Back to Journals » Journal of Inflammation Research » Volume 16
Esculetin Alleviates Inflammation, Oxidative Stress and Apoptosis in Intestinal Ischemia/Reperfusion Injury via Targeting SIRT3/AMPK/mTOR Signaling and Regulating Autophagy
Authors Shen X, Shi H, Chen X, Han J, Liu H, Yang J, Shi Y, Ma J
Received 23 March 2023
Accepted for publication 6 July 2023
Published 23 August 2023 Volume 2023:16 Pages 3655—3667
DOI https://doi.org/10.2147/JIR.S413941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Xin Shen,1,* Hai Shi,1,* Xinli Chen,1 Junwei Han,1 Haiwang Liu,1 Jie Yang,1 Yuan Shi,2 Jiajia Ma2
1Department of Gastrointestinal Surgery, Xi’an Daxing Hospital, Xi’an, 710016, People’s Republic of China; 2Department of Gynecology and Obstetrics, Xijing Hospital, Air Force Military Medical University, Xi’an, 710032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiajia Ma, Xijing Hospital, Air Force Military Medical University, No. 15 Changle Western Road, Xincheng District, Xi’an, Shaanxi, 710032, People’s Republic of China, Email [email protected]
Aim: Intestinal ischemia/reperfusion (I/R) injury is a challenging pathological phenomenon accountable for significant mortality in clinical scenarios. Substantial evidence has supported the protective role of esculetin in myocardial I/R injury. This study is designed to reveal the specific impacts of esculetin on intestinal I/R injury and disclose the underlying mechanism.
Methods: First, intestinal I/R injury model and intestinal epithelial cell line hypoxia/reoxygenation (H/R) model were established. Pathologic damages to intestinal tissues were observed through H&E staining. Serum diamine oxidase (DAO) levels were examined. RT-qPCR and Western blot examined the expression of inflammatory mediators. Commercial kits were used for detecting the levels of oxidative stress markers. TUNEL assay and caspase 3 activity assay measured cell apoptosis. Immunofluorescence (IF) staining measured autophagy levels. Western blot analyzed the expression of apoptosis-, Sirtuin 3 (SIRT3)/AMP activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) signaling- and autophagy-related proteins. Molecular docking verified the interaction of esculetin with SIRT3. Cell viability was explored via CCK-8 assay.
Results: The experimental results revealed that esculetin treatment mitigated pathological damage of intestinal tissues, reduced serum DAO level, ameliorated inflammation, oxidative stress and apoptosis and promoted autophagy in intestinal I/R rats. Moreover, esculetin bond to SIRT3 and activated SIRT3/AMPK/mTOR signaling both in vitro and in vivo. Furthermore, esculetin treatment enhanced cell viability and SIRT3 silencing reversed the impacts of esculetin on autophagy, inflammation, oxidative stress and apoptosis in H/R cell model.
Conclusion: In a word, esculetin activated SIRT3/AMPK/mTOR signaling and autophagy to protect against inflammation, oxidative stress and apoptosis in intestinal I/R injury.
Keywords: intestinal I/R injury, autophagy, esculetin, SIRT3/AMPK/mTOR signaling
Introduction
Intestine is one of the most susceptible organs to ischemia/reperfusion (I/R) injury and intestinal I/R injury is manifested as intestinal injury caused by restoration of blood supply in the intestine following interruption of intestinal blood flow.1 Intestinal I/R injury is a frequent tissue organ damage that may occur secondary to intestinal and mesenteric vascular diseases including intestinal obstruction and acute mesenteric ischemia.2 Further, intestinal I/R injury is viewed as an essential pathological process of multiple human diseases, including severe infection, trauma and hemorrhagic shock.3 Moreover, intestinal I/R injury is intensively acknowledged to be associated with increased morbidity and mortality as it may progress to multiple organ dysfunction and systemic inflammatory response syndrome.4 Thereafter, an in-depth study of discovering satisfactory treatments and effective drugs to improve the therapeutic effect of intestinal I/R injury is of great necessity.
Esculetin, also named 6,7-dihydroxy-2-chromeone (Figure 1A), is a natural derivative of coumarins available from various plants including Artemisia capillaris, Ceratostigma willmottianum and Euphorbia lathyris.5 As reported, esculetin is capable of protecting against coagulation, bacteria, inflammatory response, oxidative stress, tumors and viruses, implying the pleiotropic pharmacological effects of esculetin.6 Importantly, previous studies have underlined that esculetin may alleviate I/R injury.7,8 Meanwhile, esculetin has also been revealed to suppress intestinal injury.9 Hence, whether esculetin also plays the protective role in intestinal I/R injury remains to be elaborated.
