Back to Journals » Infection and Drug Resistance » Volume 17
Etiology, Microbiological Isolates, and Antibiotic Susceptibilities in Inpatients with Refractory Auricular Perichondritis: A 10-Year Retrospective Study
Authors Zhang X , Zhang Y, Pu C, Wang L, Ni Y, Huang T
Received 1 September 2023
Accepted for publication 16 January 2024
Published 31 January 2024 Volume 2024:17 Pages 377—386
DOI https://doi.org/10.2147/IDR.S434522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xiuwen Zhang,1,* Yibo Zhang,2– 4,* Chen Pu,5 Lehua Wang,5 Yusu Ni,2– 4 Taomin Huang1
1Department of Pharmacy, Eye & ENT Hospital, Fudan University, Shanghai, People’s Republic of China; 2Department of Otology and Skull Base Surgery, Eye & ENT Hospital, Fudan University, Shanghai, People’s Republic of China; 3NHC Key Laboratory of Hearing Medicine (Fudan University), Shanghai, People’s Republic of China; 4ENT Institute and Department of Otorhinolaryngology, Eye & ENT Hospital, Fudan University, Shanghai, People’s Republic of China; 5School of Pharmacy, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Taomin Huang, Department of Pharmacy, Eye & ENT Hospital, Fudan University, Shanghai, 200031, People’s Republic of China, Tel +86-21-64373955, Email [email protected] Yusu Ni, Department of Otology and Skull Base Surgery, Eye & ENT Hospital, Fudan University, Shanghai, People’s Republic of China, Tel +86-21-64377134, Email [email protected]
Purpose: This study aimed to elucidate the etiologies, microbiological profiles, antibiotic susceptibilities of bacteria and outcomes of patients with auricular perichondritis.
Patients and Methods: This was a single-center retrospective study. Inpatients diagnosed with auricular perichondritis at a university teaching hospital in eastern China between January 2013 and December 2022 were included in this study.
Results: A total of 127 patients were enrolled, with an average age of 50.6 ± 16.9 years. In addition to cases in which the etiology remained undetermined in 37% of the patients, postoperative infection emerged as the predominant cause (37.8%), followed by trauma (18.1%). Among the 61 cultured isolates, 21.3% were gram-positive bacteria, 55.7% were gram-negative bacteria, and 23.0% were fungal isolates. The most frequent isolate was Pseudomonas aeruginosa (30/61, 49.2%). Notably, the incidence of fungal infections was markedly higher among postoperative patients than among post-traumatic patients (41.7% vs 7.1%, p = 0.03). The proportions of gram-negative bacteria (60.0% vs 50.0%) and fungal isolates (28.6% vs 15.4%) exhibited an increasing trend during the period of 2018– 2022, as compared to the previous period of 2013– 2017. The bacterial isolates exhibited high susceptibility to vancomycin (100%), amikacin (100%), cefepime (94.6%), and ceftazidime (90.9%). In contrast, overall susceptibility to fluoroquinolones was relatively low (65.2– 67.4%), demonstrating a declining trend in the susceptibility of Pseudomonas aeruginosa. Notably, 78.7% of the patients received an initial treatment regimen covering Pseudomonas aeruginosa. Within 30 days of discharge, 8.5% (6/71) experienced an infection recurrence.
Conclusion: Auricular perichondritis predominantly originates from iatrogenic (postoperative) infections. Antibiotic therapy covering Pseudomonas aeruginosa is a sensible and appropriate empirical treatment in the majority of patients with auricular perichondritis. However, increased resistance to fluoroquinolones has become a notable concern, suggesting the need to seek new, more aggressive strategies.
Keywords: auricular perichondritis, antibiotic sensitivity, Pseudomonas aeruginosa, fluoroquinolones
Introduction
Auricular perichondritis is a potentially serious infection affecting the auricular perichondrium (and often the cartilage).1 The condition encompasses diverse etiologies, with penetrating trauma to the cartilaginous auricle constituting a definitive causal factor.2,3 Although the precise incidence of auricular perichondritis remains unknown, reports indicate a doubling of cases in England from 1990 to 1998, attributed to increased ear piercings among adolescents.4 The auricular cartilage lacks direct blood vessel nourishment and relies on the auricular perichondrium for nutrition. Consequently, auricular perichondritis can lead to gradual cartilage tissue necrosis and liquefaction secondary to ischemia. The ailment progresses rapidly, and without timely diagnosis and treatment, permanent auricular deformity can ensue, causing substantial physical and psychological harm to patients.5 Additionally, mismanagement may lead to the development of severe soft tissue or systemic infections.
