Back to Journals » Journal of Inflammation Research » Volume 17
Eugenol Inhibits Ox-LDL-Induced Proliferation and Migration of Human Vascular Smooth Muscle Cells by Inhibiting the Ang II/MFG-E8/MCP-1 Signaling Cascade
Authors He JH, Li XJ, Wang SP, Guo X, Chu HX, Xu HC, Wang YS
Received 27 October 2023
Accepted for publication 20 January 2024
Published 2 February 2024 Volume 2024:17 Pages 641—653
DOI https://doi.org/10.2147/JIR.S446960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Jia-Huan He,1,* Xiang-Jun Li,2,* Shi-Peng Wang,1 Xia Guo,1 Hao-Xuan Chu,1 Han-Chi Xu,1 Yu-Shi Wang1
1Department of Cardiology, The First Hospital of Jilin University, Changchun, 13000, People’s Republic of China; 2Department of Experimental Pharmacology and Toxicology, College of Pharmacy, Jilin University, Changchun, 130000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu-Shi Wang, Department of Cardiology, The First Hospital of Jilin University, Changchun, 13000, People’s Republic of China, Tel +86 18643199605, Email [email protected]
Objective: In this study, we investigated the effect and mechanism of action of eugenol on oxidized low-density lipoprotein (ox-LDL)-induced abnormal proliferation and migration of human vascular smooth muscle cells (HVSMCs).
Methods: HVSMCs were treated with 100 ug/mL ox-LDL for 24 hours to establish a cell model. After 1-hour pretreatment, eugenol at concentrations of 5, 25, and 50 uM was added. Cell viability was assessed using an MTT assay, PCNA expression was detected using Western blot, cell cycle distribution was analyzed using flow cytometry, and cell migration ability was evaluated using wound healing and Transwell migration assays. To investigate the mechanisms, Ang II receptors were inhibited by 1000 nM valsartan, MFG-E8 was knocked down by shRNA, MCP-1 was inhibited by siRNA, and MFG-E8 was overexpressed using plasmids.
Results: The findings from this study elucidated the stimulatory impact of ox-LDL on the proliferation and functionality of HVSMCs. Different concentrations of eugenol effectively mitigated the enhanced activity of HVSMCs induced by ox-LDL, with 50 uM eugenol exhibiting the most pronounced inhibitory effect. Flow cytometry and Western blot results showed ox-LDL reduced G1 phase cells and increased PCNA expression, while 50 uM eugenol inhibited ox-LDL-induced HVSMC proliferation. In wound healing and Transwell migration experiments, the ox-LDL group showed larger cell scratch filling and migration than the control group, both of which were inhibited by 50 uM eugenol. Inhibiting the Ang II/MFG-E8/MCP-1 signaling cascade mimicked eugenol’s effects, while MFG-E8 overexpression reversed eugenol’s inhibitory effect.
Conclusion: Eugenol can inhibit the proliferation and migration of ox-LDL-induced HVSMCs by inhibiting Ang II/MFG-E8/MCP-1 signaling cascade, making it a potential therapeutic drug for atherosclerosis.
Keywords: Ang II, eugenol, HVSMCs, MFG-E8, MCP-1, ox-LDL
Introduction
Cardiovascular disease has one of the highest incidence and mortality rates in the world. It is primarily caused by atherosclerosis.1 Numerous factors contribute to the incidence and development of atherosclerosis, with the primary initiating factors being abnormal lipid deposition and oxidation.2 When low-density lipoprotein is oxidized and modified to form oxidized low-density lipoprotein (ox-LDL), it can activate endothelial cells and vascular smooth muscle cells (VSMCs) to produce various inflammatory and chemotactic factors, thereby encouraging monocytes to aggregate using the vascular endothelium and engulf ox-LDL to form plaques.3 Therefore, inhibiting ox-LDL-induced inflammation and VSMC activation is crucial in preventing atherosclerosis.