Sirtuin 3 (SIRT3), a member of Sirtuins family located in the mitochondrial matrix,10 has been mentioned to participate in mitochondrial function, energy metabolism, signal regulation and apoptosis.11 Also, there are numerous evidence highlighting the role of SIRT3 in I/R injury.12–14 Furthermore, SIRT3 can be activated by esculetin in atherosclerosis and acute coronary artery syndrome.15,16 Noticeably, it is reported that SIRT3 can stimulate AMPK and then inactivate mTOR.17
The goal of this study is to explore the impacts of esculetin on intestinal I/R injury and to discover its potential regulatory mechanism related to SIRT3/AMPK/mTOR signaling.
Materials and Methods
Establishment of Intestinal I/R Injury Model
A total of 30 healthy male Sprague-Dawley rats (weighed 180–220 g) supplied by Beijing Vital River Laboratory Animal Technology Co., Ltd (China) were allowed free access to food and water for 1 week and housed in a specific pathogen-free room with controlled temperature of 24 ± 2°C with a 12 h light/dark cycle. The rats were randomly assigned to 5 groups (n=6 per group): Control group, Sham group, I/R group, I/R+Esculetin 10 mg/kg group and I/R+Esculetin 25 mg/kg group. Rats in I/R group received 1 h of intestinal ischemia and 2 h of reperfusion described as follows: Rats were intraperitoneally injected with pentobarbital sodium (30 mg/kg of body weight) for anesthesia. A mid-incision was made to expose the superior mesenteric artery, and a noninvasive microvascular clamp was used to occlude the superior mesenteric artery for 1 h for ischemia. Next, the clamp was removed gently for reperfusion. Rats in Sham group underwent the same protocol without vascular occlusion. Rats in I/R+Esculetin 10 mg/kg group were orally administrated with esculetin (10 mg/kg; dissolved in the 0.5% sodium carboxymethyl cellulose (CMC-Na) buffer; Sigma-Aldrich, St. Louis, MO, USA) 1 h before surgery. Rats in I/R+Esculetin 25 mg/kg group were orally administrated with 25 mg/kg of esculetin 1 h before surgery. Rats in the Control, Sham and I/R groups received the same amount of 0.5% CMC-Na buffer. At the end of the procedures, the rats were sacrificed, and intestinal tissues and blood samples from the abdominal aorta were harvested. All animal experiments were conducted in conformity with the National Institute of Health Guide for the Care and Use of Laboratory Animals,18 and were approved by the Ethics Committee of Xijing Hospital (Approval number: 20220969).
Hematoxylin and Eosin (H&E) Staining
The rat intestinal tissues fixed by 10% formalin were embedded in paraffin and cut into sections (4 μm), followed by being stained with hematoxylin solution (Shanghai Enzyme-linked Biotechnology Co., Ltd.) and eosin (Shanghai Enzyme-linked Biotechnology Co., Ltd.). With the aid of a light microscope (Keyence), the images were obtained. Histopathological lesion of intestinal tissues was assessed by the criteria of Chiu’s score method.19
Detection of Serum Diamine Oxidase (DAO) Levels
The serum was harvested following centrifugation of blood samples at 3000 g for 15 min, and DAO levels in the serum were examined with DAO assay kit (cat. no. ml092920; Mlbio, China).
Reverse Transcription-Quantitative PCR (RT-qPCR)
The extraction of total RNA from intestinal tissues and IEC-6 cells was conducted using the Trizol reagent (Beijing Leagene Biotech Co., Ltd.), followed by reverse-transcription from RNA to cDNA according to the guidelines of TRUEscript 1st Strand cDNA Synthesis Kit (CODONX, Beijing, China). Amplification of cDNA was performed via PCR using the SYBR Green qPCR Mix (CODONX, Beijing, China). PCR primer sequences used in this study were listed as follows: TNF-α, forward, 5’-ACTGAACTTCGGGGTGATTG-3’ and reverse, 5’-GCTTGGTGGTTTGCTACGAC-3’; IL-1β, forward, 5’-CAGCTTTCGACAGTGAGGAGAA-3’ and reverse, 5’-TCTTGTCGAGATGCTGCTGT-3’; IL-6, forward, 5’-TGATGGATGCTTCCAAACTG-3’ and reverse, 5’-GAGCATTGGAAGTTGGGGTA-3’; SIRT3, forward, 5’-TGCACGGTCTGTCGAAGGTC-3’ and reverse, 5’-TGTCAGGTTTCACAACGCCAG-3’; GAPDH, forward, 5’-CTCTACCCACGGCAAGTTC-3’ and reverse, 5’-GCCAGTAGACTCCACGACATA-3’. The mRNA levels of above genes were calculated with the 2−ΔΔCt method,20 and GAPDH served as the internal control.
