Back to Journals » OncoTargets and Therapy » Volume 16
Esomeprazole Alleviates Cisplatin Resistance by Inhibiting the AKT/mTOR Pathway in Ovarian Cancer Cells
Authors Duan J , Zhang Z , Du J, Zhang J, Li M, Li C
Received 3 February 2023
Accepted for publication 13 June 2023
Published 20 June 2023 Volume 2023:16 Pages 425—440
DOI https://doi.org/10.2147/OTT.S406009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Jingya Duan,1,* Zisen Zhang,2,* Jinfeng Du,2 Jihua Zhang,1 Minmin Li,1 Canyu Li1
1Department of Gynecology, the Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, People’s Republic of China; 2Department of Oncology, the Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Canyu Li, Department of Gynecology, the Third Affiliated Hospital of Zhengzhou University, No. 7, Kangfuzhong Street, Erqi District, Zhengzhou City, Henan Province, 450052, People’s Republic of China, Tel/Fax +86-0371-66903480, Email [email protected]
Purpose: Ovarian cancer is the most lethal malignancy in gynecology. Due to limited treatment strategies and platinum resistance, newer drugs and therapeutic options are needed. Esomeprazole (ESO) has been reported to have multiple anticancer activities in preclinical and clinical research. Therefore, this study aimed to explore the anticancer effects of esomeprazole on ovarian cancer and its underlying molecular mechanisms.
Methods: CCK-8 and 5-ethynyl-2′-deoxyuridine (EdU) assays were used to detect cell viability and proliferation. The Transwell assay was used to evaluate cell migration and invasion capacity. Flow cytometry was used to detect cell apoptosis. Western blotting and immunofluorescence were used to detect protein expression.
Results: ESO effectively inhibited the cell viability, proliferation, invasion, migration, and induced apoptosis of ovarian cancer cells in a concentration-dependent manner. Treatment with ESO decreased the expression of c-MYC, SKP2, E2F1, N-cadherin, vimentin, and matrix metalloproteinase 2 (MMP2), while it increased E-cadherin, caspase3, p53, BAX, and cleaved poly (ADP-ribose) polymerase (PARP) expression, and downregulated the PI3K/AKT/mTOR signaling pathway. Furthermore, ESO combined with cisplatin showed synergistic effects in inhibiting proliferation, invasion, and migration of cisplatin-resistant ovarian cancer cells. The mechanism may be related to the increased inhibition of c-MYC, epithelial-mesenchymal transition (EMT), and the AKT/mTOR signaling pathway and enhanced the upregulation of the pro-apoptotic protein BAX and cleaved PARP levels. Moreover, ESO combined with cisplatin synergistically upregulated the expression of the DNA damage marker γH2A.X.
Conclusion: ESO exerts multiple anticancer activities and has a synergistic effect in combination with cisplatin on cisplatin-resistant ovarian cancer cells. This study provides a promising strategy to improve chemosensitivity and overcome resistance to cisplatin in ovarian cancer.
Keywords: esomeprazole, ovarian cancer, cisplatin resistance, synergistic effect, AKT/mTOR pathway
Introduction
Ovarian cancer is a heterogeneous disease with complex and diverse biological characteristics.1 According to global cancer statistics in 2020, ovarian cancer has become the second leading cause of death in gynecological cancer.2 The outcomes of ovarian cancer are complicated due to hidden lesions, ineffective screening, late diagnosis, and different subtypes with distinct molecular characteristics.3,4 Relapses and platinum resistance are still obstacles to improve survival of this disease.5 Drug repositioning has been an effective strategy to find suitable chemotherapeutic candidates for multidrug-resistant cancers.6 In ovarian cancer, some old drugs have shown anticancer activity and the ability to overcome resistance to cisplatin, such as trimebutine maleate, mebendazole, arsenic trioxide, and quinacrine.7–10
Proton pump inhibitors (PPIs) have become a typical representative of recent anticancer drug repositioning.11,12 PPIs play a role in antitumor activity by influencing cell proliferation, apoptosis, autophagy, metastasis, epithelial-mesenchymal transition (EMT), drug resistance, and signaling pathways.13–16 Esomeprazole (ESO) has also been reported to enhance the cytotoxicity of taxol in cervical cancer, overcome resistance to taxol in ovarian and lung cancer, and augment the effects of chemotherapeutics in esophageal cancer.17–20 Remarkably, the benefits of ESO in clinical research of breast and esophageal cancer suggest its application prospect as a promising anti-tumor agent.21,22 Nevertheless, the role and mechanism of ESO in cisplatin-resistant ovarian cancer cells are still unclear.
The phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway, commonly activated in human cancer, is a therapeutic target for cancer treatment.23 This signaling pathway plays an important role in tumorigenesis and progression in ovarian cancer.24 A dual PI3K/mTOR inhibitor may alleviate chemoresistance of ovarian cancer cells by inhibiting both AKT and mTOR phosphorylation.25,26 Pantoprazole increases the sensitivity of drug resistant oral epidermoid carcinoma cells to vincristine by inhibiting PI3K/AKT/mTOR signaling.27 Moreover, ESO and pantoprazole have been shown to reverse multidrug resistance in gastric cancer via downregulating PI3K/AKT/mTOR/HIF-1α signaling through the TSC1/2 complex and Rheb in vitro and in vivo.28 However, the inhibitory effects of ESO in ovarian cancer cells on the PI3K/AKT/mTOR signaling pathway have not been reported.