Despite the success of lipid-lowering therapy in the treatment and prevention of cardiovascular diseases, the risk of cardiovascular disease remains high, necessitating the search for additional treatment targets. Anti-inflammatory treatment has emerged as a new strategy for anti-atherosclerosis treatment because of the central role inflammation plays in the disease.4 There is mounting evidence that the Ang II signaling cascade contributes significantly to the onset and progression of atherosclerosis. Ang II can promote abnormal proliferation, migration, and inflammatory response of VSMCs.5–7 MFG-E8 and MCP-1, two key components of the Ang II signaling cascade, have been confirmed to be involved in this process.8 MFG-E8 is a glycoprotein that exists in various cells and plays a significant role in the development of vascular aging-related diseases by promoting VSMC proliferation, migration, phenotype transformation, and inflammatory response.9–12 Ang II or MFG-E8 administration in vitro has been shown to upregulate the expression of MCP-1 and promote VSMC migration.8 Therefore, inhibiting the Ang II/MFG-E8/MCP-1 signaling axis is of immense significance in preventing the activation of VSMCs.
Eugenol is a phenolic compound that is commonly found in Chinese herbs, and possesses anti-inflammatory and antioxidant properties.13–15 Eugenol can protect vascular endothelial cells and inhibit abnormal proliferation of VSMCs.16,17 Therefore, to better understand the role of eugenol in cardiovascular protection and to provide an experimental basis for the development of eugenol and other natural medicines in the prevention of atherosclerosis, we aimed to explore the effect of eugenol on the abnormal proliferation and migration of human vascular smooth muscle cells (HVSMCs) induced by ox-LDL, as well as explore the effect of eugenol on the expression of key factors in the Ang II/MFG-E8/MCP-1 signaling cascade.
Materials and Methods
Reagents
Eugenol (purity: 98.11%) was acquired from MedChemExpress (Shanghai, China). Oxidized low-density lipoprotein was purchased from Guangzhou Yiyuan Biotechnology Co., Ltd (Cat. No. YB-002). Valsartan (purity ≥ 98%) was purchased from the Aladdin Chemical Reagent official website. 10% fetal sheep serum, 1% penicillin and streptomycin, low sugar medium, enhanced chemiluminescence (ECL) solution, SevenFast Total RNA Extraction Kit for Cells and SevenFast® Two-step RT-qPCR Kit were purchased from Seven, Beijing, China. MTT was purchased from Sigma-Aldrich in St. Louis, Missouri, United States of America. Rabbit monoclonal antibody GAPDH (380626) was purchased from ZENBIO, Chengdu, China; proliferating cell nuclear antigen (PCNA) (10205-2-AP) and AT1 (25343-1-AP) was purchased from Proteintech, Wuhan, China; MFG-E8 (DF10184) and MCP-1 (DF7577) were purchased from Affinity, Jiangsu, China. The BCA protein content determination kit was purchased from BCA kit, Beyotime Biotechnology, Shanghai, China. The flow cytometry cell cycle kit was purchased from Elabscience (Wuhan, China).
Cell Culture
HVSMCs and shMFG-E8 VSMCs were purchased from Guangzhou Landm Biotechnology Co., Ltd, (Guangzhou, China) and cultured in a low sugar medium supplemented with 10% fetal sheep serum and 1% penicillin and streptomycin.
MTT Colorimetric Method
Cells were cultivated in 96 well plates (6 × 103 cells per well), and were treated with different concentrations of eugenol and/or ox-LDL for 24 hours after cell adhesion. Subsequently, 10 ul of MTT reagent was added to each well, and the cells were incubated for another 2 hours at 37 °C. The culture medium was then removed, 150 ul of DMSO was added to each well, and the bed was shaken for 5 minutes at room temperature and low speed. Absorbance was measured using an enzyme-linked immunosorbent assay at a 570 nm wavelength.
Flow Cytometry Analysis of the Cell Cycle of VSMCs
In this study, 5×105 cells per group were collected and fixed in 70% cold ethanol and incubated at 4 °C overnight. The cells were then washed twice with phosphate-buffered saline and incubated with RNase A and propidium iodide (PI) solution in darkness at 4 °C for 30 minutes. Subsequently, the samples were analyzed using CellQuest software and FACSCalibur flow cytometry (BD, San Diego, California, United States). The PI signal was measured using an argon ion laser simulated at a 488 nm wavelength through a 630 nm filter. Data of 10,000 cells were collected and plotted in FSC/SSC scatter plots, and adherent cells and cell fragments were eliminated by gating.
