Back to Journals » Journal of Pain Research » Volume 11
Evaluation of combined radiofrequency and chemical blockade of multi-segmental lumbar sympathetic ganglia in painful diabetic peripheral neuropathy
Authors Ding Y , Yao P, Li H, Zhao R, Zhao G
Received 28 May 2018
Accepted for publication 25 June 2018
Published 26 July 2018 Volume 2018:11 Pages 1375—1382
DOI https://doi.org/10.2147/JPR.S175514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall

Yuanyuan Ding,1 Peng Yao,1 Hongxi Li,1 Rongjie Zhao,2 Guangyi Zhao3
1Department of Pain Management, Shengjing Hospital of China Medical University, Shenyang, China; 2Class 5 of 2020 Session, Shenyang No.20 High School, Shenyang, China; 3Department of Anesthesiology, Shengjing Hospital of China Medical University, Shenyang, China
Background: Painful diabetic peripheral neuropathy (PDPN) is one of the most common complications of diabetes. PDPN seriously affects the quality of life and is difficult to treat; therefore, there is an urgent need for new cost-effective treatment methods for PDPN.
Objective: To investigate the efficacy and safety of radiofrequency thermocoagulation (RF) combined with anhydrous ethanol (AE) chemical blockade of lumbar sympathetic ganglia (LSG) in patients with PDPN using computed tomography (CT).
Study design: Retrospective comparative study.
Setting: Shengjing Hospital of China Medical University.
Methods: Ninety patients diagnosed with PDPN were enrolled in this study. The patients were randomly divided into AE group (A, n=30), RF group (B, n=30), and RF+ AE group (C, n=30). The follow-up included preoperative basic conditions, visual analog scale (VAS), the total remission rate (TRR), skin temperature (ST) and the improvement of numbness and hyperalgesia in the lower extremities, complications, and degree of satisfaction (DOS) before and after surgery.
Results: Postoperative VASs were significantly decreased compared to preoperative VASs in all groups (P<0.05). The VAS in group A began to increase 3 months (3M) after surgery; VAS scores at 3M, 6 months (6M) and 1 year (1Y) were significantly different compared to group B and C (P<0.05); VAS in group B began to increase after 6M; VAS scores at 6M and 1Y were significantly different compared to group C (P<0.05); Moreover, group C maintained relatively long duration of pain relief. TRR in group A, group B and group C at 1Y after operation was 66.7%, 73.3% and 93.3%, respectively; TRR in group C was statistically different compared to groups A and B (P<0.05). Higher ST in the lower extremities was observed after surgery in all groups compared to peroration (P<0.05); nonetheless, the difference was not statistically significant. The numbness and hyperalgesia improved in all three groups after surgery compared to preoperational time, the numbness in group C was significantly higher compared to groups A and B. In addition, no severe complications were observed. At 6M and 1Y after surgery, the degree of satisfaction in patients from group C was significantly higher compared to groups A and B.
Conclusion: Radiofrequency thermocoagulation combined with AE chemical blockade of the LSG was safe and effective. Nevertheless, the details underlying analgesic mechanisms still need to be investigated.
Keywords: lumbar sympathetic ganglia, chemical lumbar sympathectomy, neurolysis, radiofrequency thermocoagulation, ablation, painful diabetic peripheral neuropathy
Introduction
As the quality of life improves, the incidence of diabetes increases on a yearly basis. Epidemiologists have estimated that the global incidence of diabetes will reach 552 million by 2030.1 Diabetic peripheral neuropathy (DPN) is one of the common complications of diabetes, accounting for 50% of diabetic neuropathy,2 and the incidence of painful diabetic peripheral neuropathy (PDPN) is 13%–26%.3 Since PDPN seriously affects the quality of life and is difficult to treat, there is urgent need for new cost-effective treatment methods for PDPN.
There are 2–6 pairs of lumbar sympathetic ganglia (LSG). The blocking of L2 and L3 LSG blocks the sympathetic fibers of the lower extremities and dilates blood vessels; L2 ganglia have an important role in this process. In addition, the position of LSG varies; the L2 sympathetic ganglia are mainly found in the lower one third of the L2 vertebrae and upper one third of the L3 vertebrae;4 therefore a multi-segment treatment is required when targeting these regions. Traditional LSG blockades include surgical or chemical sympathectomy. However, surgical sympathectomy can cause trauma and tissue damage, while chemical sympathectomy can harm surrounding vital tissues and organs due to drug diffusion. Radiofrequency therapy comprises two methods: radiofrequency thermocoagulation and pulsed radiofrequency.5 Radiofrequency thermocoagulation lumbar sympathectomy, which targets the nerve tissue by increasing the temperature, has been shown to be another effective treatment approach.6 The unmyelinated nerve fiber C-axis axons get dissolved and become necrotic due to local high temperature, which maintains a state of vasodilatation in lower extremities, increases peripheral blood flow, improves symptoms such as numbness induced by nerve injury of lower limbs, and generates long-term pain relief. Nevertheless, the accuracy of punctured target selection is extremely difficult, and the range of ablation is limited.