Western Blot
The total protein was extracted from rat intestinal tissues or IEC-6 cells adopting RIPA buffer (Mlbio, China) and then subjected to 10% SDS-PAGE for separation, followed by transferring to PVDF membranes. Thereafter, the membranes were blocked with 5% non-fat milk for 2 h and then immunoblotted with primary antibodies overnight at 4°C and goat anti-rabbit HRP antibody (cat. no. ab205718; 1/2000; Abcam) for 1 h at room temperature. The blots were visualized by the ECL reagent (Mlbio, China) and the gray analysis was implemented with ImageLab4.0 software. Inducible nitric oxide synthase (iNOS; cat. no. ab178945; 1/1000; Abcam), cyclooxygenase-2 (COX2; cat. no. ab179800; 1/1000; Abcam), cleaved caspase 3 (cat. no. #9661; 1/1000; Cell Signaling Technology), caspase 3 (cat. no. ab184787; 1/2000; Abcam), cleaved PARP1 (cat. no. ab32064; 1/1000; Abcam), PARP1 (cat. no. ab191217; 1/1000; Abcam), LC3B (cat. no. ab192890; 1/2000; Abcam), Beclin1 (cat. no. ab207612; 1/2000; Abcam), SIRT3 (cat. no. ab189860; 1/1000; Abcam), p-AMPK (cat. no. ab133448; 1/1000; Abcam), AMPK (cat. no. ab207442; 1/1000; Abcam), p-mTOR (cat. no. #5536; 1/1000; Cell Signaling Technology), mTOR (cat. no. ab134903; 1/10,000; Abcam) and GAPDH (cat. no. ab181602; 1/10,000; Abcam) primary antibodies were utilized in this study.
Evaluation of Superoxide Dismutase (SOD) and Malondialdehyde (MDA)
The SOD activity (cat. no. ml092620) and MDA content (cat. no. ml094962) were examined with corresponding kits (Mlbio, China) in the light of the manufacturer’s guidance. Absorbance value at 450 nm was determined using a microplate reader (BioTek Instruments, Inc.).
Terminal-Deoxynucleotidyl Transferase Mediated Nick End Labeling (TUNEL)
The apoptosis of intestinal tissues was examined by TUNEL assay. The sections (5 μm) were prepared from paraformaldehyde-fixed and paraffin-embedded rat intestinal tissues. After deparaffinization and hydration, the sections were digested with Proteinase K for 15 min, followed by incubation with 50 μL TUNEL reaction mixture (Beyotime, Shanghai, China) for 1 h. Finally, the images were photographed under a fluorescence microscope (Olympus, Tokyo, Japan).
Caspase 3 Activity Assay
In the light of the manufacturer’s guidance, caspase 3 activity was determined using a commercial Caspase 3 Activity kit (cat. no. C1115; Beyotime). Absorbance value at 450 nm was determined using a microplate reader (BioTek Instruments, Inc.).
Immunofluorescence (IF) Staining
After deparaffinization and hydration, the sections of rat intestinal tissues were treated with 3% hydrogen peroxide and then blocked with 10% normal goat serum in PBS for 1 h. After washing, the sections were incubated with the primary antibody against LC3B (cat. no. ab192890; Abcam) overnight at 4°C. On the second day, Alexa Fluor® 488 goat anti-rabbit IgG antibody (cat. no. ab150081; Abcam) was added and incubated for another 1 h at room temperature. Nuclear DNA was labeled with DAPI. Images were observed by a fluorescence microscope (Olympus).
