Back to Journals » Journal of Pain Research » Volume 16
Evaluation of Plasma Calcitonin Gene-Related Peptide as a Biomarker for Painful Temporomandibular Disorder and Migraine
Authors Tchivileva IE , Johnson KW, Chai X, VanDam LR, Lim PF , Slade GD
Received 10 February 2023
Accepted for publication 1 July 2023
Published 11 July 2023 Volume 2023:16 Pages 2331—2346
DOI https://doi.org/10.2147/JPR.S408044
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Alexandre F DaSilva
Inna E Tchivileva,1,2 Kirk W Johnson,3 Xiyun Chai,4 Lyndsey R VanDam,3 Pei Feng Lim,1,5 Gary D Slade1,6
1Center for Pain Research and Innovation, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; 2Division of Oral and Craniofacial Health Sciences, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; 3Pain Research, Eli Lilly and Company, Indianapolis, IN, USA; 4Precision Medicine Neuroscience, AbbVie, Chicago, IL, USA; 5Division of Diagnostic Sciences, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; 6Division of Pediatric and Public Health, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Correspondence: Inna E Tchivileva, Center for Pain Research and Innovation, Adams School of Dentistry, University of North Carolina at Chapel Hill, CB# 7455, Chapel Hill, NC, 275199-7455, USA, Tel +1 919 537 3291, Fax +1 919 966 5339, Email [email protected]
Objective: To assess associations of plasma calcitonin gene-related peptide (CGRP) with chronic temporomandibular disorder (TMD) myalgia/arthralgia or frequent/chronic migraine, alone and in combination, and to evaluate relations between the CGRP concentration and clinical, psychological, and somatosensory characteristics of participants.
Methods: The cross-sectional study selected four groups of adult volunteers: healthy controls (HCs), TMD without migraine, migraine without TMD, and TMD with migraine. Each group comprised 20 participants, providing 94% power to detect statistically significant associations with CGRP concentration for either TMD or migraine. TMD and headache were classified according to the Diagnostic Criteria for TMD and the International Classification for Headache Disorders, 3rd edition, respectively. Plasma CGRP was quantified with a validated high-sensitivity electrochemiluminescent Meso Scale Discovery assay. Questionnaires and clinical examinations were used to evaluate characteristics of TMD, headache, psychological distress, and pressure pain sensitivity. Univariate regression models quantified associations of the CGRP concentration with TMD, migraine, and their interaction. Univariate associations of the CGRP concentration with clinical, psychological, and pressure pain characteristics were also assessed.
Results: Among 80 participants enrolled, neither TMD nor migraine was associated with plasma CGRP concentration (P = 0.761 and P = 0.972, respectively). The CGRP concentration (mean ± SD) was similar in all 4 groups: HCs 2.0 ± 0.7 pg/mL, TMD 2.1 ± 0.8 pg/mL, migraine 2.1 ± 0.9 pg/mL, and TMD with migraine 2.2 ± 0.7 pg/mL. CGRP concentration was positively associated with age (P = 0.034) and marginally with body mass index (P = 0.080) but was unrelated to other participant characteristics.
Conclusion: In this well-powered study, interictal plasma concentration of CGRP was a poor biomarker for TMD and migraine.
Keywords: orofacial pain, musculoskeletal pain, neuropeptide, temporomandibular joint disorders, headache
Introduction
Painful temporomandibular disorder (TMD) often co-exists with migraine.1,2 For example, TMD prevalence was 5 times higher in people with severe headache and migraine than in people without the headache (15.6% and 2.6%, respectively).3 Likewise, migraine is highly prevalent in TMD patients: in our recent study, migraine occurred in 52% of patients with chronic myogenous TMD.4 Currently, both disorders are defined solely by clinical criteria5,6 which, in the absence of specific biomarkers, limit the capacity for screening, diagnosis, determining prognosis, and predicting response to therapy.7
One of the most promising biomarker candidates for migraine is calcitonin gene-related peptide (CGRP). CGRP consists of 37 amino acids and has 2 isoforms, α and β, that have similar biological activity but differ by 3 amino acids. Peripheral somatosensory nerves and central nervous system (CNS) neurons mostly express αCGRP (denoted as CGRP in this manuscript), while enteric and motor neurons almost exclusively express βCGRP.8 In the trigeminal system, CGRP released from peripheral nerve endings can lead to elevated synthesis of nitric oxide and sensitization of peripheral trigeminal nerves; the neuronal activation then drives central sensitization.9 A vast body of evidence supports a crucial role of CGRP in migraine pathophysiology.9–11 The initial clinical study found an elevated plasma level of CGRP in jugular venous blood during migraine attacks.12 Administration of sumatriptan aborted the CGRP increase and reduced symptoms of migraine.13 Elevated CGRP in peripheral venous blood between attacks was subsequently reported for both episodic14 and chronic migraine.15 However, other studies could not reproduce findings of increased CGRP concentrations either in the external jugular vein or peripheral blood of migraineurs.16,17 The CGRP role in migraine was further supported by research where intravenous injection of CGRP led to migraine-like headache in individuals with history of migraine.18,19 Finally, CGRP receptor antagonists and humanized antibodies to CGRP or its receptor were efficacious in the treatment of migraine.20
In contrast to compelling evidence of a causative role of CGRP in migraine, data on CGRP contribution to pathophysiology of painful TMD are scarce. CGRP-containing nerve endings are present in masseter muscle and in synovial membrane, articular disc, periosteum, and capsule of the temporomandibular joint (TMJ).21–23 Elevated CGRP concentrations were reported in the TMJ synovium in the presence of TMJ internal derangements24 and in the TMJ synovial fluid in TMJ osteoarthritis.25,26 However, the CGRP level in blood of people with painful TMD has not been investigated.
In this study with a factorial design, we aimed to assess whether plasma concentration of CGRP in peripheral venous blood was associated with a factor of present or absent chronic myogenous/arthrogenous TMD, a factor of present or absent frequent/chronic migraine, or the interaction of these factors. The second aim of the study was to evaluate associations between the CGRP concentration and participants’ phenotyping characteristics.