The absence of standardized guidelines for managing auricular perichondritis results in varying treatment strategies in clinical practice.5,6 Generally, antibiotic therapy holds predominant precedence as the primary treatment. In addition, culture-directed antibiotic approaches have been particularly advocated. Recent research has primarily focused on auricular perichondritis that arises from specific etiologies, such as piercing,3,5 postoperative,7 or those caused by distinct pathogens.8 Limited, comprehensive studies exist to encompass all the causative factors of auricular perichondritis. Moreover, most available studies have been characterized by small sample sizes, offering restricted insights into microbial characteristics and thus being inadequate to inform or guide clinical practice. Klug et al9 reported the microbiological profiles of 112 auricular perichondritis cases in central Denmark. Although potential pathogens were identified in 40 of 55 cultures, details on antibiotic susceptibility are lacking. There is increasing evidence that Pseudomonas aeruginosa is the major pathogen in auricular perichondritis caused by ear piercing.10–13 However, the predominant pathogen in auricular perichondritis, caused by other etiological factors, is controversial. Studies by Klug et al and Zhang et al showed that gram-positive bacteria were the main causative organisms, whereas Davidi et al and Prasad et al demonstrated that gram-negative bacteria were the predominant pathogen.9,14–16
Knowledge of antibiotic susceptibility in auricular perichondritis is crucial to guiding empirical antibiotic therapy. Unfortunately, information on microbial isolates, antibiotic susceptibility, and evolving trends in their microbiological attributes remains scarce. This study presents an extensive review of a sizable cohort of inpatients with auricular perichondritis (which refers to patients who, after outpatient treatment, were further transferred to inpatient care due to poor outcomes) and who were treated at a prominent tertiary university teaching hospital in eastern China over the past decade. This study aimed to: (1) delineate the etiologies and spectrum of isolated pathogens, (2) identify shifting patterns in microbiological profiles and antibiotic susceptibilities, and (3) outline treatment strategies and analyze clinical outcomes. Our findings provide valuable insights into the future management of auricular perichondritis.
Materials and Methods
Study Population
This retrospective, laboratory-based microbiological study was conducted at the Fudan University Eye and Ear, Nose, and Throat (FUEENT) Hospital between January 2013 and December 2022. The medical records of inpatients clinically diagnosed with auricular perichondritis (ICD 10 code H61.0) were reviewed. Patients with necrotizing otitis externa and those suspected of harboring noninfectious inflammatory conditions, such as relapsing perichondritis, were deliberately excluded from this investigation.
Culturing and Identification
The skin of the auricle and the external auditory canal were meticulously cleaned using 75% ethanol. Subsequently, pus or secretions were collected using a sterile cotton swab and expeditiously dispatched to the microbiology department of the FUEENT Hospital for detailed examination. These procedural steps rigorously adhered to aseptic principles. For the isolation of fungi, the specimens were inoculated on Sabouraud’s dextrose agar (SDA) and incubated at 27 °C for 7–14 d. Fungal isolates grown on SDA plates were initially identified to classify them as mold or yeast based on colony morphology and color. For bacteria culture, the specimens were inoculated into MacConkey agar and blood agar plates and incubated at 37 °C for 18–24 h. A MicroScan AutoScan system (Dade MicroScan, Inc., Sacramento, CA, USA) was used for bacterial identification and sensitivity testing. All the isolates were inoculated onto MicroScan panels and incubated according to the manufacturer’s recommendations. After incubation, the panels were loaded onto the panel drawer of the instrument for automatic reading. The results were analyzed and processed using LabPro software. Antibiotic susceptibility testing was conducted in accordance with the guidelines stipulated by the Clinical and Laboratory Standards Institute (CLSI).17
Data Collection
The ensuing data encompassed a comprehensive collection of variables, including age, sex, contributing pathogenic factors, associated comorbidities, duration of hospital stay, microbial isolates, antibiotic susceptibility profiles, treatment regimens, follow-up duration, and resultant clinical outcomes. Notably, clinical outcomes encompassed the occurrence of infection recurrence (within 30 days of discharge) and the attainment of complete alleviation of clinical symptoms upon discharge.
