (P=0.063), gender (P=0.441), height (P=0.567), weight (P=0.402), ASA status (P=0.548), New York Heart Association (NYHA) classification of cardiac function (P=0.699), surgical category (P=0.916), cardiopulmonary bypass (CPB) time (P=0.463), aortic cross clamp time (P=0.866) and transfusion requirements (P=0.428) (Table 1).
 |
Table 1 Demographic and Clinical Data of Patients |
Primary Outcome
The incidence of moderate-to-severe pain on coughing during hospitalization was 68.5% in group T and 64.8% in group M. There was no significant difference (P=0.683) (Table 2).
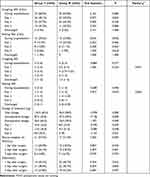 |
Table 2 Evaluation of Patients’ Opioid Dosage and Analgesic Effects |
Secondary Outcomes
The incidences of moderate-to-severe pain at rest and on coughing at the first, second and third day, discharged, and during hospitalization showed no differences between the two groups. Except that the coughing VAS score of group M was significantly lower than that of group T on the third day after surgery, there were no significant differences in VAS scores between the two groups. There was no significant difference in the dosage of sufentanil between the two groups on the first postoperative day. The intraoperative dosage of sufentanil, the dosage on the second and the third postoperative day, the postoperative dosage, and the total dosage in group M were significantly lower than those in group T. There were no significant differences in the incidence of PONV and postoperative dizziness between the two groups. The number of patients in group M who needed postoperative rescue analgesia was significantly lower than that in group T (Table 2).
There were no lost to follow-up cases at the 3-month follow-up. Only one patient in each group had chest wound pain at rest (1.9% vs 1.9%, P=1.000); 27 patients in group T (50.0%) and 23 patients (42.6%) in group M had wound pain on coughing or exercise. There was no significant difference between the two groups (χ2=0.596, P=0.440). At 1 year after surgery, 2 patients in group T and 4 patients in group M were lost to follow-up. No patient suffered chest wound pain at rest; 21 patients in group T (40.4%) and 13 patients in group M (26.0%) suffered wound pain on coughing or exercise. There were no significant differences between the two groups (χ2=2.373, P=0.123) (Table 3).
 |
Table 3 Comparison of Patients’ Prognosis Indicators |
There was no statistically significant difference in blood glucose levels (Figure 2) and four inflammatory biomarkers, including NLR (Figure 3), PLR (Figure 4), LMR (Figure 5) and SII (Figure 6), between the two groups preoperatively, on the first, second and third postoperative mornings.
 |
Figure 2 Comparison of patients’ blood glucose. Values are reported for group T (blue circles) and group M (red rhombus), with mean presented and their 95% confidence interval (error bars). |
The average mechanical ventilation times were 19.07h in group T and 17.44h in group M. The ICU length of stay was 32.08h in group T and 33.70h in group M. The average length of hospital stay was 7.46d in group T and 7.06d in group M. The differences between the two groups did not reach statistical significance (Figure 7). Two patients in group T suffered acute cerebral infarction after surgery, two patients suffered pulmonary complications, one patient suffered anxiety after surgery, and no cases suffered acute kidney insufficiency. Four patients in group M suffered pulmonary complications. There were no significant differences in in-hospital outcomes between the two groups. (χ2=3.086, P=0.243) (Table 3).
Discussion
Our study showed that this multimodal analgesia regimen is a feasible means of providing a similar analgesic effect when compared with the conventional protocol for cardiac surgery patients without increasing the incidence of complications. Multimodal analgesia significantly reduced the intraoperative opioid requirements, the total perioperative dosage of opioids, and the demand for postoperative rescue analgesia, which could improve patient comfort. However, it did not significantly reduce the incidence of moderate-to-severe pain or provide better analgesia. Whether a larger sample size will detect statistical differences remains unknown.8
The ERACS recommends a multimodal analgesia and opioid-less strategy for cardiac surgical patients.7 However, the ERACS did not give clear recommendations on specific choice of many non-opioid drugs. Among the many non-opioid drugs, acetaminophen has clear anti-inflammatory and analgesic effects.9 Pregabalin and gabapentin are both γ-aminobutyric acid (GABA) compounds, which inhibit the sensitivity of central neurons.10 Therapeutic dose of lidocaine can reduce the self-discipline of myocardium, and has no significant effect on cardiomyocyte electrical activity or myocardial contraction. With the increase in blood concentration, it can slow down conduction velocity and inhibit myocardial contractility.11 The dose of lidocaine used in our study was in a safe range, with anti-inflammatory and reduced neurological complication effects, and offering protective effects against myocardial ischemia and reperfusion injury after cardiac surgery.12 Ketamine has sedative and analgesic effects, which is known for inducing higher cardiac workload. It still can be used as a part of multimodal analgesia or multimodal general anesthesia in patients undergoing cardiac surgeries.13,14 Dexmedetomidine has dual effects of sedation and analgesia, which can also reduce the incidence of postoperative delirium.15 Magnesium has been used as a multimodal analgesic agent in some studies. But we do not think it is applicable to cardiac multimodal analgesic regimen. Signs of hypermagnesemia include flaccid paralysis, tachycardia widening of the QRS complex, and prolonged PQ interval. Bradycardia and hypotension may occur in severe cases. Furthermore, the magnesium level is not examined during our routine investigations, so magnesium is not part of our multimodal regimen in cardiac surgery.