Wound Healing Assay
A 6-well plate was inoculated with cells at a density 5×105 cells/well. When a complete saturation of cell density was reached, 10 ul pipette tips were used for the vertical marking. After washing, as suggested by the experimental protocol, the culture medium was replaced with serum-free medium, which was with or without ox-LDL and eugenol, and the cells were then further incubated for 24 hours. HVSMCs were observed and photographed at 0 and 24 hours, and the scratch area was measured using Image J software to determine the scratch healing rate.
Transwell Cell Migration Assay
Transwell cell migration was analyzed using an enhanced Boyden chamber. The cells were digested with trypsin and resuspended in serum-free medium. Subsequently, 5×104 cells were added to the upper chamber, and 700 ul culture medium containing 15% fetal bovine serum was added into the lower chamber. The cells were incubated for 24 hours under normal conditions. The cells on the lower surface of the filter were fixed with 4% paraformaldehyde and stained for 10 minutes with 0.1% crystal violet. A microscope was used to take photos of three randomly selected fields of view. Using Image J software, the ratio of the number of cells penetrated by cells in the experimental group to the number in the control group represented the change in cell migration ability.
Protein Blotting
Radioimmunoprecipitation assay was used to extract total cellular proteins, which contained protease and phosphatase inhibitors. After collecting the supernatant, the BCA protein content assay was used to quantify the total cellular protein. The protein was then denatured and inactivated in a metal bath at 95 °C for 5 minutes. An equal amount of protein (30 ug) before electrophoresis was loaded onto sodium dodecyl sulfate polyacrylamide gel, and then transferred to a polyvinylidene fluoride membrane (Millipore) using a transfer system. The membrane was sealed with 5% skim milk for one hour, then incubated overnight at 4 °C with the corresponding antibodies. After three washes with TBST, the imprint was incubated with an HRP-conjugated secondary antibody with a synergistic reaction at room temperature for one hour. The ECL solution was used to photograph and visualize immune reactive proteins. Image J software was used to quantify the density of stripes within an image.
In vitro Small Interfering RNA
Human MCP-1 siRNA was obtained from Shanghai Integrated Biotech Solutions Co., Ltd. Forward sequence: 5’ -CAGCAAGUGUCCCAAAGAAGC-3, reverse sequence: 5’ -UUCUUUGGGACACUUGCUGCU-3’. The manufacturer’s instructions were followed to transfect siRNA using Golden Tran-R (Golden TRANS TECHNOLOGY, Changchun, China).
Plasmid Construction and Transfection
The MFG-E8 overexpression plasmid was purchased from Shanghai Integrated Biotechnology Solutions Co., Ltd. GP transfer mate (Genepharma, Suzhou, China) was used to transiently transfect the construct into cells according to the manufacturer’s instructions.
RNA Extraction and Real-Time Quantitative PCR
Total RNA was extracted from cultured VSMCs using the SevenFast Total RNA Extraction Kit for Cells. Total RNA (1 ug) was reverse-transcribed into cDNA using the SevenFast® Two-step RT-qPCR Kit. RT-qPCR was amplified using a real-time fluorescence quantitative PCR instrument (qTOWER 2.0; Analytick Jena) to detect MFG-E8, MCP-1, MFG-E8 forward: 5’-CCTGCCACAACGGTGGTTTAT-3’, MFG-E8 reserve: 5’-CACATTTCGTCTCACAGTGGTT-3’, MCP-1 forward: 5’-CAGCCAGATGCAATCAATGCC-3’, MCP-1 reserve: 5’-TGGAATCCTGAACCCACTTCT-3. Relative mRNA expression was calculated using the ∆∆CT method, and then normalized to obtain the GAPDH expression values.
Statistical Analysis
All statistical data are expressed as the mean ± standard deviation. Intergroup comparisons were made by one-way ANOVA using GraphPad Prism 9.0. P < 0.05 was deemed statistically significant.
Results
Ox-LDL Stimulates the Proliferation and Migration of HVSMCs
The MTT results revealed that as the concentration of ox-LDL increased, the proliferation activity of HVSMCs also gradually increased, and the difference was statistically significant when compared with the control group (Supplementary Figure 1A). The scratch test results demonstrated that the scratch filling area of cells in the 25, 50, and 100 ug/mL ox-LDL group was significantly larger than that in the control group (Supplementary Figure 1B and C), and there was a significant dose-effect relationship. This indicated that ox-LDL could stimulate the proliferation and migration of HVSMCs in a concentration-dependent manner. A concentration of ox-LDL treatment of 100 ug/mL was selected for subsequent experiments in this study.