Statistical Analysis
All statistical analyses were conducted using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). Continuous data were presented as mean ± SD, and categorical variables were summarized using percentages (%). Unpaired t-test, Fisher’s exact test, or the chi-squared test, was used for comparisons between groups. Statistical significance was defined as a two-tailed p value <0.05.
Results
Demographic and Etiological Characteristics
During a 10-year study period, a total cohort of 127 patients diagnosed with auricular perichondritis were enrolled. Their mean age was 50.6 ± 16.9 years, and 57 (44.9%) were males. Middle-aged patients (45–59 years old) accounted for the largest proportion (44/127, 34.6%) (Table 1). Ten patients (7.9%) were suspected of having an immune-related disease, which is recorded in detail in Supplementary Table 1.
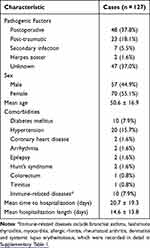 |
Table 1 Demographic and Etiological Characteristics of Patients with Auricular Perichondritis |
With regard to the underlying etiological factors, auricular perichondritis developed following aural surgery in 48 patients (37.8%). Notably, tympanoplasty was performed on 37 patients (29.1%) in this category. Trauma was the second most prevalent cause, manifesting in 23 patients (18.1%). Among these, six cases were attributed to piercing, five stemmed from frostbite, one resulted from exposure to fire burns, and the remaining 11 incidents transpired due to various other forms of mechanical injury, such as scratching or human bites. Additionally, seven cases of auricular perichondritis were traced back to secondary infections originating from conditions such as otitis externa and preauricular fistula (Table 1).
Microbiological Profiles
Culture samples were obtained from 93 (73.2%) patients. Of these, 55 (59.1%) tested positive. Six cases were polymicrobial infections. Therefore, the total number of isolates was 61. Among them, 21.3% were gram-positive bacteria, 55.7% were gram-negative bacteria, and 23.0% were fungal isolates. The most common microbial isolates were Pseudomonas aeruginosa (30/61, 49.2%), followed by Aspergillus spp. (11/61, 18.0%), and coagulase-negative staphylococci (5/61, 8.2%). A detailed overview of the microbial isolates is presented in Table 2. Notably, the prevalence of fungal auricular perichondritis markedly differed between postoperative (41.7%) and post-traumatic patients (7.1%) (p = 0.03). Fungal infections were associated with more common immune-related comorbidities (p = 0.002) and longer hospitalizations (p <0.001) (Supplementary Table 2).
 |
Table 2 Microbiological Profiles of Patients with Auricular Perichondritis |
The distribution pattern of pathogenic microorganisms exhibited notable variations, when comparing the two time periods of 2013–2017 and 2018–2022, as depicted in Figure 1. Specifically, there was an increase in the proportion of gram-negative bacteria (50.0% vs 60.0%, p = 0.44), and fungal isolates (15.4% vs 28.6%, p = 0.23), whereas the presence of gram-positive bacteria exhibited a significant decrease (34.6% vs 11.4%, p = 0.03).
 |
Figure 1 The distribution pattern of pathogenic microorganisms between the two time periods of 2013–2017 and 2018–2022. |
Antibiotic Susceptibility
A summary of the overall susceptibility of the bacterial isolates is presented in Table 3. In general, the gram-positive isolates showed the highest sensitivity (100%) to vancomycin; whereas the susceptibilities of the isolated bacteria to penicillin G, ceftriaxone, levofloxacin, ciprofloxacin, and erythromycin, were only 30.8%, 20.0%, 41.7%, 30.8%, and 15.4%, respectively. Gram-negative bacterial strains exhibited robust susceptibility (>90%) to amikacin (100%), meropenem (97.1%), cefepime (93.9%), and ceftazidime (90.9%). The susceptibility of the isolated bacteria to fluoroquinolones, particularly levofloxacin and ciprofloxacin, remained relatively low, with susceptibility rates of 67.4% and 65.2%, respectively. Fifteen isolates exhibited multidrug resistance (MDR), including five methicillin-resistant coagulase-negative staphylococci (MRCNS), four Pseudomonas aeruginosa, three Streptococcus species, one strain of methicillin-resistant Staphylococcus aureus (MRSA), one strain of Staphylococcus aureus, and one strain of Escherichia coli.