Methods
Study Design and Setting
The cross-sectional stratified sampling design selected 80 participants from 4 groups based on the presence or absence of chronic painful TMD and/or frequent/chronic migraine: (a) a healthy control group (HCs) included participants with neither painful TMD nor any migraine; (b) a TMD group included participants with chronic painful TMD, who did not have frequent/chronic migraine meeting the study migraine case definition described below; (c) a migraine (MIG) group included participants with frequent/chronic migraine who did not have chronic painful TMD (infrequent TMD, not meeting the study TMD case definition described below, was allowed); and (d) a TMD+MIG group included participants with both chronic painful TMD and frequent/chronic migraine. The study definitions for TMD and migraine cases were designed to enroll participants with clinically important painful TMD and migraine that likely reflect the population of patients seeking care in clinical settings. Each group consisted of 20 participants. Frequency matching was used during enrollment to create groups with approximately equal proportions of females (±10% among the groups) and adults younger than 30 years (±10% among the groups).
The participants were enrolled between June and December 2018 from the nearby community and the Orofacial Pain Clinic at the Adams School of Dentistry, the University of North Carolina at Chapel Hill. The study was approved by the university’s Institutional Review Board and complied with the Declaration of Helsinki. The participants attended one study visit lasting approximately 2 hours. Prior to the visit, the participants were prescreened for eligibility either in person or via telephone. Potentially eligible participants received a package of questionnaires to complete before the visit. During the visit, participants provided informed consent and were further evaluated for eligibility. All eligible participants provided a blood specimen for quantification of plasma CGRP.
Participants
Participants were females and males 18 to 64 years of age who agreed to discontinue use of any pain medication (including TMD- and migraine-specific medications) for 3 days prior to the study visit. General exclusion criteria were self-reported and comprised severe hepatic, respiratory, immunologic, cardiovascular or psychiatric diseases, fibromyalgia, trigeminal autonomic cephalalgias, painful cranial neuropathies, odontogenic pain, head trauma or surgery within 6 weeks prior to the study visit, history of treatment for drug or alcohol abuse within 1 year prior to the study visit, use of botulinum toxin or anti-CGRP monoclonal antibody injections within 4 months prior to the study visit, use of opioids or barbiturate medications, use of daily preventive medications for chronic pain conditions, and pregnancy.
The diagnoses of TMD myalgia and arthralgia were based on the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD).5 To achieve enrollment of participants with chronic and moderate-to-severe facial pain, additional inclusion criteria were established for the TMD cases: (a) the presence of facial pain for at least 3 months (this criterion constitutes chronic pain); (b) at least 10 days with facial pain in the 30 days prior to the study visit; and (c) facial pain intensity in the past week of ≥30 on a numerical rating scale of “0” to “100” (this criterion constitutes moderate-to-severe pain intensity). A specific exclusion criterion for TMD participants was use of injection therapy for facial pain within 2 weeks prior to the study visit.
The headache diagnosis was based on the International Classification for Headache Disorders, 3rd edition (ICHD-3).6 In addition, migraine cases needed to have had at least 4 migraine days per month in the last 3 months. Specific exclusion criteria for participants with migraine were migraine onset after 50 years of age and use of interventions or devices, such as nerve blocks or transcranial magnetic stimulation, within 2 months prior to the study visit.
To qualify as HCs, in addition to the overall exclusion criteria, participants must not have experienced painful TMD or migraine within 1 year prior to the study visit and must not have had tension-type headache (TTH) on more than 4 days per month in the last 3 months. The detailed list of inclusion and exclusion criteria for all groups is provided in the Supplementary Materials.
Clinical and Biopsychosocial Characteristics
Collected information comprised demographics, medical history, clinical characteristics of TMD and headache, emotional functioning, experimental pain sensitivity, and concomitant medications. Measurements of height and weight were also collected and used to calculate a body mass index (BMI).
Facial pain characteristics obtained from the DC/TMD clinical examination5 included classification of myalgia and/or arthralgia, frequency of facial pain, measurements of pain-free, maximum unassisted, and maximum assisted mouth opening, and familiar pain responses evoked by examination of the masticatory muscles and TMJs. TMD-related disability and interference in functioning were assessed using the Graded Chronic Pain Scale (GCPS),27 and jaw function was evaluated with the Jaw Functional Limitation Scale (JFLS).28,29 The GCPS grade is derived from several variables: (1) the characteristic pain intensity computed as the mean, multiplied by 10, of the 3 pain items; (2) the pain interference score, computed as the mean, multiplied by 10, of 3 pain interference items; and (3) pain disability days. Based on these 3 variables, participants were classified into 6 chronic pain grades: 0 = no pain, I = low pain intensity and low pain-related disability, IIa = high pain intensity and low pain-related disability, IIb = high pain intensity and high activity interference, III = moderate pain-related disability, IV = severe pain-related disability. For analyses, the GCPS endpoint was dichotomized into a low-grade category including grades from 0 to IIa and a high-grade category including grades from IIb to IV. The JFLS is a validated questionnaire that measures limitations on 3 subscales: mastication (6 items), vertical jaw mobility (4 items), and verbal and emotional expression (8 items). Each item is rated on an 11-point scale from “no limitation” (0 points) to “severe limitation” (10 points). The subscales are computed as a mean response for all items in the subscale, while the total score is the average of all 18 items.
Type of headache was assessed via a structured headache interview2 based on the ICHD-3.6 The structured headache interview recorded details of up to 3 different headaches. Information elicited for each headache included headache location, intensity, quality, duration, frequency, and aggravating factors. The final question asked about the average number of days per month with headache of any type during the past 3 months. For analyses, definite and probable diagnoses of primary headaches were combined into one category for each primary headache type (migraine or TTH). Headache-related disability and interference in functioning were assessed using the GCPS27 and the Headache Impact Test-6 (HIT-6).30 The 6-item HIT-6 was developed for use in both clinical practice and research. It was designed to measure the adverse impact of headache on social and role functioning, vitality, psychological wellbeing, and cognitive performance. The questionnaire also measures the severity of headache pain. Responders rate frequency of each headache-related burden using one of 5 responses: “never” (6 points), “rarely” (8 points), “sometimes” (10 points), “very often” (11 points), and “always” (13 points). These responses are summed to produce the total HIT-6 score ranging from 36 to 78 points.