In this study, we found that ESO inhibited proliferation, invasion, migration, and induced apoptosis and autophagy in ovarian cancer cells via the downregulation of the PI3K/AKT/mTOR pathway. ESO enhances the chemosensitivity of cisplatin in resistant cells by the dual inhibition of AKT/mTOR signaling. The synergistic effects of ESO combined with cisplatin suggest that it might be a new therapeutic strategy for cisplatin-resistant ovarian cancer.
Materials and Methods
Antibodies and Chemicals
PI3K (1:1000, #3011), AKT (1:1000, #4691), mTOR (1:1000, #2983), p-AKT (1:1000, #4060), p-mTOR (1:1000, #5536) and LC3B (1:1000, #3868) antibodies were purchased from Cell Signaling Technology, Inc. (USA). SKP2 (1:1000, 15010-1-AP), c-MYC (1:2000, 10828-1-AP), E-cadherin (1:10000, 20874-1-AP), PARP (1:1000, 13371-1-AP), BAX (1:2000, 505992-2-lg), caspase 3 (1:1000, 19677-1-AP), p53 (1:10000, 10442-1-AP), P62 (1:10000, 18420-1-AP), Beclin 1 (1:1000, 11306-1-AP), and E2F1 (1:1000, 12171-1-AP) antibodies were purchased from Proteintech Group (China). Antibodies for MMP2 (1:1000, T57164), vimentin (1: 1000, T55134), and γH2A.X (1:5000, T56572) were purchased from Abmart Medical Technology Co., Ltd. ESO was obtained from China AstraZeneca Pharmaceutical Co., Ltd. Cisplatin was purchased from China Qilu Pharmaceutical Co., Ltd.
Cell Culture
Human ovarian cancer cells SKOV3 and TOV112D were obtained from the Affiliated Cancer Hospital of Zhengzhou University, and were purchased from the American Type Culture Collection. The cisplatin-resistant cell line SKOV3/DDP was obtained from the Third Affiliated Hospital of Zhengzhou University, and was purchased from Shanghai Jinyuan Biotechnology Co., Ltd. SKOV3, TOV112D and SKOV3/DDP were cultured in RPMI 1640 medium, DMEM medium and McCoy’s 5A medium respectively, containing 10% fetal bovine serum (Cell-Box Biological Products Trading Co., Ltd.) and antibiotics (penicillin and streptomycin). The cells were incubated in a humidified atmosphere with 5% CO2 at 37 °C.
Cell Viability Assay
The effects of drugs on cell viability were tested using the Cell Count Kit-8 (CCK-8) (Abmole, USA). Cells in the logarithmic phase of growth were seeded in a 96-well plate (4000 cells per well). After placing the cell plate in the incubator for 12 h to allow cells to fully adhere to the dish, different concentrations of ESO and cisplatin were added. At different time points, CCK-8 solution was added and cultured for 1–4 h. The 96-well plate was placed in a microplate reader at a wavelength of 450 nm to measure the OD value. Cell viability (%) = (ODsample − ODblank)/ (ODcontrol − ODblank) × 100%.
5-Ethynyl-2’-Deoxyuridine (EdU) Incorporation Assay
The EdU assay (Beyotime Biotechnology Co., LTD, China) can measure proliferating cells by detecting replicating DNA. Log phase cells were seeded in a 24-well plate (with a built-in climbing plate), then ESO and cisplatin were added after cells adhesion. EdU was added and the culture was incubated for 2–3 h. The cells were fixed with 4% paraformaldehyde for 30 min. In the dark, cells were incubated with Click reaction mixture for 30 min and Hoechst 33342 for 15 min, and then placed under a fluorescence microscope (Olympus, IX71, Japan) to observe cell proliferation. The red fluorescence represented proliferating cells, and the blue fluorescence represented individual cells.
Migration and Invasion Assay
The Transwell assay was used to detect the migration and invasion ability of cells. For the migration assay, cells were pretreated with drugs and seeded in the upper chamber of a Transwell chamber (aperture 8 µm; Corning Inc., USA) at 2×104 cells per well. For each well, 200 µL culture medium without FBS was added to the upper chamber and 600 µL complete medium to the lower chamber. The 24-well plate was incubated for 8–10 h in a 37 °C incubator. The upper chamber was removed, and the cells were fixed with 4% paraformaldehyde for 30 min at room temperature and stained with 0.1% crystal violet for 20 min. The crystal violet was rinsed off, and the remaining cells were examined under a microscope and photographed for retention. In the invasion assay, 50 µL diluted Matrigel (Biozellen, USA) was added to the upper chamber and the plate was placed in the refrigerator at 4 °C for 3 h to wait for the matrigel to solidify. The remaining steps were the same as those of the migration assay.
Cell Apoptosis Assay
The Annexin V/PI double staining method was used to detect cell apoptosis. Cells were seeded in a 6-well plate (2×104 cells per well) and treated with different concentrations of drugs for 24 h. Cells were digested with EDTA-free trypsin, resuspended in a centrifuge tube, and centrifuged at 1000 rpm for 5 min. A 500 µL volume of buffer was added and the cells were incubated with 5 µL of Annexin V-FITC (BD, USA) for 15 min in the dark. After incubation with 5 µL PI (BD, USA) for 15 min, the samples were detected by flow cytometry (Beckman Coulter, DxFLEX, USA).
