Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Evaluation of Overactive Bladder Symptoms in Patients Recovering from Post-Acute COVID-19 Syndrome
Authors Zachariou A , Sapouna V, Kaltsas A , Dimitriadis F , Douvli E, Champilomatis I, Kounavou C, Papatsoris A, Tsounapi P, Takenaka A, Sofikitis N
Received 3 August 2022
Accepted for publication 18 October 2022
Published 26 October 2022 Volume 2022:15 Pages 2447—2452
DOI https://doi.org/10.2147/JMDH.S384436
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Athanasios Zachariou,1,2 Vagia Sapouna,2 Aris Kaltsas,1 Fotios Dimitriadis,3 Erriketi Douvli,1 Ioannis Champilomatis,1 Chrysanthi Kounavou,1 Athanasios Papatsoris,4 Panagiota Tsounapi,5 Atsushi Takenaka,5 Nikolaos Sofikitis1
1Department of Urology, University of Ioannina, Ioannina, Greece; 2Physical Medicine and Rehabilitation Centre EU PRATTEIN, Volos, Greece; 3 1st Department of Urology, Aristotle University of Thessaloniki, Thessaloniki, Greece; 4 2nd Department of Urology, Sismanogleion General Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece; 5Department of Urology, Faculty of Medicine, Tottori University, Yonago, Japan
Correspondence: Athanasios Zachariou, Department of Urology, University of Ioannina, 3 Spyridi Street, Volos, 38221, Greece, Tel +302421026937, Fax +302421026932, Email [email protected]
Purpose: Coronavirus disease (COVID-19) is a multi-organ viral infection with many manifestations. However, its impact on the genitourinary system is nowadays under investigation. This study aimed to evaluate the consequences on bladder function in patients suffering from post-acute COVID-19 syndrome (PACS) transferred to inpatient rehabilitation for long-term care after initial treatment for COVID-19 pathophysiology.
Materials and Methods: All the patients were initially asked the question (after having recovered from the acute stage of COVID-19 disease): “Have you noticed a sudden, uncontrolled need to urinate and sometimes a urine leakage accompanying the voiding desire?” Sixty-six out of 147 patients responded positively to this question and were assessed with the AUA Urology Care Foundation Overactive Bladder Assessment Tool (AUA-OAB-tool). All included men were evaluated with the IPSS score.
Results: The median age of patients was 59.5 (range 44– 72). We identified 44 patients with newly diagnosed OAB (Group A; post-COVID assessment) and 22 with worsening OAB symptoms (Group B). The mean symptom score ± standard deviation in Group A patients was 18.25 ± 2.11 (using the above AUA OAB tool). In the patients of Group B, there was an increase in the above score from 10.43 ± 1.52 (pre-COVID condition) to 17.87 ± 1.89 (post-COVID assessment). In patients of Group A, the total quality of life (QOL) score was 17.74 ± 2.34. Patients in Group B presented an escalation in total QOL score from 9.04 ± 1.41 (pre-COVID) to 18.84 ± 1.96 (post-COVID condition). There was no statistically significant difference in symptoms and QOL scores between men and women in groups A and B. There were 11 men in Group A and 5 in Group B with an IPSS score > 20.
Conclusion: OAB symptoms may be essential to PACS syndrome and influence quality of life, delaying full recovery.