Therefore, our study selected a multi-modal analgesic regimen which consists of acetaminophen, gabapentin, ketamine, lidocaine, and dexmedetomidine, in addition to sufentanil, to achieve the sedative, analgesic, and anti-inflammatory effects.
The 100mm visual analog scale (VAS) has high sensitivity to evaluate acute postoperative pain intensity.16 According to the VAS score, patients could be divided into four groups: mild or no pain (VAS score, 0–3), moderate pain (VAS score, 4–6), severe pain (VAS score, 7–8) and extreme pain (VAS score, 9–10). The last three groups are usually merged into moderate-to-severe pain group (VAS score, 4–10). Sleep disturbance, insomnia, depression, and anxiety are widespread in patients with moderate-to-severe pain and analgesics are usually required.17
We found that the incidence of moderate-to-severe pain on coughing during hospitalization after cardiac surgery was 67%; the incidence was highest (47%) on the first postoperative day, and then declined day by day, to 6.5% at discharge. Our data was consistent with the literature: that 30–75% of patients report moderate-to-severe acute pain after cardiac surgery.18–21 Inflammatory response is one of the causes in the immediate postoperative period. Non-opioid drugs are effective adjuncts for analgesia for patients undergoing cardiac surgery, through different mechanisms such as inhibition of inflammation. Unfortunately, we did not reveal a lower incidence of moderate-to-severe pain or lower VAS score in group M.
Training to use PCIA pumps was provided twice, during preoperative visit and after tracheal extubation. If pain cannot be relieved after 3 consecutive bolus, rescue analgesia was provided immediately. Therefore, although some patients had VAS equal to or greater than 4, only a few had VAS scores greater than 6. Thus, we compared the rates of rescue analgesia, instead of stratified analysis, as moderate, severe and extreme pain.
Intraoperative opioid requirement was predictably reduced in group M. The analgesic effect of dexmedetomidine and ketamine made it possible for opioids to be reduced. Moreover, anesthesiologists were not blinded to treatment allocation, that is, that they would administer reduced opioid doses in the process. And even so, PCIA demand was not only similar on the first postoperative day, but also showed a significant reduction compared to the control group on the second and third days. This means that the reduction in opioid dosage without investigator bias continued till the third postoperative day. That we did not record the point-in-time of the first PCIA and had no record of the type and dose of rescue analgesic are limitations of this study, which may also explain the reasons for the similar opioid requirements on the first postoperative day. Rescue analgesia requirements were significantly different between the two groups. The definition of rescue analgesia was the once or more occurrences of any sorts of analgesic provided by on-call physicians during hospitalization, if pain cannot be relieved after 3 PCIA consecutive bolus. Therefore, a higher rate of rescue analgesia was indicative of a poorer analgesic effect and a greater need for analgesics. Moreover, the use of rescue analgesics retarded further increases in VAS, which may also be one of the reasons for the similar analgesic effects between the two groups despite the different incidence of rescue analgesia.21
We did not show a significant advantage of our multimodal analgesia regimen in reducing PONV and dizziness. Compared with previous reports,22 the total dosages of opioids are really low in both groups, which are both close to an “opioid-sparing regimen”. In addition, we routinely use tropisetron and dexamethasone to prevent PONV. These two drugs are reported to have been effective in reducing PONV.23
Inflammatory response is one of the causes of postoperative pain. Adequate analgesia may in turn reduce the inflammatory response, creating a virtuous cycle. Opioids may affect the patient’s immune system and regulate inflammation pathways. Systemic inflammatory biomarkers, such as NLR, PLR, LMR and SII, were measured to show if our opioid-sparing multimodal analgesic regimen affected perioperative inflammatory responses. NLR, PLR, LMR and SII, which are based on neutrophil, lymphocyte, monocyte and/or platelet counts, known as systemic inflammatory biomarkers, are immune response‐related indicators and significantly associated with cardiovascular diseases, and used to assess the postoperative inflammatory response.24,25 Our study did not find differences in perioperative inflammatory response during cardiac surgery. Our regimen was feasible, without an anti-inflammatory advantage. We did not measure TNF, IL and other inflammatory biomarkers during the perioperative period, which may be one of the reasons for the negative results. In addition to acetaminophen,26 the anti-inflammatory effects of gabapentin, ketamine, lidocaine, and dexmedetomidine need to be confirmed by more prospective studies, which may be another reason for the negative results.