PDPN treatment requires the use of comprehensive treatment. Currently, there are many studies on single chemical or single radiofrequency treatment approaches for destroying LSG, while there are no studies examining the combination of chemicals and radiofrequency for PDPN. This paper investigates the combination of radiofrequency thermocoagulation and anhydrous ethanol (AE) chemical blockade of LSG for the treatment of PDPN, by comprehensively assessing their clinical efficacy and safety. We used CT-guidance for accurate positioning.
Methods
Patients
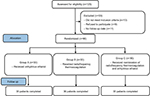
A total of 90 patients diagnosed with PDPN were enrolled at the Department of Pain Management from January 2013 to December 2016. The study was approved by the Ethics Committee of Shengjing Hospital Affiliated to China Medical University. Patients were randomly divided into three groups: AE group (A, n=30), radiofrequency thermocoagulation group (B, n=30), and radiofrequency thermocoagulation + AE group (C, n=30). All patients were informed about risks and complications before surgery, and the written informed consent was obtained from all patients (Figure 1).
Inclusion criteria were the following: 1) all patients met the criteria for diabetes diagnosis and treatment published by the American Diabetes Association (ADA) in 2016; 2) sensory impairments in the lower extremities, including symmetry or unilateral numbness and stabbing pain, knife-like, burning pain, leg cramps, decreased pain sensitivity and warm sensation, tactile abnormalities, exacerbation at night and/or sleep disorders; 3) physical examination showing pain disorder plane (below L1), diminished or disappeared knee tendon reflexes; 4) nerve conduction velocity abnormalities in the lower extremity: nerve electrophysiological examination showing motor nerve conductive velocity (MNCV) <45 m/s, sensory nerve conductive velocity (SNCV) <40 m/s; 5) excluding other causes of peripheral neuropathy; 6) conservative treatment for more than 6 months (6M), and HbA1c <8%.
Exclusion criteria were: infection of local puncture area; implantation of pacemaker/brain stimulator; mental illness, mental retardation, disturbance of consciousness preventing cooperation; blood diseases, pregnancy, severe liver and kidney dysfunction or history of heart and lung disease.
Procedures
During treatment, all patients were subjected to diet controls, while medicines were used to control neuralgia, nourish nerves and control blood glucose. Under the guidance of CT scan, patients were placed in a prone position. The middle and lower one third of the L2 vertebra, and the middle and upper one third of the L3 vertebra were identified to determine the puncture point, angle and the distance from puncture needle to the front edge of the L2 and L3 vertebra. Disinfection, draping and local anesthesia were also applied. The puncture was performed using the puncture needle at the point marked by CT. The needle was inserted at the puncture angle and it was stopped when it was close to the measurement distance. CT scan was performed for a second time to adjust the puncture point to the frontal side of vertebra. Three milliliters contrast agent was then injected and was diffused along the frontal side of the vertebra.
After injecting the radiofrequency needle core, the sensory and motor tests were done according to following criteria: 1) 50 Hz, 1.0 V electrical stimulation was performed to induce sore and swollen feeling in the deep back; 2) 2 Hz, 1.0 V electrical stimulation did not induce muscle twitch in hip and lower extremities, and no somatic nerve stimulation. Consequently, 1 mL (2%) lidocaine was injected; 5 minutes later the lower limbs became warm. Continuous radiofrequency thermocoagulation was conducted to gradually increase the temperature; the radiofrequency parameters for temperature were 70, 75 and 80°C. Radiofrequency time was 180 seconds for each temperature, and each site was submitted to radiofrequency thermocoagulation twice. The needle was withdrawn for 5 mm to test the motor and sensory response for a second time. All the other treatment parameters were done according to the protocol described above. Consequently, 1–2 mL AE was added to each L2 and L3 segment. Patients felt warm in the lower extremities, their pain was relieved and the needle was eventually completely withdrawn.