Eugenol Inhibits Ox-LDL-Mediated Excessive Proliferation of HVSMCs
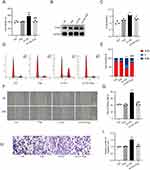
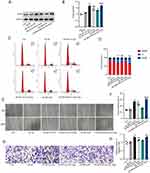
To determine the effective treatment concentration of eugenol, the cytotoxic effects of different concentrations of eugenol (5–50 uM) on HVSMCs were studied using the MTT method. Within 24 hours, different concentrations of eugenol exhibited no cytotoxicity to HVSMCs (Supplementary Figure 1D). Ox-LDL may enhance the cell viability of HVSMCs, while 5 uM, 25 uM, and 50uM concentrations of eugenol inhibited the increase in HVSMC activity brought on by ox-LDL, but there was no statistically significant difference between the different concentration groups (Supplementary Figure 1E). We subsequently selected 50 uM eugenol for subsequent experiments. As shown in Figure 1A, eugenol significantly reduced the cell viability of HVSMCs compared with the ox-LDL group (Figure 1A). Western blot results demonstrated that ox-LDL upregulated the expression of proliferative marker PCNA in HVSMCs, while eugenol inhibited this effect (Figure 1B and C). In addition, flow cytometry revealed a decrease in the number of G0/G1 phase cells and an increase in the number of S and G2/M phase cells in the ox-LDL group. However, eugenol pretreatment was able to inhibit cell cycle progression, increase the number of G0/G1 phase cells, and prevent the entry of cells into the S phase and G2/M phase (Figure 1D and E). This result suggests that eugenol increased the percentage of HVSMCs arrested in the G0/G1 phase induced by ox-LDL. In conclusion, ox-LDL-induced excessive proliferation of HVSMCs can be inhibited by eugenol.
Eugenol Inhibits the Migration of HVSMCs Stimulated by Ox-LDL
To determine the effect of eugenol on the migration of HVSMCs, experiments involving wound healing and Transwell migration were conducted. The wound healing experiment revealed that 100 ug/mL ox-LDL significantly increased the migration ability of HVSMCs, while 50 uM eugenol inhibited this effect compared to the control group (Figure 1F and G). Transwell migration experiments demonstrated that ox-LDL could stimulate transmembrane migration of HVSMCs, whereas eugenol could inhibit ox-LDL-stimulated HVSMC migration (Figure 1H and I). In conclusion, eugenol can inhibit HVSMC migration induced by ox-LDL.
Eugenol Inhibits the Excessive Proliferation and Migration of HVSMCs Stimulated by Ox-LDL by Inhibiting the Activation of AT1
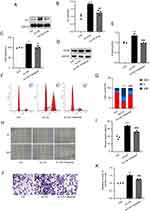
The Ang II signaling cascade plays a crucial role in the occurrence and progression of diseases associated with vascular aging. By activating type 1 receptor, Ang II primarily produces physiological and pathological effects in tissues such as vascular smooth muscle cells (AT1). Our results revealed that ox-LDL upregulated the expression of AT1 in HVSMCs, while eugenol inhibited this effect (Figure 2A and B). To determine the mechanism of eugenol inhibiting the proliferation and migration of HVSMCs induced by ox-LDL, we conducted experiments using the AT1 inhibitor valsartan. Valsartan could inhibit the increased activity of HVSMCs induced by ox-LDL (Figure 2C), the expression of PCNA (Figure 2D and E), and cell cycle progression as measured by changes in G0/G1 cell percentages (Figure 2F and G).
To study the effects of valsartan, we conducted wound healing and Transwell migration experiments. The wound healing experiment revealed that the migration ability of HVSMCs in the valsartan pre-treatment group was significantly reduced compared with the ox-LDL group, and the wound healing rate was slowed down (Figure 2H and I). Valsartan inhibited the transmembrane migration of HVSMCs that had been stimulated by ox-LDL in a Transwell migration experiment (Figure 2J and K). Eugenol inhibited the pathophysiological effects of Ang II by inhibiting the activation of AT1, thereby inhibiting the proliferation and migration of HVSMCs induced by ox-LDL, as suggested by the aforementioned results. This may be a significant mechanism by which eugenol inhibits HVSMC proliferation and migration.