 |
Table 3 Susceptibility Rate of Isolated Bacteria to Different Antibiotics |
Figure 2 presents the temporal evolution of the susceptibility of Pseudomonas aeruginosa to commonly employed antimicrobial agents from 2013 to 2017 and subsequently from 2018 to 2022. Notably, aside from meropenem, heightened susceptibility was observed to all beta-lactam antibiotics, including ceftazidime, cefepime, and aztreonam, within these two distinct timeframes (p > 0.05). However, in regard to aminoglycosides (gentamicin and tobramycin) and fluoroquinolones (levofloxacin and ciprofloxacin), a discernible decline in susceptibility was observed over the same time period (p > 0.05).
 |
Figure 2 Temporal evolution of the susceptibility of Pseudomonas aeruginosa to commonly employed antimicrobial agents from 2013 to 2017 and subsequently from 2018 to 2022. |
Treatment Strategies and Outcomes
All patients received systematic antibiotics after clinical diagnosis; an initial treatment regimen that exhibits coverage of Pseudomonas aeruginosa was administered to 78.7% of the patients. Ceftazidime alone was the most common first-line systemic antibiotic regimen used in 47.2% of patients. A total of 71 patients were followed up, of whom six had a recurrence of infection within 30 days of discharge. Complete alleviation of clinical symptoms was achieved in 62.2% of the patients upon discharge (Table 4).
 |
Table 4 Treatment Strategies and Outcomes of Patients with Auricular Perichondritis |
Discussion
Auricular perichondritis is a complication characterized by the potential for varying degrees of external deformity, thereby presenting a formidable therapeutic challenge. In this study, we investigated the etiological factors and major causative organisms of auricular perichondritis. Furthermore, our investigation encompassed an assessment of bacterial resistance to frequently employed antimicrobial agents, with the intention of providing guidance regarding first-line empirical anti-infective strategies in cases of auricular perichondritis. Notably, it is imperative to emphasize that our study, in comparison to previous cohorts, is the most extensive in scale to date, which is highlighted in Supplementary Table 3.
In a significant proportion of our patients (37.0%), a specific etiology could not be determined. Data on the concrete etiology revealed that iatrogenic (postoperative) infection (37.8%) was the most prevalent etiology, followed by trauma to the auricle (18.1%). However, it is noteworthy that the prevalence of this etiological subgroup differs significantly when compared with investigations conducted in Denmark (0.0%),9 Israel (6.0%),15 and India (7.0%).16 The observed divergence can potentially be attributed to the varying cultural norms that comprise ear-piercing practices across different regions.
Our findings revealed a substantial incidence of fungal auricular perichondritis among postoperative patients, manifesting up to 41.7% of cases, which is a percentage significantly higher than that observed in post-traumatic patients (p = 0.03). This is consistent with analogous observations from a Taiwanese study, in which 44% of postoperative auricular perichondritis cases were attributed to fungal infections.18 Plausibly, inadvertent severance of the auricular cartilage could occur during tympanoplasty, particularly when employing an intra-auricular approach, which may inadvertently expose individuals to the causative agents associated with chronic otitis media or otitis externa. These infections may subsequently proliferate from damaged cartilage, triggering comprehensive inflammation. The present study indicates an increased rate of isolation of fungal strains in recent years, with fungal infections being more pronounced in individuals with immune-related comorbidities (p = 0.002). Fungal infections play an important role in the occurrence and development of auricular perichondritis, and the culture and identification of fungi should be emphasized in the diagnosis and treatment of auricular perichondritis in the future.
In our study, the percentage of positive cultures was 59.1%, which was lower than that reported in previous studies.9,13 The reasons for this may be twofold: 1) Our study included patients with refractory auricular perichondritis, most of whom had received antimicrobial drugs on an outpatient basis; and 2) auricular perichondritis is mainly characterized by swelling and pain, with minimal discharge, which renders it difficult to obtain easily cultivable cultures. Pseudomonas aeruginosa was the predominant pathogen, accounting for 49.2% of cases, a trend that resonates with analogous reports from Israel (55.6%)15 and India (60.6%),16 but differs considerably from findings in Denmark (20.4%)9 and China (22.5%).14 These divergent patterns can be attributed to variables such as disparate study timelines, geographical and climatic considerations, and distinct regional factors. In cases associated with ear piercing, the prevalence of Pseudomonas aeruginosa skyrockets to 100%, which is consistent with findings reported in prior studies (Supplementary Table 3).10–13 Moreover, a discernible temporal shift in the distribution of pathogenic organisms was evident, with an increasing prevalence of gram-negative bacteria and fungal isolates, alongside a pronounced reduction in the proportion of gram-positive bacteria in the time period from 2018 to 2022, as compared to from 2013 to 2017. Outpatient treatment had a considerable impact on the microbiological profiles isolated during admission. We believe that the widespread use of outpatient antibiotic therapy in recent years (mainly amoxicillin-clavulanic acid, first- and second-generation cephalosporins, etc.), has eliminated gram-positive bacteria from cultures obtained during admission. Our results underscore the escalating prevalence of gram-negative bacterial and fungal infections in recent years, which warrants clinical vigilance.