Psychological characteristics were assessed with the Hospital Anxiety and Depression Scale (HADS),31 the Perceived Stress Scale (PSS),32 and the Symptom Checklist 90-Revised (SCL-90R)33 somatization subscale. The HADS was developed to screen for clinically significant anxiety and depressive symptoms in medically ill patients. Its depression and anxiety components consist of 7 items each. Responses are rated on a 4-point Likert scale and range from 0 to 3, including reversed items. The total score for each HADS component ranges from 0 to 21 points. The PSS contains 14 items and measures the degree to which situations in a responder’s life are appraised as stressful. The items in the PSS ask about feelings and thoughts during the last month. In each item, respondents rate how often they felt a certain way on a scale from “never” (0 points) to “very often” (4 points). The PSS scores are calculated by reversing responses to the 7 positively stated items and then summing across all scale items. The PSS total score ranges from 0 to 56 points. The SCL-90R instrument is used by professionals in mental health, medical, and clinical research settings. The SCL-90R somatization scale consists of 12 items and evaluates the degree of somatic symptoms experienced by responders. The responders are asked how much each symptom bothered them in the last 7 days. The responses are rated on a scale from “not at all” (0 points) to “extremely” (4 points). The total score is the average of all items.
Pressure pain thresholds (PPTs) were measured bilaterally over the temporalis, masseter, and trapezius muscles, the TMJs, and the lateral epicondyles with a pressure algometer (FDX-10, Wagner Instruments, CT), as described previously.34 One pre-trial assessment was performed at each site, followed by additional assessments until 2 measures differing by less than 0.2 kg were obtained, or 5 assessments were administered. In either case, the average of the 2 closest values was recorded as the threshold estimate. Pressure stimuli were delivered at an approximate rate of 1 kg/s. The cutoff pressure for all body sites was 5 kg. The values from the right and left sides were averaged to obtain a single PPT per anatomical site.
Blood Collection and Plasma CGRP Measurement
For migraine participants, peripheral blood was collected interictally, ie, during periods between migraine attacks. In addition, all participants refrained from taking any pain medications for 3 days prior to the study visit. Blood specimens were collected from the antecubital vein into 8.5 mL BD P100 tubes (BD Biosciences, NJ, USA) coated with a K2EDTA anticoagulant and a proprietary cocktail of protease inhibitors specifically formulated to stabilize plasma proteins. To separate plasma, the BD P100 tubes were centrifuged at 2500g for 20 minutes. Then, plasma was aliquoted into cryovials, snap-frozen in liquid nitrogen, and placed in a −80°C freezer for storage.
Plasma CGRP concentrations were quantified at Eli Lilly and Company laboratory (Indianapolis, IN, USA) using a validated high-sensitivity electrochemiluminescent Meso Scale Discovery assay described previously.35 Calibrators were run in triplicate, and samples were run in duplicate. Three quality controls were prepared in sample buffer and run on each assay plate. CGRP concentrations of 20 pg/mL, 5 pg/mL, and 1 pg/mL were denoted High, Middle, and Low quality controls, respectively. The inter-assay coefficients of variation (%CV) for High, Middle, and Low quality controls across sample plates were 3.11%, 3.74%, and 5.45%, respectively. The average lower limit of detection for the plates was 0.148 pg/mL ± 0.012 pg/mL. The laboratory staff was blinded to clinical information and group assignment.
Sample Size
For the sample size calculation, we used the results from a previous study by Cernuda-Morollón et al15 which demonstrated a mean effect estimate of 12.7 pg/mL greater CGRP concentration for participants with episodic migraine (46.4 pg/mL) compared to HCs (33.7 pg/mL) with a standard deviation of 16 pg/mL. For the current study, we used the “glmpower” procedure in SAS v9.4 to determine that a sample size of 80 participants (ie, 20 participants in each of the four groups) yielded 94% power to detect a statistically significant (P<0.05) main effect of migraine compared to controls, independent of any TMD effect. Likewise, there was 94% power to detect an independent effect of TMD if it was of the same magnitude (ie, 12.7 pg/mL increase in CGRP compared to controls). For both effects, the same sample size provided power of 79% to detect statistically significant (P<0.05) main effects as small as 10 pg/mL.
To estimate power for plausible interactions, we assumed the same mean 12.7 pg/mL increase (compared to controls) for each pain condition in the absence of the other pain condition and an increase (compared to controls) of 40.6 pg/mL for subjects with both pain conditions. The latter is equivalent to the mean 75 pg/mL CGPR concentration reported for chronic migraine cases by Cernuda-Morollón et al15 and is equivalent to synergy of 1.6-times the expected additive effects of migraine and TMD. Based on these assumptions, there was 56% power to detect the multiplicative interaction with P<0.05. Power to detect the interaction increases to 80%, when synergy is 2.0-times the expected effect under additivity.
Statistical Methods
Distributions of CGRP concentrations in each of the four study groups were inspected with the aid of violin plots. Statistical outliers were defined as any concentrations that exceeded the third quartile plus 1.5 times the interquartile range (ie, 3.934 pg/mL) and the six values identified were truncated to 3.934. Descriptive statistics for other covariates were calculated for each study group and reported as percentage distribution of categorical variables or mean values and standard deviations of continuous variables.
To evaluate the first aim, CGRP concentration was used as the dependent variable in a least squares linear model that tested for main effects of two binary indicator variables for each case classification (ie, TMD present or absent and migraine present or absent) and their interaction. Covariates in the model were sex (a binary indicator, male or female), race (a binary indicator, White or other), age (a continuous variable), and a BMI (a continuous variable). Besides CGRP, other 22 participant characteristics, that did not have their values equal to 0 in one of the study groups, were then used as the dependent variables in similar univariate regression models (linear regression for the continuous characteristics and binary logistic regression for the binary characteristics). While a Bonferroni correction of P ≤ 0.002 (ie, 0.05/23) provides sufficient protection against type I error for traditional judgments about statistical significance, P-values were reported without the Bonferroni correction to provide a more general guide to potentially important univariate associations.