Observations and follow-up
General preoperative information including the age, gender, and duration of disease, the degree of preoperative pain, the dosage of gabapentin, and the location and nature of the pain were recorded. The follow-up evaluation was performed 1 week (1W), 1 month (1M), 3 months (3M), 6M and 1 year (1Y) after surgery, and was done using double-blind method by experienced medical staff of a non-surgical group.
For pain scoring, the following visual analog scale (VAS) was used: 0 (no pain) to 10 (the most unbearable pain).
Analgesic effect was analyzed according to the WHO evaluation criteria for pain relief; the efficacy was formulated using four grades: complete remission (CR), partial remission (PR), mild remission (MR), and no response (NR). The total remission rate was analyzed using the following formula: total remission rate (%) = [(CR+ PR+ MR)/n]×100%. The subjective symptoms and clinical signs in patients were assessed 1 year after surgery.
Skin temperature changes of bilateral lower limbs, improvement of accompanied symptoms such as numbness and hyperalgesia, the nerve injury, orthostatic hypotension and other complications were also observed.
Satisfaction for symptom relief was analyzed according to a previously described approach.7 Briefly, the degree of satisfaction for symptom relief in lower extremities was assessed by the patient: I very dissatisfied, II unsatisfied, III neutral, IV satisfied, V very satisfied (I–III were defined as unsatisfactory, while IV–V were defined as satisfactory).
Statistical analysis
Statistical analysis was performed using SPSS19.0 software. Measurement data were presented as mean ± SD. One-way analysis of variance was used if the data were in line with the normal distribution and the variance was homogeneous; if the data did not meet the normal distribution, then Kruskal–Wallis test was used. Chi-squared test was used for enumeration of data (if the data did not meet the chi-square, then calibration formula or Fisher test was used). P<0.05 was considered statistically significant.
Results
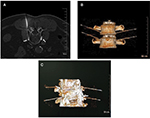
The surgical processes went smoothly in all the patients. CT imaging showed that the needle point was located at the frontal side of bilateral vertebrae, ie, lower third of L2 vertebrae and upper third of L3 vertebrae, with accurate position and in the multi-segments. The contrast agent was observed along the two sides of vertebrae, with a range covering the L2 and L3 sympathetic ganglia (Figure 2).
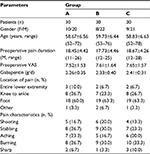
The preoperative basic conditions of patients in the three groups were then compared. No significant differences in gender, age, preoperative pain duration, preoperative VAS score, dose of analgesic drug gabapentin, location and nature of the pain were found between groups (P>0.05) (Table 1).
Furthermore, postoperative VASs were significantly decreased compared to preoperative VASs in all groups (P<0.05). In addition, at early stage, the reduction amplitude of VAS in group B was smaller compared to groups A and B, but the difference was not statistically significant (P>0.05). The VAS in group A began to increase 3M after surgery; VAS scores at 3M, 6M and 1Y were significantly different compared to group B and C (P<0.05). Additionally, VAS of group B began to increase after 6M; VAS scores at 6M and 1Y were significantly different compared to group C (P<0.05). VAS scores between different groups are shown in Figure 3. Moreover, group C maintained relatively long duration of pain relief.
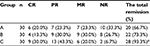
One year after operation, the total remission rates in groups A, B, and C were 66.7%, 73.3%, and 93.3%, respectively (Table 2). The total remission rate in group C was statistically different compared to groups A and B (P<0.05).
In all three groups, patients felt a warm sensation in the lower extremities after treatment. Higher skin temperatures in the lower extremities were observed after surgery in all groups compared to preoperation (P<0.05) (Table 3). The skin temperature in group A slightly decreased from the third month, but the difference was not statistically significant (P>0.05). In addition, no significant changes in temperature were observed between the three groups (P>0.05).
One year after surgery, the improvement rates of numbness in groups A, B and C were 61.6%, 73.1% and 85.2%, respectively; while the improvement rates of hyperalgesia in groups A, B and C at 1 year were 66.7% and 80.0% and 81.8%, respectively. The numbness and hyperalgesia in the three groups all improved after surgery compared to preoperational time. In addition, the numbness in group C was statistically significantly more improved compared to groups A and B (P<0.05).
Decreased blood pressure was observed in group A (four cases) and in group C (two cases) after surgery. The symptoms were relieved after rehydration. Three cases of genitofemoral neuritis occurred in group A and were relieved after 4 weeks of nutritional nerve treatment (Neurotropin, Nippon Zoki Pharmaceutical Co., Ltd, Japan). In addition, no severe complications, such as ureteric stricture, paraplegia or severe back pain were observed.