Eugenol Inhibits Excessive Proliferation and Migration of HVSMCs Stimulated by Ox-LDL by Inhibiting MFG-E8
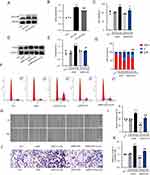
MFG-E8 is a signaling molecule downstream of angiotensin II that promotes the proliferation,9 migration,10 and phenotype transformation of VSMCs.11,12 ox-LDL was found to upregulate the expression of MFG-E8 in HVSMCs, whereas valsartan inhibited this effect (Supplementary Figure 2A and B). This suggests that MFG-E8 may promote the proliferation and migration of HVSMCs by mediating Ang II. We further discovered that eugenol significantly reduced the expression level of MFG-E8 protein induced by ox-LDL (Figure 3A and B). To clarify the effect of MFG-E8 on proliferation and migration of HVSMCs induced by ox-LDL, we reduced the expression of MFG-E8 in HVSMCs using shRNA interference (Supplementary Figure 3A–C). The low expression of MFG-E8 inhibited the increase in cell viability induced by ox-LDL (Figure 3C), PCNA expression (Figure 3D and E), cell cycle progression and as measured by changes in G0/G1 cell percentages (Figure 3F and G), and cell migration (Figure 3H–K). In conclusion, MFG-E8 is essential for the proliferation and migration of HVSMCs when ox-LDL is present, and eugenol may inhibit the proliferation and migration of HVSMCs by inhibiting the expression of MFG-E8.
Eugenol Inhibits Excessive Proliferation and Migration of HVSMCs Stimulated by Ox-LDL by Inhibiting MCP-1
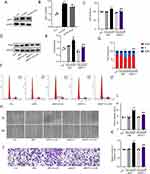
Previous studies have demonstrated that the Ang II/MFG-E8/MCP-1 signal axis is related to the migration of VSMCs.8 Firstly, we observed that MFG-E8 silencing could inhibit the MCP-1 expression upregulated by ox-LDL (Supplementary Figure 3D and E), indicating that MFG-E8 may positively regulate MCP-1. In addition, we discovered that eugenol could inhibit the ox-LDL-induced expression of MFG-E8 (Figure 4A and B). To better understand the function of MCP-1, we used siRNA technology to suppress its expression (Supplementary Figure 3F–H). Compared to the control group, the MCP-1 knockdown group exhibited weaker cell proliferation ability after ox-LDL treatment, as evidenced by a decrease in MTT value (Figure 4C), a decrease in proliferation marker PCNA expression (Figure 4D and E), and inhibition of cell cycle progression shown by increased G0/G1 cell percentages (Figure 4F and G). In the cell scratch test and the Transwell test, siMCP-1 cells exhibited a significant decrease in their migration ability (Figure 4H and I) indicating that the migration ability was poor (Figure 4J and K). Overall, MCP-1 is a key effector molecule downstream of MFG-E8 that promotes the proliferation and migration of VSMCs. Eugenol may downregulate the expression of MCP-1 by inhibiting upstream MFG-E8, thereby inhibiting the proliferation and migration of HVSMCs induced by ox-LDL.
Overexpression of MFG-E8 Rescued the Inhibitory Effect of Eugenol on the Proliferation and Migration of HVSMCs Induced by Ox-LDL
MFG-E8 overexpression experiments were conducted to determine the role of MFG-E8 in inhibition of VSMC proliferation and migration of eugenol. MFG-E8 was initially overexpressed using plasmid transfection technology (Supplementary Figure 4A–C). Then, flow cytometry was used to detect cell cycle distribution, Western blot was used to detect the expression of proliferative protein PCNA, the cell scratch test was used to observe cell migration ability, and the Transwell migration test was used to assess cell transmembrane migration ability. Compared to the control group, the MFG-E8 overexpression group exhibited upregulation of PCNA expression (Figure 5A and B), accelerated cell cycle progression as measured by changes in G0/G1 cell percentages (Figure 5C and D), and increased cell migration ability in cell scratch tests (Figure 5E and F) and Transwell (Figure 5G and H) following co-treatment with eugenol and ox-LDL. This indicated that MFG-E8 overexpression could inhibit the effect of eugenol on the proliferation and migration of HVSMCs induced by ox-LDL. Therefore, the results confirmed the significance of MFG-E8 in the inhibitory effect of eugenol on the proliferation and migration of HVSMCs. Eugenol inhibits the proliferation and migration of HVSMCs through the downregulation of MFG-E8.