For the second aim, univariate linear regression models in which the dependent variable was CGRP concentration transformed to a z-score estimated associations with age (a continuous variable, transformed to a z-score), sex (a binary indicator variable for females/males), race (a binary indicator variable for Whites/other races), and body mass index (a continuous variable, transformed to a z-score). Other participant characteristics that were continuous variables were transformed to z-scores and used as the independent variables in linear regression models with CGRP concentration, also transformed to a z-score, as the dependent variable. Each model also adjusted for sex, race, age, and a BMI.
Results
Participants
Of 154 participants prescreened, 80 met inclusion/exclusion criteria for one of the study groups and were enrolled in the study. The main reasons for non-enrollment were as follows: not meeting the study criterion for migraine frequency of at least 4 days per month (n = 21), using preventive migraine medications (n = 15), not being interested in the study (n = 9), and not meeting the study inclusion criteria for TMD (n = 7). The rest of the prescreened participants were excluded due to comorbid conditions such as cluster headache (n = 1), trigeminal neuralgia (n = 1), diabetes (n = 1), fibromyalgia (n = 4), hyperthyroidism (n = 2), and multiple sclerosis (n = 1), use of preventive pain medications (n = 2), use of pain medications within 3 days prior to the study visit (n = 3), use of opioids (n = 2), surgery within 6 weeks prior to the study visit (n = 2), alcohol abuse (n = 1), and difficulty to understand study instructions (n = 1). Most of the enrolled participants were young females (Table 1). The four study groups matched for age and sex (Table 1).
 |
Table 1 Demographic and Clinical Characteristics in Four Participant Groups, Stratified by TMD and Migraine Statusa |
Although the absence of painful TMD and any migraine was a selection criterion for the HC group, episodic TTH was allowed, and 7 (35%) HCs had episodic TTH with mild headache intensity (Table 1).
In the TMD group, all participants had TMD myalgia and 19 (95%) of them also had TMJ arthralgia. In the last month, they experienced TMD pain almost daily with average intensity of 53.2 on a 0–100 scale and exhibited a restricted pain-free jaw opening (Table 1).
In the MIG group, 15 (75%) participants met ICHD-3 criteria for episodic migraine (with and without aura) and 5 (25%) met criteria for chronic migraine. They had average headache frequency of 11.1 days per month and average headache intensity of 54.8 on the 0–100 scale (Table 1).
In the TMD+MIG group, values of TMD characteristics resembled values seen in the TMD group. Migraine was chronic in 50% of the participants with average headache frequency of 17.7 days per month and headache intensity like in the MIG group (Table 1).
Among TMD and migraine groups, analyses of data for analgesic medications used during a month prior to the study visit showed that the most frequently reported medications were non-steroidal anti-inflammatory drugs and acetaminophen. In addition, the participants with migraine used triptans and muscle relaxants (Table 2).
 |
Table 2 Psychological and Experimental Pain Sensitivity Characteristics, Plasma CGRP Concentration, and Medication Use in Four Participant Groups, Stratified by TMD and Migraine Statusa |
Participants’ Characteristics Associated with TMD and Migraine
As expected, TMD was statistically significantly associated with characteristics of facial pain, such as jaw openings and limitations in jaw function (P values < 0.002), and some characteristics of headache, such as headache monthly frequency, intensity, and impact (P < 0.002) (Table 1). Predictably, migraine was also associated with headache characteristics (P < 0.001), and TMD and migraine interacted in their associations with headache intensity and impact (P < 0.001) (Table 1).
Migraine was strongly associated with psychological factors but not with somatosensory characteristics. Compared with participants without migraine, migraineurs reported greater anxiety (P = 0.001), depression (P < 0.001), perceived stress (P = 0.002), and somatic symptoms (P < 0.001) (Table 2). In contrast, the TMD association with psychological distress was less pronounced, as the statistically significant association was noted with somatic symptoms only (P < 0.001) (Table 2). However, TMD was strongly associated with increased sensitivity to pressure pain at all sites assessed (P < 0.001).
Plasma CGRP Concentration
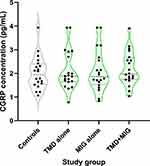
Violin plots of individual CGRP concentrations revealed similar distributions among the four study groups (Figure 1). Summary statistics for CGRP concentration (mean ± SD) were similar in all 4 groups: 2.0 ± 0.7 pg/mL in HCs, 2.1 ± 0.8 pg/mL in participants with TMD, 2.1 ± 0.9 pg/mL in participants with migraine, and 2.2 ± 0.7 pg/mL in participants with both TMD and migraine (Table 2). In regression analysis including all participants, neither TMD nor migraine as main effects were associated with the plasma CGRP concentration (P = 0.761 and P = 0.972, respectively), and no statistically significant TMD × migraine interaction was noted (P = 0.696) (Table 2).
Association Between Plasma CGRP Concentration and Participants’ Characteristics
In analyses including all participants, participant age was the only characteristic statistically significantly associated with plasma CGRP concentration (P = 0.034), while the association with BMI showed a trend towards significance (P = 0.080) (Table 3). We found no statistically significant associations between the CGRP concentration and participant characteristics related to TMD, migraine, psychological distress, and experimental pain sensitivity (Table 4).
 |
Table 3 Unadjusted Associations of Plasma CGRP Concentration with Participant Demographic Characteristics and a Body Mass Indexa |
 |
Table 4 Adjusted Associations of Plasma CGRP Concentration with Participant Characteristicsa |
As PPTs were significantly lower in TMD cases, we analyzed associations of CGRP concentrations with PPTs within each study group and found no statistically significant results (Table 5). Also, we did not observe statistically significant PPT × study group interactions in linear regression models for all study participants, where the CGRP concentration was a dependent variable (Table 5).
 |
Table 5 Adjusted Associations of Plasma CGRP Concentration with PPTs, Stratified by a Study Group |
Discussion
Summary of the Main Findings
To our knowledge, this is the first study measuring plasma CGRP concentrations in peripheral venous blood of participants with chronic painful TMD. Given frequent co-occurrence of TMD and migraine, we sampled enough participants from four study groups to have ample statistical power to distinguish between potential influences of either condition or their co-occurrence, on circulating CGRP. Yet, neither chronic painful TMD nor migraine was associated with plasma CGRP concentrations. Moreover, the CGRP concentration was not associated with any of participant clinical, psychological, or pressure pain sensitivity characteristics. A positive association was found only with age, and a trend towards significance was observed for association with obesity.