Keywords: post-acute COVID-19, quality of life, AUA-OAB tool, overactive bladder, nocturia
Introduction
Infection with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) causes Coronavirus Disease 2019 (COVID-19) and demonstrates an extensive range of symptoms. The most common are fever, cough, fatigue, and dyspnea. Although most infected patients develop minor to moderate symptoms, a minority of individuals present acute respiratory distress syndrome and multiple organ dysfunction syndromes.1 Post-acute COVID-19 (PACS) is defined as persistent symptoms and/or delayed or long-term complications beyond four weeks from the onset of symptoms. Care for the condition usually does not conclude at the time of hospital discharge. Still, multidisciplinary medical collaboration is required for those patients in Rehabilitation Centres or outpatient settings.2
A urologist is usually involved in the initial assessment of patients presenting with fever without other symptoms of COVID-19, as it is sometimes taken as urosepsis. The placement of urethral catheters, ureteral stents or nephrostomy tubes to relieve urinary obstruction contribute to the incidence of infections. Furthermore, encrustations and high-resistant infections are frequent disadvantages of indwelling devices and can lead to sepsis in frail patients.3
Mumm et al recently reported increased urinary frequency as a symptom of COVID-19.4 As current data indicates, patients can present with less typical symptoms involving the lower urinary tract and the male genital system. These are chiefly lower urinary tract symptoms (LUTS) (be it de novo or the worsening of a pre-existing condition) and epididymal and/or testis discomfort.5
There are increasing reports of COVID-19 patients presenting with onset or an exacerbation of baseline urinary symptoms, especially overactive bladder (OAB). This is alluded to as COVID-19-associated cystitis (CAC).6 What is not well understood are the contributing factors of urinary symptoms in those presenting with COVID-19. However, there are hypotheses aimed at explaining the effect of COVID-19 on the genitourinary system.7 As regards so-called “long COVID” or PACS and its association with urinary symptoms or discomfort, this has not been comprehensively studied.
Therefore, this study aimed to evaluate the consequences on bladder function in patients suffering from PACS. They were transferred to inpatient rehabilitation for long-term care following initial hospitalised treatment for COVID-19 pathophysiology.
Materials and Methods
This descriptive study was carried out between December 2020 and May 2021 and involved patients in an inpatient department of a Rehabilitation Centre in Volos, Greece. Looking specifically at the hospital activity data for Greece, pressures during the latest COVID-19 wave in January 2021 significantly surpassed the burden observed during the first peak in March 2020. The Rehabilitation Centre admitted patients with PACS for long-term care during this period. The local ethics committee of the Physical Medicine and Rehabilitation Centre EU PRATTEIN approved the study, which was conducted following the Declaration of Helsinki. All participants gave informed consent for the use of their data prior to study commencement.
The study group assessed participants’ cognition with the Mini-Mental State Examination (MMSE).8 The MMSE is the most common tool used to evaluate various cognitive disorders quickly. A total MMSE score of 24 or more was an initial inclusion criterion.
After hospitalisation and recovering from the acute stage of COVID-19, all the patients were initially asked upon admission to Rehabilitation Center: “Have you noticed a sudden, uncontrolled need to urinate, and sometimes a urine leakage accompanying the voiding desire?”. The study included patients that responded positively and continued with the AUA Urology Care Foundation Overactive Bladder Assessment Tool (AUA-OAB-tool) (Supplemental Table 1). Subjects were excluded from participation if they had active UTI, bladder or pelvic tumours, stress urinary incontinence, bladder or kidney stones, neurologic disorders affecting bladder function and postvoid residual volume over 100 mL.
The AUA OAB tool consists of five questions about the frequency of the following storage symptoms: urgency, urge incontinence, incontinence, frequency, nocturia and overall satisfaction regarding the current urinary condition. The answers range from 0 to 5, with 0 describing not at all and five always. The total symptom score varies from 0 (absence of symptoms) to 25 (most severe). Additionally, there are four quality of life (QoL) questions describing the patients’ bother from urgency, urge incontinence, frequency, nocturia and the fifth question about overall satisfaction with their current urinary condition. The answers range from 0 to 5; 0 referred to “I am not bothered at all” and 5 to “I am bothered a great deal”. The patients presenting a history of OAB symptoms completed two questionnaires, one for the pre-COVID-19 OAB symptoms and the second for their current situation. The final question examines the QoL issue, “How have your symptoms changed your life?”. Patients have a free-response option in questions relating to everyday life activities that are affected by their OAB (eg Keeping you from getting a good night’s sleep? Causing you to stay home more than you would like? etc.).
Patients that reported a deterioration of their OAB symptoms (Group A) answered the questionnaire twice; the first time for their previous condition before COVID-19 disease and the second time for their present state. Individuals reporting onset of OAB symptoms (Group B) once fulfilled the AUA OAB tool, describing their current condition. All included men fulfilled an additional questionnaire, the IPSS score.