Western Blotting Assay
Cells pretreated with the drugs were transferred to a centrifuge tube with a scraper and centrifuged. RIPA cell lysis (China Kangwei Century Biotechnology Co., Ltd.) was added to the centrifuge tube on ice for 30 min to lyse the cells. After centrifugation for 15 min, the supernatant was added to SDS buffer to obtain the protein lysate. The protein was separated by electrophoresis on 10%SDS-PAGE gel plates and transferred to PVDF membranes (Millipore, Kenilworth, NJ, USA). A solution of 5% skim milk at room temperature was used to block the membranes for 1 h. The primary antibody was incubated at 4 °C overnight, and the secondary antibody was incubated for 1 h at room temperature. The ECL chemiluminescence solution (Biosharp, China) was added to the PVDF membrane, and the experimental band images were obtained by chemical capture software (Clinx Science, Shanghai, China).
Cellular Immunofluorescence Assay
Cells in logarithmic phase were seeded in a 24-well plate (with built-in climbing plate) and placed in a 37 °C incubator for 12 h. After cells adhesion, the different drugs were added for 24 h. The cells were fixed with 4% paraformaldehyde for 30 min. Each well was permeated with 300 µL of permeant at room temperature for 20 min and then blocked with 5% BSA for 1 h. The primary antibody prepared with 3% BSA was added and incubated at 4 °C overnight. The secondary antibody (Abcam, US) was added and incubated for 1 h, then DAPI was added and incubated for 15 min, both at room temperature in the darkness. The climbing plate was carefully extracted and placed on the slide for immediate observation under confocal microscopy.
Statistical Analysis
All experiments were repeated three times under the same treatment conditions. All results were processed with SPSS21.0 software and all graphs were prepared using GraphPad Prism 8.0. Measurement data were expressed as mean ± standard deviation, and data between multiple groups were analyzed by one-way analysis of variance. P-values <0.05 indicated statistical significance.
Results
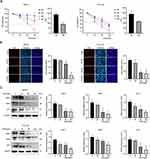
ESO Treatment Inhibited Proliferation of Ovarian Cancer Cells
Cell viability was detected by the CCK-8 assay. Treatment with ESO decreased the viability of SKOV3 and TOV112D cells in a concentration- and time-dependent manner (Figure 1A). Compared to the 24 h timepoint, ESO treatment for 48 h had a stronger effect on cell viability with a lower IC50 value (Figure 1A). EdU was used to detect proliferating cells undergoing DNA replication. The results showed that the proportion of EdU-positive cells decreased with an increase in the concentration of ESO. There was a significant difference at the concentration ≥80 mg/L (Figure 1B). Western blotting analysis showed that ESO significantly inhibited the expression of c-MYC, SKP2, and E2F1 in a concentration-dependent manner (Figure 1C). These findings consistently indicated that ESO exerted antiproliferative activity in ovarian cancer cells.
ESO Treatment Inhibited Invasion and Migration in Ovarian Cancer Cells
The Transwell assay showed that the number of SKOV3 and TOV112D cells migrating and invading decreased, respectively, with the increase in ESO concentration (Figure 2A). Western blotting indicated that ESO treatment significantly decreased the expression of MMP2, N-cadherin, and vimentin, and increased E-cadherin significantly (Figure 2B and C). These results suggested that ESO had inhibitory effects on migration, invasion, and EMT in ovarian cancer cells.
ESO Treatment Induced Apoptosis in Ovarian Cancer Cells
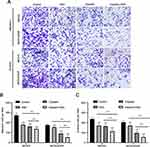
The Annexin V/PI-double staining assay demonstrated that the proportion of apoptotic cells increased after ESO treatment, with a significant difference at the concentration of 120 mg/L both in SKOV3 and TOV112D cells (Figure 3A). Western blotting showed that ESO treatment induced the protein expression of cleaved PARP, BAX, caspase 3, and p53 (Figure 3B and C). These results provided molecular evidence for the induction of apoptosis by ESO in ovarian cancer cells.
Effects of ESO Treatment on the Expression of Autophagy-Related Proteins in Ovarian Cancer Cells
Autophagy is closely related to the proliferation, apoptosis, and drug resistance of ovarian cancer cells. LC3B is considered a molecular marker reflecting the level of autophagy. To estimate the effects of ESO on autophagy in ovarian cancer cells, we detected the expression of autophagy-related proteins LC3B, P62, and Beclin 1 by Western blotting. The results showed that the expression of LC3B and P62 increased, while the expression of Beclin1 decreased after treatment with ESO (Figure 4). Thus, ESO could induce autophagy in ovarian cancer cells, but the underlying molecular mechanisms remain to be explored.
ESO Treatment Inhibited the PI3K/AKT/mTOR Signaling Pathway in Ovarian Cancer Cells
The PI3K/AKT/mTOR signaling pathway plays an important role in regulating the biological behavior of cancer cells. To explore the effects of ESO treatment on this pathway in ovarian cancer cells, we evaluated the expression of related proteins by Western blotting. ESO treatment downregulated the expression of PI3K, AKT, p-AKT, mTOR, and p-mTOR, with a significant difference achieved at the concentration of 120 mg/L in both SKOV3 and TOV112D cells (Figure 5). In combination with the multiple effects mentioned above, ESO might influence the growth, invasion, migration, and apoptosis in ovarian cancer cells by inhibiting the PI3K/AKT/mTOR signaling pathway.