Comparison of Psychological and Somatosensory Profiles of TMD and Migraine Participants
A vast body of evidence demonstrated associations of painful TMD and migraine with psychological factors, such as depression, anxiety, stress, and somatic awareness.36–42 Moreover, assessment of the patient psychological status is recommended by the Axis II protocol in the DC/TMD.5 However, not many studies compared psychological profiles of TMD and migraine patients. One recent research, utilizing the same factorial design as our study, found elevated anxiety and somatization in TMD patients and greater anxiety, depression, and somatization in migraineurs.43 Our results are in full agreement with these findings, as we also observed an across-group gradient (HC < TMD < MIG < TMD+MIG) of increasing estimates for all four psychological factors assessed. Additionally, our analysis also showed a more pronounced association of anxiety, depression, stress, and somatization with migraine than with painful TMD.
Elevated somatosensory perception in painful TMD and migraine, compared with healthy controls, was reported by many studies, although with some discrepancies between the reports.34,44–48 In both conditions, the differences in pain sensitivity were found not only in orofacial region but also in remote body sites, suggesting a generalized upregulation of nociceptive processing. In TMD, the largest effects were observed for PPTs, and that is why these quantitative sensory tests were chosen for our study.34 As stated in a recent meta-analysis, PPTs were also reliably lower in patients with migraine than in healthy controls.48 To our knowledge, none of the studies compared PPTs in participants with TMD and/or migraine. One study evaluated sensitivity to cold, heat, and mechanical cutaneous stimuli among healthy, TMD, and migraine participants stratified in a factorial design.49 While cold and heat hyperalgesia was observed in all 3 patient groups, mechanical cutaneous allodynia was demonstrated only in TMD patients, with and without migraine. For all experimental modalities, the most pronounced hyperalgesia/allodynia was found in patients with concomitant TMD and migraine. Our PPT results agree with the above study, as we also observed an across-group gradient in PPTs (HC > MIG > TMD > TMD+MIG) with the statistically significant effect of TMD.
CGRP as a Biomarker for Migraine
A biomarker is
a characteristic that can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention.50
In clinical practice, a reliable biomarker could be used to identify risk of developing a disease, to screen for a subclinical disease, to diagnose an overt disease, to categorize disease severity, or to predict response to therapy.51
CGRP has been actively investigated as a putative biomarker for migraine.7 It was evaluated in ictal (ie, during the attacks) and interictal (ie, between the attacks) phases of migraine, in peripheral and jugular venous blood, and in episodic and chronic migraine.52 However, the results of the studies are inconsistent. The first study found elevated CGRP plasma concentrations in the external jugular vein of migraineurs during the attacks.12 Another study confirmed the finding in peripheral blood collected from migraine patients ictally but not in the blood collected interictally.53 However, the third study measuring CGRP concentrations in the jugular and peripheral blood both ictally and interictally did not reproduce the previous positive findings.17 Regarding interictal CGRP concentrations in peripheral blood, several more studies reported higher CGRP concentrations in patients with episodic and chronic migraine compared with healthy volunteers,14,15,54,55 but another study found no difference between migraineurs and HCs, between migraine with and without aura, between episodic and chronic migraine, and between ictal and interictal phases.16 Our study results also did not show interictally elevated CGRP concentrations in participants with frequent or chronic migraine. Further, we did not find any associations between the CGRP concentration and headache monthly frequency, intensity, chronic pain grade, and impact on quality of life.
The discrepancies between studies can be explained by differences in sample sizes, designs, participant selection criteria, blood processing protocols, and assays used for the CGRP measuring. For example, the blood CGRP has a short half-life of less than 10 minutes,56 and that is why the use of protease inhibitors is important to prevent the peptide degradation. However, some studies used the protease inhibitors and others did not. Additionally, commercially available CGRP assays are variable and not well validated. Most assays might detect not only an entire peptide but also its fragments and relative peptides, such as βCGRP and amylin.7 Standardization of blood processing protocols and thorough validation of assays are needed to achieve better comparisons between studies.
CGRP as a Biomarker for TMD
The average CGRP concentration in our study was not elevated in participants with chronic painful TMD, and it was not associated with TMD characteristics. Despite our negative results, we consider CGRP’s contribution to painful TMD plausible. This hypothesis is supported by findings of CGRP-containing nerve endings in the masseter muscle and TMJ21–23 and elevated CGRP concentrations in the TMJ with disc displacement or degenerative joint disorders.24–26 Additional evidence for the CGRP role in painful TMD comes from animal studies. Supporting CGRP contribution to myogenous TMD, increased CGRP concentrations were found in the rat masseter muscle following injection of complete Freund’s adjuvant (CFA).57 In another study, pretreatment with a CGRP receptor antagonist before the CFA injection in the mouse masseter muscle reduced animal orofacial pain behavior and neuronal activation in the spinal trigeminal nucleus.58 Establishing CGRP involvement in arthrogenous TMD, an increased number of TMJ-innervating CGRP-immunoreactive neurons was detected in the rat trigeminal ganglion in a carrageenan-induced TMJ arthritis model.59 The elevated CGRP level in the trigeminal ganglion and spinal trigeminal nucleus was also confirmed in another model of TMJ arthritis in rats.60 Additionally, injecting CGRP in the rat TMJ stimulated neuronal and glial expression of proteins involved in the development of peripheral and central sensitization.61 Considering all the evidence, we recognize the importance of CGRP’s contribution to pathophysiology of painful TMD but regard the plasma concentration of CGRP in peripheral blood to be a poor biomarker for the disorder. Potentially, local CGRP concentrations in the masticatory muscles, TMJ, trigeminal ganglion, spinal trigeminal nucleus, and other regions of CNS implicated in central sensitization would be better correlates of painful TMD than the plasma CGRP concentration, although CGRP concentrations in those regions were not studied here.