Molecular Docking
The crystal structure of SIRT3 protein was retrieved from the Protein Data Bank (https://www.rcsb.org/). The three-dimensional structure of esculetin was obtained in SDF format from the PubChem database (https://pubchem.ncbi.nlm.nih.gov). The molecular docking between esculetin and SIRT3 was performed using Autodock software (The Scripps Research Institute, La Jolla, CA, USA) and the interaction models were obtained by evaluating the best binding energies. The conformation was visualized utilizing PyMoL software.
Cell Culture and Hypoxia/Reoxygenation (H/R) Model
Rat intestinal epithelial IEC-6 cells from American Type Culture Collection (ATCC, Manassas, VA) were kept in Dulbecco’s modified Eagle’s medium (DMEM; Biochrom AG, Berlin, Germany) supplemented with 10% fetal bovine serum (FBS; Biochrom AG) and incubated at 37°C with 5% CO2. For H/R induction, IEC-6 cells were cultured under hypoxic conditions (5% CO2 and 95% N2) at 37°C for 6 h, after which the cells were transferred to normal oxygen conditions (95% air and 5% CO2) in a humidified incubator at 37°C for 6 h.21 To measure the impacts of esculetin on H/R cell model, cells were pre-treated by 5, 10 and 20 μM of esculetin prior to H/R induction.7
Cell Counting Kit-8 (CCK-8)
Cell activity was assessed by CCK-8 assay. IEC-6 cells were placed into 96-well plates (5 × 103 cells/well). After the indicated treatment, 10 μL CCK-8 solution (Mlbio, China) was added to each well and the plates were incubated at 37°C with 5% CO2 for extra 2 h. Cell activity was assessed through detecting absorbance value at 450 nm with the application of a microplate reader (BioTek Instruments, Inc.).
Plasmid Transfection
The specific shRNAs targeting SIRT3 (ShRNA-SIRT3-1/2) and the negative control (ShRNA-NC) were synthesized by GenePharma (Shanghai, China). IEC-6 cells were subjected to plasmid transfection adopting Lipofectamine 3000 (Invitrogen) as per the manufacturer’s recommendation. Subsequent experiments were carried out 48 h post-transfection.
Statistical Analyses
GraphPad Prism 8 software (GraphPad Software, Inc.) was utilized to carry out statistical analyses. All data were presented as the mean ± standard deviation. Statistical significances were measured by using of one-way ANOVA along with Tukey’s post hoc test. The significance level was P<0.05.
Results
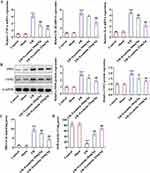
Esculetin Alleviates Tissue Pathological Damage and Reduces Serum DAO Levels in Intestinal I/R Rats
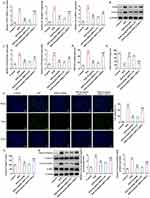
To uncover the effects of esculetin on intestinal I/R injury in vivo, the pathological changes of rat intestinal tissues were observed by H&E staining. As displayed in Figure 1B and C, no apparent alternations were observed in the morphology of rat intestinal tissues in the Control group and Sham group. Swelling, shortening, and loss of intestinal villi and the increased Chiu’s score were noticed in the I/R group, suggesting the severe pathological damage of rat intestinal tissues following I/R. After administration of 10 or 25 mg/kg of esculetin, the rat intestinal I/R injury was mitigated and Chiu’s score was prominently declined. Also, relative to the Sham group, serum DAO levels were significantly elevated in I/R group and esculetin treatment markedly decreased serum DAO levels (Figure 1D). Taken together, esculetin might play the protective role in I/R-induced intestinal injury.
Esculetin Ameliorates Inflammatory Response, Oxidative Stress and Apoptosis in Intestinal I/R Rats
Further, through RT-qPCR and Western blot, it was noted that I/R treatment remarkably raised the expression of inflammatory factors TNF-α, IL-1β, IL-6, iNOS and COX2 by contrast with Sham group and esculetin dose-dependently cut down TNF-α, IL-1β, IL-6, iNOS and COX2 expression (Figure 2A and B). Additionally, relative to the Sham group, MDA level was elevated, whereas SOD level was lessened in I/R group. Treatment with esculetin conversely declined MDA level and augmented SOD level in I/R group in a concentration-dependent manner (Figure 2C and D). Besides, the results from TUNEL assay elucidated that the apoptotic rate was strengthened in the I/R group, accompanied by enhanced caspase 3 activity and upregulated cleaved caspase 3/caspase 3 and cleaved PARP1/PARP1 expression in contrast with Sham group. Following esculetin administration, the apoptotic rate was attenuated, and Caspase 3 activity, caspase 3/caspase 3 and cleaved PARP1/PARP1 expression were declined (Figure 3A–C). To conclude, esculetin suppressed inflammatory response, oxidative stress and apoptosis in intestinal I/R rat model.