Overall, patients were satisfied with the degree of symptom remission when discharged from the hospital. Degree of satisfaction was assessed at 1M, 3M, 6M, and 1Y post-surgery. During long-term follow-up, degree of satisfaction decreased with the regeneration of damaged nerve fibers in patients. One month after operation, the degree of satisfaction in groups A, B and C was 86.7%, 77.3% and 93.3%; 3M after operation was 73.3%, 70.0% and 90.0%; 6M after operation was 56.7%, 60.0% and 86.7%; and 1Y after operation was 33.3%, 40.0% and 80.0%, respectively. At 6M and 1Y after surgery, the degree of satisfaction in patients from group C was significantly higher compared to groups A and B (P<0.05).
Discussion
PDPN, which is defined as neuropathic pain, is a common type of diabetic peripheral neuropathy. PDPN is mainly manifested as burning pain and numbness, deep dull pain and stabbing pain in the distal limbs, and especially the lower limbs. The pattern of pain can vary, and spontaneous allodynia and hyperalgesia with various forms of pain can occur in the affected area.8 The pain is continuous and persistent with unclear etiology and mechanisms. Spinal cord stimulation can remit the pain,9,10 nonetheless this method is expensive, and thus very difficult to apply widely, since most patients cannot afford it.
PDPN is a nerve dysfunction of distal limbs that occurs due to metabolic disturbance, microcirculation disorders and immune abnormalities. The pathological manifestations include capillary basement membrane thickening, endothelial cell swelling and hyperplasia, hyaline degeneration, glycoprotein deposition, luminal stenosis, which in turn lead to nerve ischemia, hypoxia, and then axonal atrophy, or even to disappearance of axons, myelin segmental or diffused shrinkage or demyelination.11 With disease progression, foot skin ulcers, necrosis and other symptoms on the basis of neuropathy, and severe pain in lower extremities can also occur. A series of pathological changes can also occur when the peripheral sensory nerve gets damaged. The sympathetic-sensory contacts are observed, and sympathetic stimulation can repeatedly activate neurons, prompting a significant increase in ectopic discharge.12,13 The primary sensory neurons are particularly sensitive to the output of sympathetic pulse. Sympathetic ectopic discharge can cause sensitization and excitation of primary sensory nerves, leading to primary neuronal sensitization, which is displayed as hyperalgesia and allodynia.14 Sympathetic nerves can also induce the release of pain-related inflammatory mediators such as substance P.15 Accordingly, sympathetic nerves have an important role in neuralgia mechanisms. Therefore, this study selected the lumbar sympathetic ganglion as the study subject.
Previous studies have found that blocking sympathetic nerves can inhibit pain transmission and relieve neuropathic pain.16–18 LSG excision or ablation can block the pain stimulus conducting to central nervous system through the nerve fibers, and can also relieve the vascular spasm in lower extremities. It can also promote the recovery of artery pulse in the feet, regulate an abnormal neuroendocrine system, improve immune function and relieve the stress state of the body,19 all of which should be treated at early stage of the disease.20–22 Chemical lumbar sympathectomy (CLS) uses the nerve damaging agent on the lumbar sympathetic nerve to induce damage and neurolysis, thus achieving lumbar sympathetic nerve resection. The blood vessels are dilated, peripheral vascular resistance is reduced, collateral circulation is increased and the skin and muscle blood perfusion in lower extremities is increased through the “loss of sympathetic effect”.23 However, the duration of the neurolysis effects is relatively short, and the symptoms mostly recur within 3–6 months. The clinical remission time of phenol is short, and it easily forms secondary neuroma.24 Therefore, in this study, AE was chosen as chemical damage agent and it was found that pain symptoms repeatedly appeared in the AE group after 3M, gradually becoming worse with the prolongation of time. This was consistent with the studies reported by Jackson and Gaeta,24 and it may be related to the regeneration of nerves using the AE. Although complications are significantly reduced under visual guidance, permanent damage of the genitofemoral nerve or lateral