Forty patients of the Rehabilitation Center suffering from OAB and negative for COVID −19 formed a comparison group (Control Group). The patients answered, like the individuals with worsening OAB symptoms, the AUA OAB tool twice. The first reported OAB symptoms at their facilities and the second after admission to Rehabilitation Center.
To evaluate the statistically significant differences between the two measurements in Group A and Control Group, we used the paired-samples t-test; p <0.050 was considered significant. The mean and standard deviation of the quantitative data (symptom score, QoL score) were calculated. Age is presented with a median value and range. Data were analysed using IBM SPSS Statistics for Windows, Version 24.0.
Results
One hundred forty-seven patients were referred to a Rehabilitation Centre for long-term care. Our study identified 75 patients who developed either de novo or exacerbated symptoms associated with OAB somewhere between 4 and 7 weeks after presenting with COVID-19 (Table 1). Although nine patients declined to answer questionnaires, 66 patients enrolled in the study and submitted answers to the AUA OAB tool. We identified 44 patients with newly diagnosed OAB (Group A; post-COVID assessment) and 22 with worsening OAB symptoms (Group B).
 |
Table 1 Demographics of Patients’ Study |
Patients ranged from 44 to 72, with a median of 59.5 years old. The median duration of hospitalisation was 36 days (range 28–48 days), and the median stay in the Rehabilitation centre was 31 days (range 14–58 days). Benign Prostatic Hyperplasia (BPH) was identified in 16 men, representing 44.4% of our sample male population.
The primary outcomes regarding mean symptoms score and QoL score with standard deviation (mean score ± standard deviation) in the different patients’ groups are presented in Table 2. All patients complained about nocturia episodes (median episodes 3, range between 2 and 5) and had specific arguments concerning their QoL. There were 11 men in Group A and 5 in Group B with an IPSS score>20.
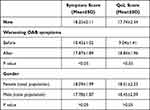 |
Table 2 Primary Outcomes for Symptoms and Quality of Life Scores |
The patients in the Control Group ranged from 46 to 71, with the median being 58.8 years old. The median length of hospital stay before their admission for rehabilitation was 34 days (range between 28 and 43 days), and the median stay in the Rehabilitation Centre was 29 days (range between 15 and 59 days). A total of 8 men had BPH, representing 33.3% sampled male population.
No statistically significant difference in symptoms and QOL scores between men and women in groups A and B. An assessment of the Control Group failed to identify any statistically significant changes in OAB symptoms before and during their stay at the Rehabilitation Center (Table 3).
 |
Table 3 Patients’ Evaluation for Symptoms and Quality of Life Scores in Control Group |
Discussion
The outcomes of the present study emphasise OAB symptoms and the impaired quality of life in patients recovering from COVID-19. The presence of OAB was a significant issue, and a suitable questionnaire was the pertinent way to prove it. In our study, the chronological characteristics of the alterations of urinary symptoms suggest that COVID-19 affects the lower urinary tract and that PACS can induce OAB.