Discussion
Atherosclerosis, a persistent inflammatory ailment characterized by the accumulation of lipid-rich plaques within the arterial intima, has the potential to disrupt blood flow to various vital organs and tissues, including the heart and brain. ox-LDL stands as a pivotal factor in initiating the onset of atherosclerosis.3 The deposition and aggregation of ox-LDL in the vascular wall activates endothelial cells and mononuclear macrophages, which in turn stimulates the abnormal proliferation and migration of smooth muscle cells, thereby promoting arterial intimal hypertrophy and plaque formation.2,18 VSMCs are the predominant cell type in all atherosclerotic plaque stages.19 The utilization of ox-LDL in the treatment of VSMCs represents a well-established in vitro model for investigating the pathogenesis of atherosclerosis. In the course of this study, it was observed that ox-LDL treatment markedly augmented the viability and migratory potential of HVSMCs, thereby affirming the successful establishment of the experimental model.
Furthermore, eugenol was found to inhibit the ox-LDL-induced abnormal proliferation and migration of HVSMCs. Eugenol is a phenolic compound, which exist in various plants such as cinnamon and cloves, with extensive pharmacological activities and multiple protective effects on the cardiovascular system.20–26 Eugenol can improve blood lipid metabolism, reduce oxidative stress, thereby enhancing vascular elasticity, stabilize vascular inflammation, and reduce the risk of cardiovascular disease.16,27 Our investigation revealed that eugenol effectively suppressed the proliferation and migratory responses of HVSMCs induced by ox-LDL, thereby substantiating the anti-atherosclerotic potential of eugenol.
The importance of inflammatory response in the development of atherosclerosis is growing. A new strategy for the treatment of atherosclerosis involves targeting key inflammatory factors or signaling cascades.28 Ang II is an important signaling molecule in the inflammatory response associated with atherosclerosis.29 Ang II primarily activates downstream pathways by binding to AT1 to promote VSMC proliferation, migration, and release of inflammatory factors.30 Ang II signaling can stimulate the production of MFG-E8, which in turn stimulates the expression of MCP-1 as revealed by a recent study.8 MFG-E8, Ang II, and MCP-1 are highly co-localized in VSMCs of atherosclerotic plaques in the aorta of elderly rats and humans and play a role in the development of atherosclerosis syndrome (AS).8,9,31 The silencing of MFG-E8 can inhibit the Ang II–induced migration of VSMCs. MFG-E8 administration in vitro can upregulate MCP-1 expression and stimulate VSMC migration, which is inhibited by MCP-1 antagonists.8 Ang II/MFG-E8/MCP-1 therefore forms a positive feedback loop of inflammatory amplification, playing a crucial role in the development of atherosclerosis. The results of this study also confirmed that the Ang II/MFG-E8/MCP-1 axis can stimulate abnormal HVSMC proliferation. It has been revealed that ox-LDL can activate this signaling axis, promote the proliferation and migration of HVSMCs, and significantly inhibit this effect by using Ang II receptor antagonists or inhibiting the expression of MFG-E8 and MCP-1. Therefore, targeting Ang II/MFG-E8/MCP-1 axis may become an effective new strategy for treating atherosclerosis.
Numerous studies have confirmed that eugenol has anti-inflammatory effects that can inhibit the occurrence of cyclooxygenase-2 (COX-2) and tumor necrosis factor-α (TNF-α) as well as various inflammatory factors.15,32 As a potential mechanism of eugenol against AS, the effect of eugenol on the expression of Ang II/MFG-E8/MCP-1 signaling axis was investigated. The results revealed that eugenol could downregulate the protein level of Ang II/MFG-E8/MCP-1 axis in HVSMCs, which was activated by ox-LDL, and overexpression of MFG-E8 could weaken the inhibitory effect of eugenol on the proliferation and migration of HVSMCs, indicating that the Ang II/MFG-E8/MCP-1 signal axis may be an important mechanism for eugenol to achieve its anti-inflammatory and cardiovascular protective effects. This research establishes a fundamental groundwork for further experimental advancements involving eugenol and other natural therapeutic agents in the prevention and treatment of atherosclerosis. Consequently, this study not only builds upon and broadens the existing body of knowledge concerning ox-LDL and the Ang II signaling cascade but also introduces noteworthy novel findings with meaningful clinical translational implications. While our study has yielded valuable insights, it is important to acknowledge certain limitations. Firstly, the statistical analysis, particularly concerning the normality assumption for one-way ANOVA, may not have been rigorous enough. This should be addressed in future studies with larger sample sizes to ensure robust and reliable results. Moreover, the interpretation of results derived from the current methodology should be approached with caution, considering the aforementioned limitations. These considerations are essential for the comprehensive evaluation and appropriate contextualization of our findings.