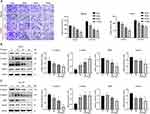
Effects of Cisplatin and ESO on Cell Viability in SKOV3/DDP Cells
To evaluate the effects and potential application value of ESO on cisplatin sensitivity and drug resistance, the ovarian cancer cell line SKOV3 and its cisplatin-resistant cell line SKOV3/DDP were used for the experiments in this study. The results of the CCK-8 assay showed that cisplatin inhibited the viability of SKOV3 and SKOV3/DDP cells in a concentration and time dependent manner (Figure 6A and B). Compared to SKOV3 cells, the IC50 value of cisplatin in SKOV3/DDP cells increased significantly (Figure 6C). ESO also suppressed the viability of SKOV3/DDP cells in a concentration-dependent and time-dependent manner (Figure 6D), suggesting its antiproliferative effect on cells resistant to cisplatin. Furthermore, ESO (80mg/L) combined with cisplatin significantly inhibited cell viability at 24 h, compared to cisplatin alone (Figure 6E and F). These results suggested that ESO in combination with cisplatin may have a synergistic effect on cisplatin-resistant cells under certain conditions.
ESO and Cisplatin Synergistically Inhibited Proliferation in SKOV3 and SKOV3/DDP Cells
To evaluate the interaction between ESO and cisplatin, we calculated the drug combination index (CI) using Compusyn software.29 As shown in Supplementary Table 1, ESO combined with cisplatin can produce synergistic and additive effects on SKOV3 and SKOV3/DDP. Considering cell viability in case of drug combination, SKOV3 cells were treated with ESO 80 mg/L and cisplatin 4 mg/L (CI=0.742), and SKOV3/DDP cells were treated with ESO 80 mg/L and cisplatin 8 mg/L (CI=0.783) in the following experiments. As shown in the CCK-8 assay, ESO combined with cisplatin inhibited cell viability in SKOV3 and SKOV3/DDP cells in a time-dependent manner (Figure 7A and B). Furthermore, the cell viability of the combined group was significantly lower than that of the cisplatin group (Figure 7A and B). The proportion of EdU-positive cells in SKOV3 and SKOV3/DDP cells decreased significantly in the ESO and combination group (Figure 7C and D). Furthermore, the proportion of EdU positive cells in the combination group was significantly lower than in the cisplatin group (Figure 7C and D). These results demonstrated that ESO could improve the sensitivity of cisplatin in ovarian cancer cells, including cisplatin-resistant cells.
ESO and Cisplatin Synergistically Inhibited Invasion and Migration in SKOV3 and SKOV3/DDP Cells
Transwell assays showed that invasion and migration cells decreased significantly after treatment with ESO, cisplatin and their combination, both in SKOV3 and SKOV3/DDP cells, respectively (Figure 8A). The number of invasive and migratory cells in the combined group was less than that in the ESO or cisplatin group (Figure 8B and C). These results indicated that ESO combined with cisplatin could synergistically inhibit the migration and invasion of ovarian cancer cells, implying the potential reversal effect of ESO on cisplatin resistance from a different perspective.
Effects of ESO and Cisplatin on Growth-Regulated Proteins in SKOV3 and SKOV3/DDP Cells
As shown in the Western blotting analysis, in both SKOV3 and SKOV3/DDP cell lines, the expression of c-MYC, N-cadherin, and vimentin were downregulated. Meanwhile, the expression of cleaved-PARP and BAX increased. They were statistically significant in the combined dosing group (Figure 9). In particular, compared to cisplatin alone, ESO in combination with cisplatin further decreased the expression of N-cadherin, vimentin and increased cleaved-PARP, BAX (Figure 9). These results provided molecular evidence to explain the synergistic effects of ESO combined with cisplatin on the proliferation, invasion, and migration of ovarian cancer cells and cisplatin-resistant cells.
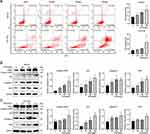
ESO Promoted Cisplatin Inhibitory Effects on AKT/mTOR Signaling in SKOV3 and SKOV3/DDP Cells
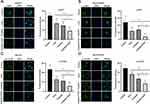
As mentioned above (Figure 5), ESO could significantly inhibit AKT/mTOR signaling. Herein, we further evaluated the synergistic effects of ESO combined with cisplatin on the AKT/mTOR signaling pathway by Western blotting. The results showed that the relative expressions of PI3K, AKT, p-AKT, mTOR, and p-mTOR in SKOV3 and SKOV3/DDP cells were significantly downregulated in the combination treatment group (Figure 10). More importantly, compared to either ESO or cisplatin alone, the relative expression of p-AKT and p-mTOR in the combined group was also significantly downregulated (Figure 10).
Subsequently, we observed the expression of p-AKT and p-mTOR in cells using cellular immunofluorescence. The results demonstrated that the average fluorescence intensity in cells decreased significantly after treatment with ESO, cisplatin, and their combination (Figure 11). Compared to ESO or cisplatin alone, the mean fluorescence intensity in cells of the combination group also decreased with a significant difference (Figure 11). These data indicated that the synergistic biological effect of ESO combined with cisplatin may be achieved by co-inhibiting the AKT/mTOR signaling pathway.