Our study also evaluated the occurrence of chronic pain. The overall incidence of chronic pain was 46.3% at three months and 33.3% at one year after surgery. The incidence of chronic pain in this study was on the high side of prospective studies, which ranges between 22.9–61.0% at 3 months and 7.3–34.2% at 12 months, more in the range that can be expected when thoracotomy is performed.27,28 High incidence of moderate-to-severe pain, as well as high opioid requirements, have been associated with postoperative persistence of pain.29 The incidence of acute pain in our study was 67%, and rescue analgesia was 44%, which may be the reason for the high incidence of chronic pain. Another reason could be that the patients were more likely to express pain and discomfort when they received more attention via telephone follow-up. There was no statistically significant difference in chronic pain between the two groups. The reasons were small sample size or the similar incidence of acute postoperative pain.
Our study did not find significant differences regarding mechanical ventilation and length of stay between the two groups. Although the comprehensive ERAS program achieved shorter length of hospital stay, this may not be attributed to multimodal analgesia.30
Limitations
There are several limitations of our clinical trial. First, moderate statistical power due to relatively small sample size. Second, the non-blinding method during surgery may affect the intraoperative medication, which could be a bias issue. We also missed recording the first opioid dose after surgery. Third, the drug, dosage, times for rescue analgesia and QoR-15 have not been further analyzed for conformity.
Conclusions
The multimodal analgesic regimen of paracetamol, gabapentin, ketamine, lidocaine, dexmedetomidine and sufentanil in cardiac surgery was feasible. It was not superior to those of a traditional sufentanil-based regimen but did effectively reduce perioperative opioid consumption along with the rescue analgesia needed. Moreover, it showed the same inflammatory response markers, length of stay and incidences of chronic pain, PONV and postoperative complications.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Ethics Approval and Consent to Participate
The trial was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (Ethics approval number: B2018-039R, Chairperson Xinyu Qin, approved on 17 April 2018) and written informed consent was obtained from all subjects participating in the trial. All methods were performed in accordance with the relevant guidelines and regulations.
Acknowledgments
We want to show our appreciation to Dr. Yi Lyu for assistance in study design and proofreading.
Author Contributions
All authors contributed to study design, execution, acquisition of data, data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by Zhongshan Hospital Clinical Special Research Fund [Grant number 2020ZSLC13] and Shanghai Municipal Health Commission Clinical Research Program [Grant number 202140270].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Extracorporeal Circulation Division of Chinese Society of Biomedical Engineering. White paper on data of cardiac surgery and extracorporeal circulation in China 2019. Chin J Extracorporeal Circulat. 2020;18:193–196.
2. Fuchs A, Heinisch PP, Luedi MM, et al. Pain after cardiac surgery: time to include multimodal pain management concepts in ERAS protocols. J Clin Anesth. 2022;76:110583. doi:10.1016/j.jclinane.2021.110583
3. Kleiman AM, Sanders DT, Nemergut EC, et al. Chronic poststernotomy pain: incidence, risk factors, treatment, prevention, and the anesthesiologist’s role. Reg Anesth Pain Med. 2017;42:698–708. doi:10.1097/AAP.0000000000000663
4. Guertin JR, Pagé MG, Tarride JÉ, et al. Just how much does it cost? A cost study of chronic pain following cardiac surgery. J Pain Res. 2018;11:2741–2759. doi:10.2147/JPR.S175090
5. Myles PS, Boney O, Botti M, et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: patient comfort. Br J Anaesth. 2018;120:705–711. doi:10.1016/j.bja.2017.12.037
6. Khademi H, Kamangar F, Brennan P, et al. Opioid therapy and its side effects: a review. Arch Iran Med. 2016;19:870–876.