Conclusion
The outcomes of this investigation demonstrate that eugenol exerts inhibitory effects on the proliferation and migratory behavior of HVSMCs stimulated by ox-LDL. Mechanistic insights have unveiled that eugenol achieves this regulatory effect by suppressing the activation of the Ang II/MFG-E8/MCP-1 signaling cascade. While eugenol shows promise as a potential anti-atherosclerotic therapeutic agent, its clinical viability necessitates further scrutiny. Future studies should aim to ascertain the optimal effective dose, administration modalities, safety profile, and potential interactions with alternative treatment modalities associated with eugenol.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval
The immortalised cell line used in this study were purchased. Ethical approval for the use of these cells is not required in accordance with national guidelines.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
Jilin Province Medical and Health Talents Special Fund, Grant/Award Number: JLSWSRCZX2023-75.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630–1645. doi:10.1016/j.cell.2022.04.004
2. Kattoor AJ, Goel A, Mehta JL. LOX-1: regulation, signaling and its role in atherosclerosis. Antioxidants. 2019;8(7):218. doi:10.3390/antiox8070218
3. Kattoor AJ, Kanuri SH, Mehta JL. Role of Ox-LDL and LOX-1 in atherogenesis. Curr Med Chem. 2019;26(9):1693–1700. doi:10.2174/0929867325666180508100950
4. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi:10.1161/ATVBAHA.108.179705
5. Huynh DTN, Jin Y, Van Nguyen D, Myung CS, Heo KS. Ginsenoside Rh1 inhibits angiotensin II-induced vascular smooth muscle cell migration and proliferation through suppression of the ROS-mediated ERK1/2/p90RSK/KLF4 signaling pathway. Antioxidants. 2022;11(4):643. doi:10.3390/antiox11040643
6. Zhang M, Xu Y, Qiu Z, Jiang L. Sulforaphane attenuates angiotensin ii-induced vascular smooth muscle cell migration via suppression of NOX4/ROS/Nrf2 signaling. Int J Biol Sci. 2019;15(1):148–157. doi:10.7150/ijbs.28874
7. Wang M, Monticone RE, McGraw KR. Proinflammatory arterial stiffness syndrome: a signature of large arterial aging. J Vasc Res. 2018;55(4):210–223. doi:10.1159/000490244
8. Fu Z, Wang M, Gucek M, et al. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ Res. 2009;104(12):1337–1346. doi:10.1161/CIRCRESAHA.108.187088
9. Wang M, Fu Z, Wu J, et al. MFG-E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging Cell. 2012;11(3):500–508. doi:10.1111/j.1474-9726.2012.00813.x
10. Chiang HY, Chu PH, Chen SC, Lee TH. MFG-E8 regulates vascular smooth muscle cell migration through dose-dependent mediation of actin polymerization. J Am Heart Assoc. 2021;10(11):e020870. doi:10.1161/JAHA.121.020870
11. Chiang HY, Chu PH, Chen SC, Lee TH. MFG-E8 promotes osteogenic transdifferentiation of smooth muscle cells and vascular calcification by regulating TGF-β1 signaling. Commun Biol. 2022;5(1):364. doi:10.1038/s42003-022-03313-z
12. Chiang HY, Chu PH, Lee TH. MFG-E8 mediates arterial aging by promoting the proinflammatory phenotype of vascular smooth muscle cells. J Biomed Sci. 2019;26(1):61. doi:10.1186/s12929-019-0559-0
13. Zhao X, Zheng S, Wei S, et al. The protective effect and potential mechanisms of eugenol against Salmonella in vivo and in vitro. Poult Sci. 2022;101(5):101801. doi:10.1016/j.psj.2022.101801
14. Wang K, Tang Y, Wu X, et al. Eugenol attenuates transmissible gastroenteritis virus-induced oxidative stress and apoptosis via ROS-NRF2-ARE signaling. Antioxidants. 2022;11(9):1838.