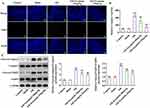
Esculetin Activates Autophagy and SIRT3/AMPK/mTOR Signaling in Intestinal I/R Rats
Importantly, Western blot analysis revealed that SIRT3 and p-AMPK/AMPK were downregulated while p-mTOR/mTOR protein level was upregulated following I/R injury, which was partly hindered by esculetin administration (Figure 4A). Through IF assay, it was discovered that the autophagy level decreased in I/R group was elevated by esculetin administration (Figure 4B). This finding was also further validated by the results that the LC3II/I and Beclin1 expression was lowered following I/R injury while was conversely elevated under esculetin administration (Figure 4C). Overall, esculetin stimulated SIRT3/AMPK/mTOR signaling and autophagy in intestinal I/R injury in vivo.
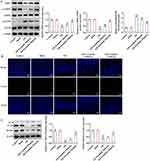
Esculetin Regulates Autophagy in H/R Cell Model via Activating SIRT3/AMPK/mTOR Signaling
Via molecular docking, the interaction between esculetin and SIRT3 was confirmed (Figure 5A). As CCK-8 assay depicted, various concentrations of esculetin elicited no obvious effects on the viability of IEC-6 cells (Figure 5B). Moreover, the suppressed IEC-6 cell viability in H/R cell model was restored by esculetin in a concentration-dependent manner (Figure 5C). In agreement with the in vivo results, SIRT3 and p-AMPK/AMPK expressions were downregulated, while p-mTOR/mTOR expression was up-regulated in H/R-exposed IEC-6 cells, which were then partly reversed by esculetin treatment dose-dependently (Figure 5D).
To validate the involvement of SIRT3 in esculetin-mediated H/R cell injury, SIRT3 was knocked down by transfection of ShRNA-SIRT3-1/2 plasmids. Moreover, ShRNA-SIRT3-1 was chosen for the ensuing experiments as it exhibited a more remarkable interference efficacy than ShRNA-SIRT3-2 (Figure 5E). It was observed from IF assay that esculetin enhanced the autophagy level in H/R cell model, which was partly hindered by further depletion of SIRT3 (Figure 5F). As expected, LC3II/I and Beclin1 expression were diminished in IEC-6 cells exposed to H/R induction, and this result was reversed by esculetin. Further, the effect of esculetin was abolished by SIRT3 knockdown (Figure 5G). Collectively, esculetin activated SIRT3/AMPK/mTOR signaling to modulate autophagy in H/R cell model.
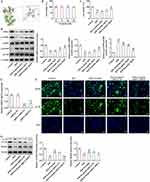
Esculetin Stimulates SIRT3/AMPK/mTOR Signaling to Relieve Inflammation, Oxidative Stress and Apoptosis in H/R Cell Model
Furthermore, esculetin administration noticeably lowered H/R exposure-induced TNF-α, IL-1β, IL-6, iNOS and COX2 expression and SIRT3 deficiency improved TNF-α, IL-1β, IL-6, iNOS and COX2 expression again (Figure 6A–C). Besides, esculetin decreased MDA level, whereas raised SOD level in H/R cell model and SIRT3 conversely elevated MDA level and declined SOD level (Figure 6D and E). At the same time, esculetin obstructed the apoptosis of IEC-6 cells exposed to H/R and this effect was abrogated by SIRT3 knockdown (Figure 6F). The inhibitor effects of esculetin treatment on Caspase 3 activity, cleaved caspase 3/Caspase 3 and cleaved PARP1/PARP1 expression in H/R-induced IEC-6 cells were also weakened after SIRT3 knockdown (Figure 6G and H). All these results pointed out that esculetin protected against H/R-induced inflammation, oxidative stress and apoptosis in IEC-6 cells via activating SIRT3.