7. Engelman DT, Ben Ali W, Williams JB, et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154:755–766. doi:10.1001/jamasurg.2019.1153
8. Guinot PG, Spitz A, Berthoud V, et al. Effect of opioid-free anesthesia on post-operative period in cardiac surgery: a retrospective matched case-control study. BMC Anesthesiol. 2019;19:136. doi:10.1186/s12871-019-0802-y
9. Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine postoperative delirium among older patients following cardiac surgery: the DEXACET randomized clinical trial. JAMA. 2019;321:686–696. doi:10.1001/jama.2019.0234
10. Miyazaki T, Sakai T, Sato S, et al. Is early postoperative administration of pregabalin beneficial for patients with lung cancer? - randomized control trial. J Thorac Dis. 2016;8:3572–3579. doi:10.21037/jtd.2016.12.04
11. Tsuboi M, Chiba S. Effects of lidocaine on isolated, blood-perfused ventricular contractility in the dog. Heart Vessels. 1999;14(6):289–294. doi:10.1007/BF03257241
12. Gholipour Baradari A, Habibi MR, Habibi V, et al. Administration of lidocaine to prevent cognitive deficit in patients undergoing coronary artery bypass grafting and valve plasty: a systematic review and meta-analysis. Expert Rev Clin Pharmacol. 2017;10(2):179–185. doi:10.1080/17512433.2017.1266252
13. Anwar S, Cooper J, Rahman J, et al. Prolonged perioperative use of pregabalin and ketamine to prevent persistent pain after cardiac surgery. Anesthesiology. 2019;131:119–131. doi:10.1097/ALN.0000000000002751
14. Aguerreche C, Cadier G, Beurton A, et al. Feasibility and postoperative opioid sparing effect of an opioid-free anaesthesia in adult cardiac surgery: a retrospective study. BMC Anesthesiol. 2021;21:166. doi:10.1186/s12871-021-01362-1
15. Fondeur J, Escudero Mendez L, Srinivasan M, et al. Dexmedetomidine in prevention of postoperative delirium: a systematic review. Cureus. 2022;14:e25639. doi:10.7759/cureus.25639
16. Breivik EK, Björnsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000;16:22–28. doi:10.1097/00002508-200003000-00005
17. Schepman P, Thakkar S, Robinson R, et al. Moderate to severe osteoarthritis pain and its impact on patients in the United States: a national survey. J Pain Res. 2021;14:2313–2326. doi:10.2147/JPR.S310368
18. Milgrom LB, Brooks JA, Qi R, et al. Pain levels experienced with activities after cardiac surgery. Am J Crit Care. 2004;13(2):116–125. doi:10.4037/ajcc2004.13.2.116
19. Raksamani K, Wongkornrat W, Siriboon P, et al. Pain management after cardiac surgery: are we underestimating post sternotomy pain? J Med Assoc Thai. 2013;96(7):824–828.
20. King M, Stambulic T, Hassan SMA, et al. Median sternotomy pain after cardiac surgery: to block, or not? A systematic review and meta-analysis. J Card Surg. 2022;37:3729–3742. doi:10.1111/jocs.16882
21. Rafiq S, Steinbrüchel DA, Wanscher MJ, et al. Multimodal analgesia versus traditional opiate based analgesia after cardiac surgery, a randomized controlled trial. J Cardiothorac Surg. 2014;9:52. doi:10.1186/1749-8090-9-52
22. Bartholmes F, Malewicz NM, Ebel M, et al. Pupillometric monitoring of nociception in cardiac anesthesia. Dtsch Arztebl Int. 2020;117:833–840. doi:10.3238/arztebl.2020.0833
23. Weibel S, Rücker G, Eberhart LH, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database Syst Rev. 2020;10:CD012859. doi:10.1002/14651858.CD012859.pub2
24. Yayla Ç, Akboğa MK, Canpolat U, et al. Platelet to lymphocyte ratio can be a predictor of infarct-related artery patency in patients with ST-segment elevation myocardial infarction. Angiology. 2015;66(9):831–836. doi:10.1177/0003319715573658
25. Fu XT, Tang Z, Chen JF, et al. Laparoscopic hepatectomy enhances recovery for small hepatocellular carcinoma with liver cirrhosis by postoperative inflammatory response attenuation: a propensity score matching analysis with a conventional open approach. Surg Endosc. 2021;35:910–920. doi:10.1007/s00464-020-07710-5
26. Liu Y, Yao W, Xu J, et al. The anti-inflammatory effects of Acetaminophen and N-acetylcysteine through suppression of the NLRP3 inflammasome pathway in LPS-challenged piglet mononuclear phagocytes. Innate Immun. 2015;21:587–597. doi:10.1177/1753425914566205
27. Marcassa C, Faggiano P, Greco C, et al. A retrospective multicenter study on long-term prevalence of chronic pain after cardiac surgery. J Cardiovasc Med. 2015;16:768–774. doi:10.2459/JCM.0000000000000271
28. Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta-analysis. J Pain. 2014;15:887–897. doi:10.1016/j.jpain.2014.06.005
29. Krakowski JC, Hallman MJ, Smeltz AM. Persistent pain after cardiac surgery: prevention and management. Semin Cardiothorac Vasc Anesth. 2021;25(4):289–300. doi:10.1177/10892532211041320
30. Grant MC, Isada T, Ruzankin P, et al. Opioid-sparing cardiac anesthesia: secondary analysis of an enhanced recovery program for cardiac surgery. Anesth Analg. 2020;131(6):1852–1861. doi:10.1213/ANE.0000000000005152
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