The cellular expression of angiotensin‐converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) are the pathways for SARS‐CoV‐2 to enter host cells.10 Although there is no established theory, several mechanisms are currently being considered. SARS-CoV-2 pathophysiology encompasses viral spike protein binding to ACE2 receptors placed on pneumocytes, urothelial cells, and testis.11 SARS‐CoV‐2 could infect the bladder by a hematogenous route or by spreading from urethral cells, where there is proof of the presence of ACE2 receptors. As to which cells, luminal or basal urothelial, efficiently express the ACE2 receptor is a matter for further investigation. The SARS‐CoV‐2 detection in the urine accounts for only 4.5% of COVID-19 patients.12 SARS‐CoV‐2 is only infrequently found in the urine samples of infected patients. This gives some credence to the idea that the virus spreads to the bladder from the urethral endothelium.4
It has been proposed that COVID-19 patients presenting with severe de novo urinary symptoms do so following the release of inflammatory cytokines into the urine. A direct bladder or urothelium insult could possibly amount for COVID‐19 Associated Cystitis (CAC) and its associated bladder dysfunctions.7 Indeed, COVID-19 patients presenting with severe de novo urinary symptoms have been found to have increased pro-inflammatory cytokines. By inference, it seems that COVID-19-associated inflammation can cause bladder dysfunction.7
Finally, it has also been suggested that COVID-19 can cause neurocognitive symptoms post-infection, most likely because of damage rendered to the nervous system.13 Reports support that SARS-CoV-2 is responsible for the initiation of neuroinflammation, which causes neuronal demyelination in the central and peripheral nervous system. The exaggerated release of pro-inflammatory molecules also identified as “cytokine storm” can stimulate inflammation and demyelination of the pudendal nerve triggering bladder and bowel incontinence.14 In contrast, it has been reported that an identical structural disorder can also lead to urinary and fecal retention through not entirely understood mechanisms.15
This study is one of the few investigating OAB symptoms in patients with PACS who receive long-term care in a Rehabilitation Centre. Interestingly, 68.5% of patients described the commencement of urinary symptoms after COVID-19 infection. Moreover, of patients with OAB symptoms, 31.5% reported worsening their clinical symptomatology after COVID-19. Given that all these patients were hospitalised for COVID-19 and later presented with PACS, these are demonstrative results of severe acute disease and not of asymptomatic, mild, or moderate illness.
As aforementioned, the median age of our patients was 59.5 years. Pre-existing genitourinary (GU) symptoms generally appear in the +65 year old demographic. Based on the IPSS, another prospective study reported augmentation of lower urinary tract symptoms (LUTS) in older men during COVID-19 hospitalisation. Therefore, elderly male patients with subclinical or nonspecific symptoms should be additionally evaluated for COVID-19 when presenting with increased LUTS of an indeterminate cause.16 Future studies with younger adult patients will clarify the relation of the genitourinary system with SARS-CoV-2 infection. A smaller study included younger patients (mean ± standard deviation of the age was 32.3 ± 8.9 (female) and 38.9 ± 13 (male) years old). It concluded that symptoms related to storage in the lower urinary tract were more widespread during acute COVID-19.17 Our study points to the effect of PACS and presents similar results to smaller studies, raising awareness of the urologic impact of the disease.18,19 The existence of a Control Group further strengthens our protocol because it evaluates the changes in OAB symptoms in COVID-19 patients in response to pandemic-related stress or lifestyle changes during the same period.
Even though they reported OAB symptoms for the first time during hospitalisation, 16 men were confirmed to have BPH using the IPSS questionnaire. Luciani et al outlined the case of three men with pre-existing LUTS (ie BPH and radiation cystitis) whose urinary tract was severely impacted during COVID-19 hospitalisation. The supposition was that a pre-existing urological condition made patients especially vulnerable to damage to the urinary tract by COVID-19, including hematuria.20
Remarkably, Welk et al presented no LUTS increase in COVID-19 patients compared to the corresponding cohort.21 However, as their study excluded patient-reported measures of urinary symptoms, it was somewhat limited. It is likely that their subjects did not seek medical care or receive medical treatment for their urological condition. What is more, their study encompassed patients with a positive SARS-CoV-2 nasopharyngeal swab, whilst ours only focused on patients hospitalised for COVID-19 and later presented with PACS.
At this time, it is uncertain if symptoms of OAB following COVID-19 infection vary in a more extended follow-up period. It is felt that further investigation, including urodynamic studies, could shed more light on the primary pathophysiology involved. Furthermore, patients in Group A who reported a deterioration of their OAB symptoms answered the same questionnaire twice. There is a possibility of recall bias, which we should refer to as a limitation of our study.
Conclusion
Future prospective studies in multi-centre settings and a long-term follow-up are seen as being essential to managing these clinical topics. Our research indicates that COVID-19 may lead to de novo or deteriorating urinary symptoms. Most importantly, medication may help in the management of urinary symptoms in COVID-19 recovered patients, and these treatments may differ from those prescribed for patients not affected by COVID-19. Therefore, it may be essential to differentiate the diagnosis as either an independent urinary disease or a COVID-19 sequela.