CGRP and Ageing
In animal research, circulating concentrations of CGRP have been found to both increase and decrease with ageing.62 One possible explanation why plasma concentration of CGRP elevates as age advances could be increased production of CGRP by thyroid.63,64 On the other hand, decreased circulating CGRP in aged females could be a consequence of decreased production of sex steroid hormones.65 Further, aging enhances negative effect of hypertension on CGRP expression in neurons and its release from nerves.66 In two earlier studies that assessed a relation between circulating CGRP and age in people with and without migraine, no association was noted.15,16 The discrepancy with positive association found in our study could be explained by differences in participant selection criteria, eg, uncontrolled hypertension was exclusionary in our study but not in two other studies.
CGRP and Obesity
Although we found only borderline positive association of the CGRP concentration with a BMI, our finding is in line with the existing evidence on the role of CGRP in obesity. Mice lacking CGRP were protected against obesity induced by high-fat diet.67 Plasma CGRP concentration was elevated in obese Zucker rats prior to the onset of obesity68 and in obese women.69 Relevant to our study population, obesity constitutes a risk factor for migraine70 and was associated with frequency, severity, and clinical features of migraine attacks.71 Research on the role of obesity in painful TMD is scarce. One study found that TMD pain was associated with total body fat percentage in a univariate analysis, but the association was lost when corrected for co-variates in a multivariable model.72 Collectively, current evidence points towards CGRP as an important link between obesity and migraine.73
Strengths and Limitations
Important strengths of this study were the use of the validated DC/TMD5 for the classification of TMD myalgia and arthralgia and the use of the structured, ICHD-3-based interview for classification of headache.2 The use of the validated Meso Scale Discovery assay and blinding of the laboratory staff to participants’ clinical data also strengthened the validity of study findings. Another strength was that the sample size was calculated a priori, providing sufficient statistical power to detect group differences that were plausible based on a previous study. Hence, the observed lack of statistically significant differences in CGRP concentrations between the study groups cannot be attributed to a type II error. The limitation of the study is reliance on participants’ retrospective self-report for assessment of TMD and headache characteristics. The generalizability of our data was supported by enrollment of all consecutive eligible volunteers of any sex, race, and ethnicity. However, the selection criteria were designed for enrollment of participants with advanced symptoms typical for clinical settings and not fully representative of the conditions themselves.
Conclusion
In this well-powered study, the interictal concentration of CGRP in peripheral blood was a poor biomarker of these disorders. Further, the CGRP concentration was not associated with any of the clinical characteristics of painful TMD or migraine, characteristics of psychological distress, or measures of pressure pain sensitivity. Interestingly, it was positively associated with participant age and, borderline, with obesity.
Abbreviations
TMD, temporomandibular disorder; CGRP, calcitonin gene-related peptide; CNS, central nervous system; TMJ, temporomandibular joint; HCs, healthy controls; MIG, migraine group; DC/TMD, the Diagnostic Criteria for TMD; ICHD-3, the International Classification of Headache Disorders, 3rd edition; TTH, tension-type headache; BMI, body mass index; GCPS, Graded Chronic Pain Scale; JFLS, Jaw Functional Limitation Scale; HIT-6, Headache Impact Test-6; HADS, Hospital Anxiety and Depression Scale; PSS, Perceived Stress Scale; SCL-90R, Symptom Checklist 90-Revised; PPT, pressure pain threshold; SD, standard deviation; UNC-CH, University of North Carolina at Chapel Hill.
Acknowledgments
The authors wish to thank Dr. Shreya Nayak, the UNC-CH Orofacial Pain Program resident, and Sonya Capps, the study coordinator, for their valuable contribution to the study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
Funding for this study was provided by the UNC-CH Adams School of Dentistry to IET. The sponsor was not involved in any stage of the project.
Disclosure
KWJ and LRV are employees of Eli Lilly and Company and may or may not hold stock in the company. XC is an employee of AbbVie. The authors report no other conflicts of interest in this work.
References
1. Goncalves DA, Camparis CM, Speciali JG, Franco AL, Castanharo SM, Bigal ME. Temporomandibular disorders are differentially associated with headache diagnoses: a controlled study. Clin J Pain. 2011;27(7):611–615. doi:10.1097/AJP.0b013e31820e12f5
2. Tchivileva IE, Ohrbach R, Fillingim RB, Greenspan JD, Maixner W, Slade GD. Temporal change in headache and its contribution to the risk of developing first-onset temporomandibular disorder in the Orofacial Pain: prospective Evaluation and Risk Assessment (OPPERA) study. Pain. 2017;158(1):120–129. doi:10.1097/j.pain.0000000000000737
3. Plesh O, Adams SH, Gansky SA. Self-reported comorbid pains in severe headaches or migraines in a US national sample. Headache. 2012;52(6):946–956. doi:10.1111/j.1526-4610.2012.02155.x
4. Tchivileva IE, Ohrbach R, Fillingim RB, et al. Effect of comorbid migraine on propranolol efficacy for painful TMD in a randomized controlled trial. Cephalalgia. 2021;41(7):839–850. doi:10.1177/0333102421989268
5. Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network* and orofacial pain special interest group. J Oral Facial Pain Headache. 2014;28(1):6–27. doi:10.11607/jop.1151
6. Olesen J. Headache classification Committee of the International Headache Society (I) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
7. Ashina M, Terwindt GM, Al-Karagholi MA, et al. Migraine: disease characterisation, biomarkers, and precision medicine. Lancet. 2021;397(10283):1496–1504. doi:10.1016/S0140-6736(20)32162-0
8. Mulderry PK, Ghatei MA, Spokes RA, et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience. 1988;25(1):195–205. doi:10.1016/0306-4522(88)90018-8
9. Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the trigeminal system in migraine. Headache. 2019;59(5):659–681.
10. Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338–350. doi:10.1038/s41582-018-0003-1
11. Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622. doi:10.1152/physrev.00034.2015
12. Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183–187. doi:10.1002/ana.410280213
13. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33(1):48–56. doi:10.1002/ana.410330109
14. Ashina M, Bendtsen L, Jensen R, Schifter S, Olesen J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain. 2000;86(1–2):133–138. doi:10.1016/S0304-3959(00)00232-3
15. Cernuda-Morollon E, Larrosa D, Ramon C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81(14):1191–1196. doi:10.1212/WNL.0b013e3182a6cb72
16. Lee MJ, Lee SY, Cho S, Kang ES, Chung CS. Feasibility of serum CGRP measurement as a biomarker of chronic migraine: a critical reappraisal. J Headache Pain. 2018;19(1):53. doi:10.1186/s10194-018-0883-x
17. Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58(4):561–568. doi:10.1002/ana.20605
18. Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–1186. doi:10.1177/0333102410368444
19. Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. doi:10.1046/j.1468-2982.2002.00310.x
20. Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet. 2019;394(10210):1765–1774. doi:10.1016/S0140-6736(19)32504-8
21. Kido MA, Kiyoshima T, Kondo T, et al. Distribution of substance P and calcitonin gene-related peptide-like immunoreactive nerve fibers in the rat temporomandibular joint. J Dent Res. 1993;72(3):592–598. doi:10.1177/00220345930720030701
22. Uddman R, Grunditz T, Kato J, Sundler F. Distribution and origin of nerve fibers in the rat temporomandibular joint capsule. Anat Embryol. 1998;197(4):273–282. doi:10.1007/s004290050137
23. Azuma Y, Sato I. The localization of calcitonin gene-related peptide in the human trigeminal ganglion and masseter muscle. Okajimas Folia Anat Jpn. 2017;93(4):127–138. doi:10.2535/ofaj.93.127
24. Sato J, Segami N, Kaneyama K, Yoshimura H, Fujimura K, Yoshitake Y. Relationship of calcitonin gene-related peptide in synovial tissues and temporomandibular joint pain in humans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(5):533–540. doi:10.1016/j.tripleo.2004.02.057
25. Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Neuropeptides in the arthritic TMJ and symptoms and signs from the stomatognathic system with special consideration to rheumatoid arthritis. J Orofac Pain. 1995;9(3):215–225.
26. Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Relation between intra-articular temperature of the arthritic temporomandibular joint and presence of calcitonin gene-related peptide in the joint fluid. A clinical study. Acta Odontol Scand. 1993;51(5):285–291. doi:10.3109/00016359309040579
27. Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–149. doi:10.1016/0304-3959(92)90154-4
28. Ohrbach R, Granger C, List T, Dworkin S. Preliminary development and validation of the jaw functional limitation scale. Community Dent Oral Epidemiol. 2008;36(3):228–236. doi:10.1111/j.1600-0528.2007.00397.x
29. Ohrbach R, Larsson P, List T. The jaw functional limitation scale: development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain. 2008;22(3):219–230.
30. Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12(8):963–974. doi:10.1023/A:1026119331193
31. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
32. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi:10.2307/2136404
33. Krogstad BS, Jokstad A, Dahl BL, Soboleva U. Somatic complaints, psychologic distress, and treatment outcome in two groups of TMD patients, one previously subjected to whiplash injury. J Orofac Pain. 1998;12(2):136–144.
34. Greenspan JD, Slade GD, Bair E, et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. 2011;12(11 Suppl):T61–74. doi:10.1016/j.jpain.2011.08.006
35. Sloan JH, Ackermann BL, Carpenter JW, et al. A novel, high-sensitivity and drug-tolerant sandwich immunoassay for the quantitative measurement of circulating proteins. Bioanalysis. 2012;4(3):241–248. doi:10.4155/bio.11.312
36. Gatchel RJ, Garofalo JP, Ellis E, Holt C. Major psychological disorders in acute and chronic TMD: an initial examination. J Am Dent Assoc. 1996;127(9):1365–1370. doi:10.14219/jada.archive.1996.0450
37. Carlson CR, Reid KI, Curran SL, et al. Psychological and physiological parameters of masticatory muscle pain. Pain. 1998;76(3):297–307. doi:10.1016/S0304-3959(98)00063-3
38. Fillingim RB, Ohrbach R, Greenspan JD, et al. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12(11 Suppl):T46–60. doi:10.1016/j.jpain.2011.08.007
39. Karamat A, Smith JG, Melek LNF, Renton T. Psychologic impact of chronic orofacial pain: a critical review. J Oral Facial Pain Headache. 2022;36(2):103–140.