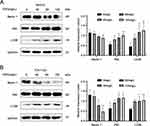
Effects of ESO and Cisplatin on the Expression of γH2A.X Protein in Ovarian Cancer Cells
The response to DNA damage is an important mechanism of regulation of cell growth, and γH2A.X is a sensitive molecular marker of DNA damage and repair. Cisplatin is the first-line drug for ovarian cancer, and its mechanism of action is to cross-link with DNA.30 This can cause DNA damage in cancer cells, thus inhibiting the process of DNA replication. Thus, we detected the expression of γH2A.X in ovarian cancer cells under ESO and combined treatment. The results showed that the expression of γH2A.X increased with the concentration of ESO in SKOV3 and TOV112D cells, with a significant difference at 120 mg/L (Figure 12A), suggesting that ESO might induce a response to DNA damage in ovarian cancer cells. Furthermore, the expression of γH2A.X in SKOV3 and SKOV3/DDP cells increased significantly in groups of cisplatin alone and ESO combined with cisplatin (Figure 12B). While these results indicated that the DNA damage response was the basic mechanism of cisplatin activity, these results also suggested that the synergistic anticancer mechanism of ESO and cisplatin may be related to the aggravation of the DNA damage response.
Discussion
Ovarian cancer is the most lethal gynecological malignancy. Recurrence and drug resistance often lead to therapeutic failure. Platinum exhibits the main type of drug resistance, which remains a huge challenge.31,32 Patients with platinum resistance have limited treatment options and poor survival.33,34 Newer therapies must be developed to improve platinum sensitivity or overcome resistance. ESO has a variety of antitumor activities in cancer, including enhancing chemosensitivity and reducing drug resistance.19,21,28 Our previous research demonstrated that ESO can downregulate EGFR through the PI3K/FOXO3a pathway to inhibit gastric cancer cell growth.35 This study further explored the effects of ESO on ovarian cancer cells.
In this study, ESO significantly inhibited the viability and proliferation of ovarian cancer cells and simultaneously decreased the expression of c-MYC, SKP2, and E2F1, which played a key role in the regulation of ovarian cancer cell growth.36–38 C-MYC, SKP2, and E2F1 are important transcription factors, and are also considered as oncogenes and potential therapeutic targets.39,40 Furthermore, their interactions determine the survival and death of cells.42–44 These results suggest that the significant anticancer activity of ESO may have complex mechanisms that need to be further clarified.
The PI3K/AKT/mTOR signaling pathway is a biological mechanism and therapeutic target of ovarian cancer.24,45,46 Inhibition of the PI3K/AKT/mTOR pathway both enhances the sensitivity of chemotherapy drugs,25,47 and overcomes platinum resistance in ovarian cancer cells.26,48 Our results show that ESO can effectively reduce the activity of the PI3K/AKT/mTOR signaling pathway in cisplatin-resistant ovarian cancer cells, thereby enhancing cisplatin sensitivity. It has also been reported that ESO can reverse multidrug resistance of gastric cancer cells by targeting the PI3K/AKT/mTOR pathway.27,28 Altogether, these data provide evidence of a signaling pathway responsible for the anti-tumor effects of ESO and overcoming drug resistance.
In SKOV3 cells, c-MYC is a key regulator that affects the expression of downstream proteins after AKT/mTOR is inhibited.49,50 We found that ESO significantly promoted cisplatin inhibition of the AKT/mTOR pathway and c-MYC expression in SKOV3 and SKOV3/DDP cells, which may be the potential mechanism of their synergy.
Apoptosis and autophagy are the core mechanisms that regulate cell growth, and have become important targets for cancer therapy.51–53 Inhibition of the PI3K/AKT/mTOR pathway can induce cell apoptosis and autophagy.54,55 However, drugs have different effects on autophagy despite inducing apoptosis of ovarian cancer cells.56,57 Our findings confirm that ESO induces autophagy and apoptosis of tumor cells,58,59 but we also show that this activity in ovarian cancer cells is associated with a downregulated PI3K/AKT/mTOR pathway, which may be a mechanism of ESO to resist inhibit cancer and overcome resistance to cisplatin. Nevertheless, whether ESO promotes cisplatin to induce autophagic apoptosis needs further investigation.
The mechanism of cisplatin resistance in ovarian cancer is complex. In clinical treatment settings, increasing sensitivity is an effective strategy to alleviate cisplatin resistance in ovarian cancer.60 Our results show that ESO promotes the chemosensitivity of cisplatin, synergistically suppressing proliferation, invasion, and migration in ovarian cancer and cisplatin-resistant cells.
EMT is closely related to cisplatin resistance, which involves AKT/mTOR signaling.61 Inhibition of the AKT/mTOR pathway can reduce cisplatin resistance by ovarian cancer cells by reversing EMT.26 We found that ESO can significantly inhibit cell invasion, migration, and EMT. Moreover, in cisplatin-resistant cells, ESO combined with cisplatin also synergistically inhibits EMT and AKT/mTOR signaling. This provides a strategic option for the combination of ESO in the treatment of metastatic ovarian cancer and the chemoprevention of metastasis of ovarian cancer.
Induction of DNA damage is the basis of the cisplatin activity, in turn, which is also one of the mechanisms of resistance to cisplatin in ovarian cancer.62,63 Targeting the PI3K/AKT/mTOR signaling pathway and the DNA damage response is a promising strategy.64 In this study, ESO combined with cisplatin synergistically upregulated the expression of the DNA damage marker γH2A.X and the pro-apoptotic proteins BAX and cleaved PARP. Furthermore, c-MYC is a potential therapeutic target for platinum-resistant ovarian cancer.65 We observed that ESO promoted inhibition of c-MYC expression by cisplatin and increased sensitivity to cisplatin in resistant cells.