Discussion
Intestinal I/R injury is a complex and grave disorder, the pathological process of which may involve extensive fields such as inflammatory response, apoptosis, autophagy, oxidative stress and so on.22–24 DAO is an intracellular enzyme existing in the cytoplasm of upper villus cells of the intestinal mucosa.25 Serum DAO level may indirectly reflect the permeability and barrier function of intestinal mucosa and the integrity of intestinal epithelial cells lining mucosal surfaces as DAO may be released into blood when intestinal villus is damaged.25 Consistently, in the present study, severe pathological damage of rat intestinal tissues, enhanced Chiu’s score and serum DAO levels were observed following I/R injury. Previous study has introduced a variety of pharmacological properties of esculetin, a natural dihydroxy coumarin.26 In this study, esculetin was discovered to dose-dependently reduce pathological injury of intestinal tissues and decrease Chiu’s score and serum DAO levels in intestinal I/R rat model. Hence, esculetin might play the protective role in I/R-induced intestinal injury.
Intestine can release a great number of pro-inflammatory mediators and induce inflammatory response following I/R injury.27 TNF-α is an initiating factor of inflammatory response and a leading cause of inflammatory response-induced intestinal injury.28 IL-1β and IL-6 are also inflammatory factors which are produced secondary to endothelial injury and may damage intestinal mucosa and intestinal functions.29 As reported, up-regulated iNOS and COX2 expression are closely associated with inflammatory response and even intestinal I/R injury.30 As expected, we found that the production of TNF-α, IL-1β, IL-6 and the expression of iNOS and COX2 were greatly increased following I/R treatment, demonstrating that I/R triggered inflammatory response in intestinal tissues. Anyway, this inflammatory response was remarkably restricted by esculetin in a concentration-dependent manner. Additionally, it is commonly acknowledged that the overproduction of oxidative stress marker MDA or absence of antioxidant SOD may contribute to acute lung injury and experimental acute liver lesion elicited by intestinal I/R injury.31,32 Intestinal epithelial cell apoptosis is also deemed as a pivotal mechanism leading to loss of intestinal barrier function stimulated by intestinal I/R injury.13 Here, the diminished SOD activity and the elevated MDA level and apoptotic rate confirmed the occurrence of oxidative stress and cell apoptosis in intestinal tissues following I/R injury. These changes were partly abolished by esculetin treatment, demonstrating that esculetin ameliorated oxidative stress and apoptosis in intestinal I/R rats, in line with the previous report that esculetin was able to protect against oxidative stress and apoptosis to alleviate myocardial I/R injury.7
Autophagy is a degradation process of cellular components mediated by lysosomes.33 Recent research has exposed that autophagy has been widely recognized to be implicated in intestinal I/R injury and activation of autophagy can ameliorate intestinal I/R injury.34,35 In this study, the autophagy level was decreased in the intestinal tissues of intestinal I/R rat model, while esculetin treatment could promote the autophagy level, partly accounting for the protective role of esculetin against intestinal I/R injury. SIRT3 is a pivotal deacetylase mainly modulating mitochondrial action and ROS generation.36 Further research has proposed that SIRT3 can stimulate AMPK signaling to inhibit mTOR and regulate autophagy.17 Accumulating evidence has revealed that SIRT3 may act as a suppressor in I/R injury, including intestinal I/R injury.13,14,37 Further, SITR3 can be activated by esculetin in atherosclerosis.15 Consistent with these findings, the interaction between esculetin and SIRT3 was also verified by molecular docking in this study. Meanwhile, esculetin promoted the activation of SIRT3/AMPK/mTOR signaling in both I/R-caused intestinal injury and H/R-induced IEC-6 cells, and the inhibitory effects of esculetin on inflammatory response, oxidative stress, apoptosis and the promotive effect of esculetin on autophagy in H/R-induced IEC-6 cells were weakened by SIRT3 knockdown, indicating that the protective role of esculetin against H/R-induced cell injury was partly achieved by up-regulating SIRT3.
Taken together, esculetin activated SIRT3/AMPK/mTOR signaling and autophagy to relieve inflammatory response, oxidative stress and apoptosis in intestinal I/R injury both in vitro and in vivo. This study expounded the protective role of esculetin against intestinal I/R injury and uncovered its potential molecular mechanism, suggesting the potential of esculetin as an effective drug for the treatment of intestinal I/R injury.
Data Sharing Statement
All data in this study have been included in this article.