15. Mateen S, Rehman MT, Shahzad S, et al. Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. Eur J Pharmacol. 2019;852:14–24. doi:10.1016/j.ejphar.2019.02.031
16. Huang MZ, Yang YJ, Liu XW, Qin Z, Li JY. Aspirin eugenol ester attenuates oxidative injury of vascular endothelial cells by regulating NOS and Nrf2 signalling pathways. Br J Pharmacol. 2019;176(7):906–918. doi:10.1111/bph.14592
17. Kwon H, Lee -J-J, Lee J-H, et al. Cinnamon and its components suppress vascular smooth muscle cell proliferation by up-regulating cyclin-dependent kinase inhibitors. Am J Chin Med. 2015;43(04):621–636. doi:10.1142/S0192415X1550038X
18. Tian K, Ogura S, Little PJ, Xu SW, Sawamura T. Targeting LOX-1 in atherosclerosis and vasculopathy: current knowledge and future perspectives. Ann N Y Acad Sci. 2019;1443(1):34–53. doi:10.1111/nyas.13984
19. Basatemur GL, Jørgensen HF, Clarke MCH, Bennett MR, Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16(12):727–744. doi:10.1038/s41569-019-0227-9
20. Ulanowska M, Olas B. Biological properties and prospects for the application of eugenol a review. Int J Mol Sci. 2021;22(7):3671. doi:10.3390/ijms22073671
21. Chen X, et al. Antioxidant activities of essential oils and their major components in scavenging free radicals, inhibiting lipid oxidation and reducing cellular oxidative stress. Molecules. 2023;28(11):4559.
22. Ma L, Liu J, Lin Q, Gu Y, Yu W. Eugenol protects cells against oxidative stress via Nrf2. Exp Ther Med. 2021;21(2):107. doi:10.3892/etm.2020.9539
23. Wang K, Chen D, Yu B, et al. Eugenol alleviates transmissible gastroenteritis virus-induced intestinal epithelial injury by regulating NF-κB signaling pathway. Front Immunol. 2022;13:921613. doi:10.3389/fimmu.2022.921613
24. Li Z, Veeraraghavan VP, Mohan SK, et al. Apoptotic induction and anti-metastatic activity of eugenol encapsulated chitosan nanopolymer on rat glioma C6 cells via alleviating the MMP signaling pathway. J Photochem Photobiol B. 2020;203:111773. doi:10.1016/j.jphotobiol.2019.111773
25. Qian W, Sun Z, Wang T, et al. Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb Pathog. 2020;139:103924. doi:10.1016/j.micpath.2019.103924
26. Huang M-Z, Zhang Z-D, Yang Y-J, et al. Aspirin eugenol ester protects vascular endothelium from oxidative injury by the apoptosis signal regulating kinase-1 pathway. Front Pharmacol. 2020;11:588755. doi:10.3389/fphar.2020.588755
27. Venkadeswaran K, Thomas PA, Geraldine P. An experimental evaluation of the anti-atherogenic potential of the plant, Piper betle, and its active constitutent, eugenol, in rats fed an atherogenic diet. Biomed Pharmacother. 2016;80:276–288. doi:10.1016/j.biopha.2016.03.028
28. Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. 2022;7(1):131. doi:10.1038/s41392-022-00955-7
29. Forrester SJ, Booz GW, Sigmund CD, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–1738. doi:10.1152/physrev.00038.2017
30. Wang M, Khazan B, Lakatta EG. Central arterial aging and angiotensin II signaling. Curr Hypertens Rev. 2010;6(4):266–281. doi:10.2174/157340210793611668
31. Georgakis MK, van der Laan SW, Asare Y, et al. Monocyte-chemoattractant protein-1 levels in human atherosclerotic lesions associate with plaque vulnerability. Arterioscler Thromb Vasc Biol. 2021;41(6):2038–2048. doi:10.1161/ATVBAHA.121.316091
32. Kaur G, Athar M, Alam MS. Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Mol, Carcinog. 2010;49(3):290–301. doi:10.1002/mc.20601
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.