ESO and cisplatin are commonly used drugs in clinical practice. High-dose ESO (80mg/L) is well tolerated with no apparent safety concerns in several clinical trials.66,67 We have verified the synergistic effects of ESO combined with cisplatin in vitro, and further exploration of the feasibility and stability of the drugs combination is needed in preclinical trials.
Conclusion
ESO exhibits multiple anti-cancer activities in ovarian cancer cells. The mechanism of ESO enhancing cisplatin sensitivity is related to the synergistic regulation of cell proliferation, invasion, migration, apoptosis, the DNA damage response, EMT, and regulation of the AKT/mTOR signaling pathway. As a chemosensitizer, ESO in combination with cisplatin might be a new therapeutic strategy for cisplatin-resistant ovarian cancer.
Funding
This work was supported by the Science and Technology Project of Henan Province (grant number 212102310618) and the Key Scientific Research Project Plan of Henan Province (grant number 22A320005).
Disclosure
Dr Zisen Zhang reports grants from Department of Education of Henan Province, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Achimas-Cadariu P, Kubelac P, Irimie A, Berindan-Neagoe I, Ruhli F. Evolutionary perspectives, heterogeneity and ovarian cancer: a complicated tale from past to present. J Ovarian Res. 2022;15(1):67. doi:10.1186/s13048-022-01004-1
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253. doi:10.1016/S0140-6736(18)32552-2
4. Yang Y, Yu Y, Chen H, et al. Illuminating platinum transportation while maximizing therapeutic efficacy by gold nanoclusters via simultaneous near-infrared-I/II imaging and glutathione scavenging. ACS Nano. 2020;14(10):13536–13547. doi:10.1021/acsnano.0c05541
5. Flynn MJ, Ledermann JA. Ovarian cancer recurrence: is the definition of platinum resistance modified by PARPi and other intervening treatments? The evolving landscape in the management of platinum-resistant ovarian cancer. Cancer Drug Resist. 2022;5(2):424–435. doi:10.20517/cdr.2022.13
6. Dinic J, Efferth T, Garcia-Sosa AT, et al. Repurposing old drugs to fight multidrug resistant cancers. Drug Resist Updat. 2020;52:100713. doi:10.1016/j.drup.2020.100713
7. Huang L, Zhao L, Zhang J, et al. Antiparasitic mebendazole (MBZ) effectively overcomes cisplatin resistance in human ovarian cancer cells by inhibiting multiple cancer-associated signaling pathways. Aging. 2021;13(13):17407–17427. doi:10.18632/aging.203232
8. Lee H, Kwon OB, Lee JE, et al. Repositioning trimebutine maleate as a cancer treatment targeting ovarian cancer stem cells. Cells. 2021;10(4). doi:10.3390/cells10040918
9. Oien DB, Pathoulas CL, Ray U, Thirusangu P, Kalogera E, Shridhar V. Repurposing quinacrine for treatment-refractory cancer. Semin Cancer Biol. 2021;68:21–30. doi:10.1016/j.semcancer.2019.09.021
10. Tang S, Shen Y, Wei X, Shen Z, Lu W, Xu J. Olaparib synergizes with arsenic trioxide by promoting apoptosis and ferroptosis in platinum-resistant ovarian cancer. Cell Death Dis. 2022;13(9):826. doi:10.1038/s41419-022-05257-y
11. Ikemura K, Hiramatsu S, Okuda M. Drug repositioning of proton pump inhibitors for enhanced efficacy and safety of cancer chemotherapy. Front Pharmacol. 2017;8:911. doi:10.3389/fphar.2017.00911
12. Spugnini EP, Fais S. Drug repurposing for anticancer therapies. A lesson from proton pump inhibitors. Expert Opin Ther Pat. 2020;30(1):15–25. doi:10.1080/13543776.2020.1704733
13. Ihraiz WG, Ahram M, Bardaweel SK. Proton pump inhibitors enhance chemosensitivity, promote apoptosis, and suppress migration of breast cancer cells. Acta Pharm. 2020;70(2):179–190. doi:10.2478/acph-2020-0020
14. Lee YY, Jeon HK, Hong JE, et al. Proton pump inhibitors enhance the effects of cytotoxic agents in chemoresistant epithelial ovarian carcinoma. Oncotarget. 2015;6(33):35040–35050. doi:10.18632/oncotarget.5319
15. Su G, Chen X, Yang H. Pantoprazole promotes the sensitivity of cervical cancer cells to cisplatin by inhibiting cisplatin-induced autophagy. J Cancer Res Ther. 2022;18(2):362–369. doi:10.4103/jcrt.jcrt_968_21
16. Zhang B, Yang Y, Shi X, et al. Proton pump inhibitor pantoprazole abrogates Adriamycin-resistant gastric cancer cell invasiveness via suppression of Akt/GSK-beta/beta-catenin signaling and epithelial-mesenchymal transition. Cancer Lett. 2015;356(2 Pt B):704–712. doi:10.1016/j.canlet.2014.10.016
17. Bai Z, Ding N, Ge J, et al. Esomeprazole overcomes paclitaxel-resistance and enhances anticancer effects of paclitaxel by inducing autophagy in A549/Taxol cells. Cell Biol Int. 2021;45(1):177–187. doi:10.1002/cbin.11481