femoral cutaneous nerve can easily occur due to the fluidity of damage agent and variation of neural pathway,25 which may even lead to acute renal failure in severe conditions.26 In the present study, four cases of genitofemoral nerve damage occurred, which resolved after conservative treatment. The relatively short duration of pain relief maintenance and relatively high incidence of complications limit the application of CLS. CT-guided radiofrequency thermocoagulation of LSG has advantages such as clear images, accurate target position for puncture, scanning and confirming the location of needle point position so as to avoid injury, and sensory and motor neurological tests that can further prevent the occurrence of complications.27 It can also be repeatedly performed; nonetheless, the requirements for accuracy of nerve target are relatively high, and the number and location of lumbar sympathetic ganglia largely vary with absent division of sympathetic stem and communicating branches.28 This can easily result in incomplete nerve ablation, where the degree of ablation is affected by temperature, location and action duration. So, the effect of simple radiofrequency ablation of LSG is often not stable. In the present study we found that the analgesic effect in radiofrequency group in the early stage is lower compared to the AE group and the combination group. The combination group (group C) can maintain better analgesic effect for up to 1 year, and its 1-year total effective rate is also higher compared to the other two groups (groups A and B). The remission rate of numbness symptoms in the late stage was also superior to the other two groups, with higher satisfaction and fewer complications than the AE group. Since there was no significant difference in skin temperature between the three groups, the symptom relief was not simply attributed to expansion of blood vessels to improve circulation in group C. Studies have shown that disruption of lumbar sympathetic nerve can regulate the regeneration of cutaneous vascular cells by inhibiting the proliferation of parietal cells and increasing the expression of angiopoietin-1.29 It can reduce the inflammatory reaction in the sympathetic nerve denervation area,30 decrease the adrenergic release in the dorsal root ganglion, inhibit sympathetic activity by stimulating α2-adrenergic receptors and/or upregulating α2-adrenoceptors,31 inhibit spinal microglia activation and reduce the expression of inflammatory cytokines (IL-1β, IL-6, and TNF-α).32 Therefore, the mechanisms of sympathetic nerve blockade for pain relief are complex and need to be further studied. Multi-segmental AE combined with radiofrequency thermocoagulation blockade of LSG can be precisely positioned, reduce complications and achieve long-term analgesic effects.
Conclusion
Radiofrequency thermocoagulation combined with AE chemical blockade of the lumbar sympathetic ganglia was more effective compared to the single use of chemical or radiofrequency blockade for the treatment of PDPN. This method appeared to be safe and effective. It significantly alleviated the symptoms of PDPN and improved the patients’ degree of satisfaction. The details of analgesic mechanisms need to be further investigated.
Acknowledgment
This study was supported by the Natural Science Foundation of Liaoning Province (no. 20170541032).
Disclosure
The authors report no conflicts of interest in this work.
References
Scanlon PH, Aldington SJ, Stratton IM. Epidemiological issues in diabetic retinopathy. Middle East Afr J Ophthalmol. 2013;20(4):293–300. | ||
Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28 Suppl 1(Suppl 1):8–14. | ||
Ziegler D. Painful diabetic neuropathy: advantage of novel drugs over old drugs? Diabetes Care. 2009;32 Suppl 2:S414–S419. | ||
Murata Y, Takahashi K, Yamagata M, Takahashi Y, Shimada Y, Moriya H. Variations in the number and position of human lumbar sympathetic ganglia and rami communicantes. Clin Anat. 2003;16(2):108–113. | ||
Kim YH, Lee CJ, Lee SC, et al. Effect of pulsed radiofrequency for postherpetic neuralgia. Acta Anaesthesiol Scand. 2008;52(8):1140–1143. | ||
Manchikanti L. The role of radiofrequency in the management of complex regional pain syndrome. Curr Rev Pain. 2000;4(6):437–444. | ||
Triantafyllou K, Gkolfakis P, Triantafyllou M, et al. Long-term patient satisfaction of gastrointestinal endoscopic procedures. Ann Gastroenterol. 2016;29(2):188–195. | ||