40. Baskin SM, Smitherman TA. Migraine and psychiatric disorders: comorbidities, mechanisms, and clinical applications. Neurol Sci. 2009;30(Suppl 1):S61–65. doi:10.1007/s10072-009-0071-5
41. Buse DC, Silberstein SD, Manack AN, Papapetropoulos S, Lipton RB. Psychiatric comorbidities of episodic and chronic migraine. J Neurol. 2013;260(8):1960–1969. doi:10.1007/s00415-012-6725-x
42. Minen MT, Begasse De Dhaem O, Kroon Van Diest A, et al. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry. 2016;87(7):741–749. doi:10.1136/jnnp-2015-312233
43. Vinals Narvaez AC, Sanchez-Sanchez T, Garcia-Gonzalez M, et al. Psychological and behavioral factors involved in temporomandibular myalgia and migraine: common but differentiated profiles. Int J Environ Res Public Health. 2023;20(2):1545. doi:10.3390/ijerph20021545
44. Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63(3):341–351. doi:10.1016/0304-3959(95)00068-2
45. Fernandez-de-las-penas C, Galan-del-Rio F, Fernandez-Carnero J, Pesquera J, Arendt-Nielsen L, Svensson P. Bilateral widespread mechanical pain sensitivity in women with myofascial temporomandibular disorder: evidence of impairment in central nociceptive processing. J Pain. 2009;10(11):1170–1178. doi:10.1016/j.jpain.2009.04.017
46. Grossi DB, Chaves TC, Goncalves MC, et al. Pressure pain threshold in the craniocervical muscles of women with episodic and chronic migraine: a controlled study. Arq Neuropsiquiatr. 2011;69(4):607–612. doi:10.1590/S0004-282X2011000500007
47. Peng KP, May A. Migraine understood as a sensory threshold disease. Pain. 2019;160(7):1494–1501. doi:10.1097/j.pain.0000000000001531
48. Nahman-Averbuch H, Shefi T, Schneider VJ, et al. Quantitative sensory testing in patients with migraine: a systematic review and meta-analysis. Pain. 2018;159(7):1202–1223. doi:10.1097/j.pain.0000000000001231
49. Chaves TC, Dach F, Florencio LL, et al. Concomitant migraine and temporomandibular disorders are associated with higher heat pain hyperalgesia and cephalic cutaneous allodynia. Clin J Pain. 2016;32(10):882–888. doi:10.1097/AJP.0000000000000369
50. Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi:10.1067/mcp.2001.113989
51. Puntmann VO. How-to guide on biomarkers: biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad Med J. 2009;85(1008):538–545. doi:10.1136/pgmj.2008.073759
52. Riesco N, Cernuda-Morollon E, Pascual J. Neuropeptides as a marker for chronic headache. Curr Pain Headache Rep. 2017;21(4):18. doi:10.1007/s11916-017-0618-8
53. Gallai V, Sarchielli P, Floridi A, et al. Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia. 1995;15(5):384–390. doi:10.1046/j.1468-29821995.1505384.x
54. Fusayasu E, Kowa H, Takeshima T, Nakaso K, Nakashima K. Increased plasma substance P and CGRP levels, and high ACE activity in migraineurs during headache-free periods. Pain. 2007;128(3):209–214. doi:10.1016/j.pain.2006.09.017
55. Rodriguez-Osorio X, Sobrino T, Brea D, Martinez F, Castillo J, Leira R. Endothelial progenitor cells: a new key for endothelial dysfunction in migraine. Neurology. 2012;79(5):474–479. doi:10.1212/WNL.0b013e31826170ce
56. Edvinsson L, Ekman R, Goadsby PJ. Measurement of vasoactive neuropeptides in biological materials: problems and pitfalls from 30 years of experience and novel future approaches. Cephalalgia. 2010;30(6):761–766. doi:10.1177/0333102409351807
57. Carleson J, Lundeberg T, Appelgren B. Muscle and brain changes of calcitonin gene-related peptide in experimentally induced unilateral rat masseter myositis. J Orofac Pain. 2004;18(3):246–252.
58. Romero-Reyes M, Pardi V, Akerman S. A potent and selective calcitonin gene-related peptide (CGRP) receptor antagonist, MK-8825, inhibits responses to nociceptive trigeminal activation: role of CGRP in orofacial pain. Exp Neurol. 2015;271:95–103. doi:10.1016/j.expneurol.2015.05.005
59. Damico JP, Ervolino E, Torres KR, et al. Phenotypic alterations of neuropeptide Y and calcitonin gene-related peptide-containing neurons innervating the rat temporomandibular joint during carrageenan-induced arthritis. Eur J Histochem. 2012;56(3):e31. doi:10.4081/ejh.2012.e31
60. Jiang H, Xu L, Liu W, Xiao M, Ke J, Long X. Chronic pain causes peripheral and central responses in MIA-induced TMJOA rats. Cell Mol Neurobiol. 2022;42(5):1441–1451. doi:10.1007/s10571-020-01033-8
61. Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi:10.1186/1744-8069-7-94
62. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–1142. doi:10.1152/physrev.00034.2013
63. Kendall CH, Homer CE, Bishop AE, Polak JM. Age-related peptide production by human thyroid C cells. An immunohistochemical study. Virchows Arch a Pathol Anat Histopathol. 1986;410(2):97–101. doi:10.1007/BF00713511
64. Wimalawansa SJ. Age-related increase of calcitonin gene-related peptide in rat thyroid and circulation. Peptides. 1991;12(5):1143–1147. doi:10.1016/0196-9781(91)90071-V
65. Gangula PR, Chauhan M, Reed L, Yallampalli C. Age-related changes in dorsal root ganglia, circulating and vascular calcitonin gene-related peptide (CGRP) concentrations in female rats: effect of female sex steroid hormones. Neurosci Lett. 2009;454(2):118–123. doi:10.1016/j.neulet.2009.02.068
66. Yamaga N, Kawasaki H, Inaizumi K, Shimizu M, Nakamura A, Kurosaki Y. Age-related decrease in calcitonin gene-related peptide mRNA in the dorsal root ganglia of spontaneously hypertensive rats. Jpn J Pharmacol. 2001;86(4):448–450. doi:10.1254/jjp.86.448
67. Walker CS, Li X, Whiting L, et al. Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology. 2010;151(9):4257–4269. doi:10.1210/en.2010-0284
68. Gram DX, Hansen AJ, Wilken M, et al. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur J Endocrinol. 2005;153(6):963–969. doi:10.1530/eje.1.02046
69. Zelissen PM, Koppeschaar HP, Lips CJ, Hackeng WH. Calcitonin gene-related peptide in human obesity. Peptides. 1991;12(4):861–863. doi:10.1016/0196-9781(91)90147-H
70. Martami F, Jayedi A, Shab-Bidar S. Primary headache disorders and body mass index categories: a systematic review and dose-response meta-analysis. Headache. 2022;62(7):801–810. doi:10.1111/head.14356
71. Bigal ME, Liberman JN, Lipton RB. Obesity and migraine: a population study. Neurology. 2006;66(4):545–550. doi:10.1212/01.wnl.0000197218.05284.82
72. Jordani PC, Campi LB, Circeli GZ, Visscher CM, Bigal ME, Goncalves DA. Obesity as a risk factor for temporomandibular disorders. J Oral Rehabil. 2017;44(1):1–8. doi:10.1111/joor.12453
73. Recober A, Goadsby PJ. Calcitonin gene-related peptide: a molecular link between obesity and migraine? Drug News Perspect. 2010;23(2):112–117. doi:10.1358/dnp.2010.23.2.1475909
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.