18. He J, Shi XY, Li ZM, et al. Proton pump inhibitors can reverse the YAP mediated paclitaxel resistance in epithelial ovarian cancer. BMC Mol Cell Biol. 2019;20(1):49. doi:10.1186/s12860-019-0227-y
19. Lindner K, Borchardt C, Schopp M, et al. Proton pump inhibitors (PPIs) impact on tumour cell survival, metastatic potential and chemotherapy resistance, and affect expression of resistance-relevant miRNAs in esophageal cancer. J Exp Clin Cancer Res. 2014;33:73. doi:10.1186/s13046-014-0073-x
20. Song T, Jeon HK, Hong JE, et al. Proton pump inhibition enhances the cytotoxicity of paclitaxel in cervical cancer. Cancer Res Treat. 2017;49(3):595–606. doi:10.4143/crt.2016.034
21. Matsumura S, Ishikawa T, Yoshida J, et al. Proton pump inhibitors enhance the antitumor effect of chemotherapy for esophageal squamous cell carcinoma. Cancers. 2022;14(10):2395. doi:10.3390/cancers14102395
22. Wang BY, Zhang J, Wang JL, et al. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J Exp Clin Cancer Res. 2015;34:85. doi:10.1186/s13046-015-0194-x
23. Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi:10.1016/j.semcancer.2019.07.009
24. Ediriweera MK, Tennekoon KH, Samarakoon SR. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin Cancer Biol. 2019;59:147–160. doi:10.1016/j.semcancer.2019.05.012
25. Choi HJ, Heo JH, Park JY, et al. A novel PI3K/mTOR dual inhibitor, CMG002, overcomes the chemoresistance in ovarian cancer. Gynecol Oncol. 2019;153(1):135–148. doi:10.1016/j.ygyno.2019.01.012
26. Deng J, Bai X, Feng X, et al. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer. 2019;19(1):618. doi:10.1186/s12885-019-5824-9
27. Lu ZN, Shi ZY, Dang YF, et al. Pantoprazole pretreatment elevates sensitivity to vincristine in drug-resistant oral epidermoid carcinoma in vitro and in vivo. Biomed Pharmacother. 2019;120:109478. doi:10.1016/j.biopha.2019.109478
28. Chen M, Lu J, Wei W, et al. Effects of proton pump inhibitors on reversing multidrug resistance via downregulating V-ATPases/PI3K/Akt/mTOR/HIF-1alpha signaling pathway through TSC1/2 complex and Rheb in human gastric adenocarcinoma cells in vitro and in vivo. Onco Targets Ther. 2018;11:6705–6722. doi:10.2147/OTT.S161198
29. Yang Y, Liu X, Ma W, et al. Light-activatable liposomes for repetitive on-demand drug release and immunopotentiation in hypoxic tumor therapy. Biomaterials. 2021;265:120456. doi:10.1016/j.biomaterials.2020.120456
30. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi:10.1124/pr.58.3.10
31. De Giorgi U, Casadei C, Bergamini A, et al. Therapeutic challenges for cisplatin-resistant ovarian germ cell tumors. Cancers. 2019;11(10):1584. doi:10.3390/cancers11101584
32. Pujade-Lauraine E, Banerjee S, Pignata S. Management of platinum-resistant, relapsed epithelial ovarian cancer and new drug perspectives. J Clin Oncol. 2019;37(27):2437–2448. doi:10.1200/JCO.19.00194
33. Bogani G, Lopez S, Mantiero M, et al. Immunotherapy for platinum-resistant ovarian cancer. Gynecol Oncol. 2020;158(2):484–488. doi:10.1016/j.ygyno.2020.05.681
34. Le Saux O, Ray-Coquard I, Labidi-Galy SI. Challenges for immunotherapy for the treatment of platinum resistant ovarian cancer. Semin Cancer Biol. 2021;77:127–143. doi:10.1016/j.semcancer.2020.08.017
35. Du J, Xu Q, Zhao H, et al. PI3K inhibitor 3-MA promotes the antiproliferative activity of esomeprazole in gastric cancer cells by downregulating EGFR via the PI3K/FOXO3a pathway. Biomed Pharmacother. 2022;148:112665. doi:10.1016/j.biopha.2022.112665
36. Ghaffarnia R, Nasrollahzadeh A, Bashash D, Nasrollahzadeh N, Mousavi SA, Ghaffari SH. Inhibition of c-Myc using 10058-F4 induces anti-tumor effects in ovarian cancer cells via regulation of FOXO target genes. Eur J Pharmacol. 2021;908:174345. doi:10.1016/j.ejphar.2021.174345
37. Liu H, Yue Q, He S. Amentoflavone suppresses tumor growth in ovarian cancer by modulating Skp2. Life Sci. 2017;189:96–105. doi:10.1016/j.lfs.2017.09.026
38. Zhan L, Zhang Y, Wang W, Song E, Fan Y, Wei B. E2F1: a promising regulator in ovarian carcinoma. Tumour Biol. 2016;37(3):2823–2831. doi:10.1007/s13277-015-4770-7
39. Dhanasekaran R, Deutzmann A, Mahauad-Fernandez WD, Hansen AS, Gouw AM, Felsher DW. The MYC oncogene - The grand orchestrator of cancer growth and immune evasion. Nat Rev Clin Oncol. 2022;19(1):23–36. doi:10.1038/s41571-021-00549-2
40. Hershko DD. Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer. 2008;112(7):1415–1424. doi:10.1002/cncr.23317
41. Wu Z, Yu Q. E2F1-mediated apoptosis as a target of cancer therapy. Curr Mol Pharmacol. 2009;2(2):149–160. doi:10.2174/1874467210902020149