Scholz J, Rathmell JP, David WS, et al. A standardized clinical evaluation of phenotypic diversity in diabetic polyneuropathy. Pain. 2016;157(10):2297–2308. | ||
de Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014;155(11):2426–2431. | ||
van Beek M, Geurts JW, Slangen R, et al. Severity of Neuropathy Is Associated With Long-term Spinal Cord Stimulation Outcome in Painful Diabetic Peripheral Neuropathy: Five-Year Follow-up of a Prospective Two-Center Clinical Trial. Diabetes Care. 2018;41(1):32–38. | ||
Gonçalves NP, Vægter CB, Andersen H, Østergaard L, Calcutt NA, Jensen TS. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol. 2017;13(3):135–147. | ||
Xing JL, Hu SJ, Jian Z, Duan JH. Subthreshold membrane potential oscillation mediates the excitatory effect of norepinephrine in chronically compressed dorsal root ganglion neurons in the rat. Pain. 2003;105(1-2):177–183. | ||
Mclachlan EM, Jänig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363(6429):543–546. | ||
Hu SJ, Yang HJ, Jian Z, et al. Adrenergic sensitivity of neurons with non-periodic firing activity in rat injured dorsal root ganglion. Neuroscience. 2000;101(3):689–698. | ||
Lee BY, da Silva MC, Aquino-Chu G, Herz BL. Surgery of the sympathetic nervous system. J Spinal Cord Med. 1996;19(1):20–26. | ||
Yoo HS, Nahm FS, Lee PB, Lee CJ. Early thoracic sympathetic block improves the treatment effect for upper extremity neuropathic pain. Anesth Analg. 2011;113(3):605–609. | ||
Gunduz OH, Kenis-Coskun O. Ganglion blocks as a treatment of pain: current perspectives. J Pain Res. 2017;10:2815–2826. | ||
Abramov R. Lumbar sympathetic treatment in the management of lower limb pain. Curr Pain Headache Rep. 2014;18(4):403. | ||
Mclachlan EM, Hu P. Inflammation in dorsal root ganglia after peripheral nerve injury: effects of the sympathetic innervation. Auton Neurosci. 2014;182:108–117. | ||
Kokhan EP, Batrashov VA, Mitroshin GE, Kokhan VE. Lumbar sympathectomy in patients with arteriosclerosis obliterans of the lower extremities and diabetes mellitus. Klin Khir. 1990;7(7):69. | ||
Dev S, Yoo Y, Lee HJ, Kim DH, Kim YC, Moon JY. Does Temperature Increase by Sympathetic Neurolysis Improve Pain in Complex Regional Pain Syndrome? A Retrospective Cohort Study. World Neurosurg. 2018;109:e783–e791. | ||
Tay VK, Fitridge R, Tie ML. Computed tomography fluoroscopy-guided chemical lumbar sympathectomy: simple, safe and effective. Australas Radiol. 2002;46(2):163–166. | ||
Nickel J, Brinckmann W, Andresen R, Ambulatory AR. Ambulatory, CT-assisted lumbar sympathicolysis in patients with severe peripheral artery disease: influence on peripheral blood flow and clinical outcome. Zentralbl Chir. 2008;133(4):349–354. | ||
Jackson TP, Gaeta R. Neurolytic blocks revisited. Curr Pain Headache Rep. 2008;12(1):7–13. | ||
Pennekamp W, Krumova EK, Feigl GP, et al. Permanent lesion of the lateral femoral cutaneous nerve after low-volume ethanol 96% application on the lumbar sympathetic chain. Pain Physician. 2013;16(4):391–397. | ||
Ranjan P, Kumar J, Chipde SS. Acute renal failure due to bilateral ureteric necrosis following percutaneous chemical lumbar sympathectomy. Indian J Nephrol. 2012;22(4):292–294. | ||
Noe CE, Haynsworth RF. Lumbar radiofrequency sympatholysis. J Vasc Surg. 1993;17(4):801–806. | ||
Jain P, Raza K, Singh S, Kumari C, Kaler S, Rani N. Lumbar sympathetic chain: anatomical variation and clinical perspectives. Clin Ter. 2016;167(6):185–187. | ||
Zheng Z, Wan Y, Liu Y, et al. Lumbar sympathectomy regulates vascular cell turnover in rat hindfoot plantar skin. Clin Hemorheol Microcirc. 2017;67(2):149–157. | ||
Xie W, Chen S, Strong JA, Li AL, Lewkowich IP, Zhang JM. Localized Sympathectomy Reduces Mechanical Hypersensitivity by Restoring Normal Immune Homeostasis in Rat Models of Inflammatory Pain. J Neurosci. 2016;36(33):8712–8725. | ||
Ogon I, Takebayashi T, Iwase T, et al. Sympathectomy and Sympathetic Blockade Reduce Pain Behavior Via Alpha-2 Adrenoceptor of the Dorsal Root Ganglion Neurons in a Lumbar Radiculopathy Model. Spine. 2015;40(24):E1269–E1275. | ||
Zhang JH, Yang CX, Zhong JY, et al. The influence of lumbar sympathetic ganglion radiofrequency thermocoagulation on the activation of microglia in rats with diabetic neuropathic pain. Zhonghua Yi Xue Za Zhi. 2016;96(24):1934–1938. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.