Antibiotic susceptibility models are pivotal for orchestrating the selection of optimal therapeutic regimens for auricular perichondritis. Unfortunately, there is a paucity of literature regarding antibiotic susceptibility patterns and trends in patients with auricular perichondritis. Notably, fluoroquinolones, 3rd- and 4th-generation cephalosporins, and aminoglycosides have historically been used as first-line empirical treatments for gram-negative bacteria within the ambit of auricular perichondritis.5,13,19 Our findings corroborate the heightened susceptibility of bacterial strains to amikacin (100%), cefepime (94.6%), and ceftazidime (90.9%). However, a notable caveat resides in the relatively diminished susceptibility of bacteria to fluoroquinolones (65.2–67.4%). Notably, a discernible downward trend in the susceptibility of Pseudomonas aeruginosa to fluoroquinolones was observed. This concurs with an earlier analysis focusing on chronic suppurative otitis media within our institutional context.20 MDR Pseudomonas aeruginosa was isolated from four patients, accounting for 22.2% of all patients with Pseudomonas aeruginosa in 2018–2022 and 0.0% of all patients with Pseudomonas aeruginosa in 2013–2017 (p = 0.13). All MDR Pseudomonas aeruginosa were resistant to gentamicin, ciprofloxacin and levofloxacin. The current data suggest that a cautious approach is warranted when considering fluoroquinolones as primary empirical therapeutic agents for auricular perichondritis.
It has been reported that auricular perichondritis caused by Pseudomonas aeruginosa is associated with more severe clinical manifestations and results in longer hospitalizations.15 Therefore, empirical treatment of hospitalized patients with anti-pseudomonal antimicrobials is recommended. In our study, 78.7% of the patients received initial antimicrobial therapy for Pseudomonas aeruginosa. Of these patients, 38 (29.9%) were treated with fluoroquinolones, and 87 (68.5%) were treated with ceftazidime as first-line empirical therapy. Considering the increasing resistance of Pseudomonas aeruginosa to fluoroquinolones, the optimal treatment regimen needs to be investigated, especially in patients with allergies to cephalosporins.
This study had several limitations. The retrospective nature of this study inevitably introduced inherent limitations. As a tertiary referral center situated in eastern China, the FUEENT Hospital attracts patients from all over the country, consequently impeding rigorous follow-up. Consequently, exhaustive evaluation of the factors influencing clinical outcomes remains limited, due to the paucity of available follow-up data. Second, the existing records pertaining to initial out-of-hospital interventions in selected patients are partial, which implies incomplete insights into antibiotic utilization in community settings. The consequent ramifications implicate the inability to decipher whether insufficient outpatient anti-infective interventions increase the risk of hospitalization.
Conclusion
In conclusion, we analyzed the clinical data of 127 patients with auricular perichondritis in eastern China. Postoperative infection was the main etiology, and Pseudomonas aeruginosa was the most common pathogenic bacterium. The occurrence of fungal auricular perichondritis following aural surgery has recently become a notable concern. The proportions of gram-negative bacteria and fungal isolates exhibited an increasing trend from 2013–2017 to the more recent time period of 2018–2022. Susceptibility to fluoroquinolones has relatively diminished and has tended to decrease in recent years, suggesting that a cautious approach is warranted when considering fluoroquinolones as primary empirical therapeutic agents for auricular perichondritis. Given the increase in MDR in Pseudomonas aeruginosa, future studies must continue to monitor changes in resistance patterns and the effectiveness of current treatments.
Data Sharing Statement
All data generated or analyzed during this study are included in this article (and its Supplementary Information Files). Further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
This study was conducted in accordance with the principles of the Declaration of Helsinki. This study was a retrospective study and did not involve personal privacy or commercial interests. Patients were not required to give informed consent to the study because the analysis used non-identifiable clinical data that were obtained from the electronic medical record system. In addition, the study protocol, exemption of informed consent document and all study materials were reviewed and approved by the Ethics Committee of the Fudan University Eye and Ear, Nose, and Throat Hospital in Shanghai, China (No. 2023082).