42. Hydbring P, Castell A, Larsson LG. MYC Modulation around the CDK2/p27/SKP2 Axis. Genes. 2017;8(7):174. doi:10.3390/genes8070174
43. Matsumura I, Tanaka H, Kanakura Y. E2F1 and c-Myc in cell growth and death. Cell Cycle. 2003;2(4):333–338. doi:10.4161/cc.2.4.428
44. Zhang L, Wang C. F-box protein Skp2: a novel transcriptional target of E2F. Oncogene. 2006;25(18):2615–2627. doi:10.1038/sj.onc.1209286
45. Dobbin ZC, Landen CN. The importance of the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int J Mol Sci. 2013;14(4):8213–8227. doi:10.3390/ijms14048213
46. van der Ploeg P, Uittenboogaard A, Thijs A, et al. The effectiveness of monotherapy with PI3K/AKT/mTOR pathway inhibitors in ovarian cancer: a meta-analysis. Gynecol Oncol. 2021;163(2):433–444. doi:10.1016/j.ygyno.2021.07.008
47. Xiao Y, Yu Y, Jiang P, Li Y, Wang C, Zhang R. The PI3K/mTOR dual inhibitor GSK458 potently impedes ovarian cancer tumorigenesis and metastasis. Cell Oncol. 2020;43(4):669–680. doi:10.1007/s13402-020-00514-8
48. Rinne N, Christie EL, Ardasheva A, et al. Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. Cancer Drug Resist. 2021;4(3):573–595. doi:10.20517/cdr.2021.05
49. Jones HM, Fang Z, Sun W, et al. Atorvastatin exhibits anti-tumorigenic and anti-metastatic effects in ovarian cancer in vitro. Am J Cancer Res. 2017;7(12):2478–2490.
50. Mazzoletti M, Bortolin F, Brunelli L, et al. Combination of PI3K/mTOR inhibitors: antitumor activity and molecular correlates. Cancer Res. 2011;71(13):4573–4584. doi:10.1158/0008-5472.CAN-10-4322
51. Das S, Shukla N, Singh SS, Kushwaha S, Shrivastava R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis. 2021;26(9–10):512–533. doi:10.1007/s10495-021-01687-9
52. Sorice M. Crosstalk of Autophagy and Apoptosis. Cells. 2022;11(9):1479. doi:10.3390/cells11091479
53. Tilija PN, Jang WJ, Jeong CH. Role of autophagy in regulation of cancer cell death/apoptosis during anti-cancer therapy: focus on autophagy flux blockade. Arch Pharm Res. 2020;43(5):475–488. doi:10.1007/s12272-020-01239-w
54. Ji Y, Hu W, Jin Y, Yu H, Fang J. Liquiritigenin exerts the anti-cancer role in oral cancer via inducing autophagy-related apoptosis through PI3K/AKT/mTOR pathway inhibition in vitro and in vivo. Bioengineered. 2021;12(1):6070–6082. doi:10.1080/21655979.2021.1971501
55. Yang J, Pi C, Wang G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed Pharmacother. 2018;103:699–707. doi:10.1016/j.biopha.2018.04.072
56. Liu M, Bamodu OA, Huang WC, et al. 4-Acetylantroquinonol B suppresses autophagic flux and improves cisplatin sensitivity in highly aggressive epithelial cancer through the PI3K/Akt/mTOR/p70S6K signaling pathway. Toxicol Appl Pharmacol. 2017;325:48–60. doi:10.1016/j.taap.2017.04.003
57. Zhou J, Jiang YY, Chen H, Wu YC, Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53(2):e12739. doi:10.1111/cpr.12739
58. Chueca E, Apostolova N, Esplugues JV, Garcia-Gonzalez MA, Lanas A, Piazuelo E. Proton pump inhibitors display antitumor effects in barrett’s adenocarcinoma cells. Front Pharmacol. 2016;7:452. doi:10.3389/fphar.2016.00452
59. Marino ML, Fais S, Djavaheri-Mergny M, et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010;1:e87. doi:10.1038/cddis.2010.67
60. Song M, Cui M, Liu K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur J Med Chem. 2022;232:114205. doi:10.1016/j.ejmech.2022.114205
61. Ashrafizadeh M, Zarrabi A, Hushmandi K, et al. Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin resistance. Int J Mol Sci. 2020;21(11):4002. doi:10.3390/ijms21114002
62. Damia G, Broggini M. Platinum resistance in ovarian cancer: role of DNA repair. Cancers. 2019;11(1):119. doi:10.3390/cancers11010119
63. Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi:10.1038/onc.2011.384
64. Huang TT, Lampert EJ, Coots C, Lee JM. Targeting the PI3K pathway and DNA damage response as a therapeutic strategy in ovarian cancer. Cancer Treat Rev. 2020;86:102021. doi:10.1016/j.ctrv.2020.102021
65. Reyes-Gonzalez JM, Armaiz-Pena GN, Mangala LS, et al. Targeting c-MYC in platinum-resistant ovarian cancer. Mol Cancer Ther. 2015;14(10):2260–2269. doi:10.1158/1535-7163.MCT-14-0801
66. Kuipers EJ, Sung JJ, Barkun A, et al. Safety and tolerability of high-dose intravenous esomeprazole for prevention of peptic ulcer rebleeding. Adv Ther. 2011;28(2):150–159. doi:10.1007/s12325-010-0095-5
67. Jankowski J, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet. 2018;392(10145):400–408. doi:10.1016/S0140-6736(18)31388-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.