Acknowledgments
The abstract of this paper was presented at the 37th EAU Annual Congress, 1–4 July 2022 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Eur Urol Suppl 2022;81(S 1):S178.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alimohamadi Y, Sepandi M, Taghdir M, et al. Determine the most common clinical symptoms in COVID-19 patients: a systematic review and meta-analysis. J Prev Med Hyg. 2020;61(3):E304–12.
2. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi:10.1038/s41591-021-01283-z
3. Sighinolfi MC, Rocco B, Mussini C. COVID-19: importance of the awareness of the clinical syndrome by urologists. Eur Urol. 2020;78(1):e40–e41. doi:10.1016/j.eururo.2020.03.029
4. Mumm JN, Osterman A, Ruzicka M, et al. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does Sars-Cov-2 cause viral cystitis? Eur Urol. 2020;78(4):624–628. doi:10.1016/j.eururo.2020.05.013
5. Creta M, Sagnelli C, Celentano G, et al. Sars-Cov-2 infection affects the lower urinary tract and male genital system: a systematic review. J Med Virol. 2022;93(5):3133–3142. doi:10.1002/jmv.26883
6. Dhar N, Dhar S, Timar R, et al. De novo urinary symptoms associated with COVID-19: COVID-19 associated cystitis (Cac). J Clin Med Res. 2020;12(10):681–682. doi:10.14740/jocmr4294
7. Lamb LE, Dhar N, Timar R, et al. Covid-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (Cac). Med Hypotheses. 2020;145:110377. doi:10.1016/j.mehy.2020.110375
8. Devenney E, Hodges JR. The mini-mental state examination: pitfalls and limitations. Pract Neurol. 2017;17(1):79–80. doi:10.1136/practneurol-2016-001520
9. Overactive bladder assessment tool. Available from: https://www.urologyhealth.org/educational-resources/overactive-bladder-assessment-tool.
10. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):
11. Zou X, Chen K, Zou J, et al. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):
12. Kashi AH, De la Rosette J, Amini E, et al. Urinary viral shedding of COVID‐19 and its clinical associations: a systematic review and meta‐analysis of observational studies. Urol J. 2020;17(5):
13. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi:10.1016/S1474-4422(20)30221-0
14. Selvi I, Donmez MI, Ziylan O, et al. Urodynamically proven lower urinary tract dysfunction in children after COVID-19: a case series. LUTS. 2022;14:301–304. doi:10.1111/luts.12436
15. Nath A. Neurologic complications of coronavirus infections. Neurology. 2020;94(19):1–2. doi:10.1212/WNL.0000000000009455
16. Can O, Erkoc M, Ozer M, et al. The effect of COVID-19 on lower urinary tract symptoms in elderly men. Int J Clin Pract. 2021;75(6):E14110. doi:10.1111/ijcp.14110
17. Kaya Y, Kaya C, Kartal T, et al. Could luts be early symptoms Of Covid-19. Int J Clin Pract. 2021;75(3):E13850. doi:10.1111/ijcp.13850
18. Khaliq F, Wills M, Dhar N, et al. Assessment of overactive bladder symptoms in deconditioned patients recovering from post-acute COVID-19 syndrome. J Urol. 2021;206(3S):e18. doi:10.1097/JU.0000000000001963.17
19. Chen W, Komnenov D, Timar R, et al. New or worsening overactive bladder symptoms after recovery from COVID-19. J Urol. 2021;206(3S):e1101. doi:10.1097/JU.0000000000002103.02
20. Luciani LG, Gallo F, Malossini G, et al. Urinary frequency as a possible overlooked symptom in COVID-19 patients: Does SARS-CoV-2 cause viral cystitis? . Eur Urol. 2020;78(3):129–130. doi:10.1016/j.eururo.2020.06.006
21. Welk B, Richard L, Braschi E, et al. Is coronavirus disease 2019 associated with indicators of long-term bladder dysfunction? Neurourol Urodynam. 2021;40(5):1200–1206. doi:10.1002/nau.24682
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