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Author Contributions
Yusu Ni and Taomin Huang are co-corresponding authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82271169).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cheng X, Peng KA, Chen B, Shu Y. A teenager with auricular infection secondary to piercing. BMJ. 2023;380:e071715. doi:10.1136/bmj-2022-071715
2. Nojoumi A, Woo BM. Management of Ear Trauma. Oral Maxillofac Surg Clin North Am. 2021;33(3):305–315. doi:10.1016/j.coms.2021.04.001
3. Kim MM, Goldman RD. Ear-piercing complications in children and adolescents. Can Fam Physician. 2022;68(9):661–663. doi:10.46747/cfp.6809661
4. Hanif J, Frosh A, Marnane C, Ghufoor K, Rivron R, Sandhu G. Lesson of the week: ”High” ear piercing and the rising incidence of perichondritis of the pinna. BMJ. 2001;322(7291):906–907. doi:10.1136/bmj.322.7291.906
5. Sosin M, Weissler JM, Pulcrano M, Rodriguez ED. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125(8):1827–1834. doi:10.1002/lary.25238
6. Mitchell S, Ditta K, Minhas S, Dezso A. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272(11):3163–3167. doi:10.1007/s00405-014-3346-2
7. Fang L, Xu J, Wang W, Huang Y. Auricular suppurative perichondritis secondary to exclusive endoscopic ear surgery for tympanoplasty: a case report and literature review. Am J Otolaryngol. 2020;41(6):102571. doi:10.1016/j.amjoto.2020.102571
8. Sistla S, Mohapatra DP, Sugumaran R, Gupta S, Thiruvoth FM, Reddy L. Auricular perichondritis of an unusual etiology. Indian J Otolaryngol Head Neck Surg. 2022;74(Suppl 1):307–310. doi:10.1007/s12070-020-02080-9
9. Klug TE, Holm N, Greve T, Ovesen T. Perichondritis of the auricle: bacterial findings and clinical evaluation of different antibiotic regimens. Eur Arch Otorhinolaryngol. 2019;276(8):2199–2203. doi:10.1007/s00405-019-05463-z
10. Kent SE, Rokade AV, Premraj K, Butcher C. ”High” ear piercing and perichondritis of the pinna. BMJ. 2001;323(7309):400. doi:10.1136/bmj.323.7309.400
11. Cicchetti S, Skillman J, Gault DT. Piercing the upper ear: a simple infection, a difficult reconstruction. Br J Plast Surg. 2002;55(3):194–197. doi:10.1054/bjps.2001.3799
12. Keene WE, Markum AC, Samadpour M. Outbreak of Pseudomonas aeruginosa infections caused by commercial piercing of upper ear cartilage. JAMA. 2004;291(8):981–985. doi:10.1001/jama.291.8.981
13. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127(5):505–508. doi:10.1017/S0022215113000248
14. Zhang F, Zhang Y, Bao Y. Pathogens of suppurative perichondritis of ear auricle and their clinical significances. J Clin Otorhinolaryngol Head Neck Surg(China). 2015;29(2):168–170.
15. Davidi E, Paz A, Duchman H, Luntz M, Potasman I. Perichondritis of the auricle: analysis of 114 cases. Isr Med Assoc J. 2011;13(1):21–24.
16. Prasad HK, Sreedharan S, Prasad HS, Meyyappan MH, Harsha KS. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121(6):530–534. doi:10.1017/S0022215107005877
17. CLSI. Preference Standard for Antimicrobial Susceptibility Testing. CLSI suplement M; 2022:100.
18. Tseng CC, Shiao AS. Postoperative auricular perichondritis after an endaural approach tympanoplasty. J Chin Med Assoc. 2006;69(9):423–427. doi:10.1016/S1726-4901(09)70285-0
19. Mordach V, Little A. Perichondritis: a case of swollen ear. J Osteopath Med. 2023;123(2):123–124. doi:10.1515/jom-2022-0044
20. Xu J, Du Q, Shu Y, Ji J, Dai C. Bacteriological profile of chronic suppurative otitis media and antibiotic susceptibility in a tertiary care hospital in Shanghai, China. Ear Nose Throat J. 2021;100(9):NP391–NP396. doi:10.1177/0145561320923823
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