Funding
This study was supported by National key research and development program (2018YFF01012100).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Li G, Wang S, Fan Z. Oxidative stress in intestinal ischemia-reperfusion. Front Med. 2021;8:750731. doi:10.3389/fmed.2021.750731
2. Li G, Zhang Y, Fan Z. Cellular signal transduction pathways involved in acute lung injury induced by intestinal ischemia-reperfusion. Oxid Med Cell Longev. 2021;2021:9985701. doi:10.1155/2021/9985701
3. Akbari G. Emerging roles of microRNAs in intestinal ischemia/reperfusion-induced injury: a review. J Physiol Biochem. 2020;76(4):525–537. doi:10.1007/s13105-020-00772-y
4. Playford RJ, Marchbank T. Pancreatic secretory trypsin inhibitor reduces multi-organ injury caused by gut ischemia/reperfusion in mice. PLoS One. 2020;15(1):e0227059. doi:10.1371/journal.pone.0227059
5. Masamoto Y, Ando H, Murata Y, Shimoishi Y, Tada M, Takahata K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci Biotechnol Biochem. 2003;67(3):631–634. doi:10.1271/bbb.67.631
6. Garg SS, Gupta J, Sharma S, Sahu D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur J Pharm Sci. 2020;152:105424. doi:10.1016/j.ejps.2020.105424
7. He Y, Li C, Ma Q, Chen S. Esculetin inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Biochem Biophys Res Commun. 2018;501(1):139–144. doi:10.1016/j.bbrc.2018.04.195
8. Wang C, Pei A, Chen J, et al. A natural coumarin derivative esculetin offers neuroprotection on cerebral ischemia/reperfusion injury in mice. J Neurochem. 2012;121(6):1007–1013. doi:10.1111/j.1471-4159.2012.07744.x
9. Witaicenis A, Seito LN, Di Stasi LC. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chem Biol Interact. 2010;186(2):211–218. doi:10.1016/j.cbi.2010.03.045
10. Ji Z, Liu GH, Qu J. Mitochondrial sirtuins, metabolism, and aging. J Genet Genomics. 2022;49(4):287–298. doi:10.1016/j.jgg.2021.11.005
11. Zhang J, Xiang H, Liu J, Chen Y, He RR, Liu B. Mitochondrial Sirtuin 3: new emerging biological function and therapeutic target. Theranostics. 2020;10(18):8315–8342. doi:10.7150/thno.45922
12. Zheng Y, Shi B, Ma M, Wu X, Lin X. The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion. J Cell Physiol. 2019;234(5):5488–5495. doi:10.1002/jcp.27329
13. Wang Z, Sun R, Wang G, et al. SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol. 2020;28:101343. doi:10.1016/j.redox.2019.101343
14. Liu L, Chen H, Jin J, et al. Melatonin ameliorates cerebral ischemia/reperfusion injury through SIRT3 activation. Life Sci. 2019;239:117036. doi:10.1016/j.lfs.2019.117036
15. Karnewar S, Vasamsetti SB, Gopoju R, et al. Mitochondria-targeted esculetin alleviates mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3 regulation in endothelial cells: potential implications in atherosclerosis. Sci Rep. 2016;6:24108. doi:10.1038/srep24108
16. Katta S, Karnewar S, Panuganti D, Jerald MK, Sastry BKS, Kotamraju S. Mitochondria-targeted esculetin inhibits PAI-1 levels by modulating STAT3 activation and miR-19b via SIRT3: role in acute coronary artery syndrome. J Cell Physiol. 2018;233(1):214–225. doi:10.1002/jcp.25865
17. Zhao W, Zhang L, Chen R, et al. SIRT3 protects against acute kidney injury via AMPK/mTOR-regulated autophagy. Front Physiol. 2018;9:1526. doi:10.3389/fphys.2018.01526
18. National Research Council Institute for Laboratory Animal R. Guide for the Care and Use of Laboratory Animals. National Academies Press (US) Copyright 1996 by the National Academy of Sciences; 1996.
19. Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101(4):478–483. doi:10.1001/archsurg.1970.01340280030009
20. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi:10.1038/nprot.2008.73
21. Du J, Fan X, Yang B, Chen Y, Liu KX, Zhou J. Irisin pretreatment ameliorates intestinal ischemia/reperfusion injury in mice through activation of the Nrf2 pathway. Int Immunopharmacol. 2019;73:225–235. doi:10.1016/j.intimp.2019.05.011
22. Gonzalez LM, Moeser AJ, Blikslager AT. Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol. 2015;308(2):G63–75. doi:10.1152/ajpgi.00112.2013
23. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329–354. doi:10.1152/physrev.00040.2012
24. Lenaerts K, Ceulemans LJ, Hundscheid IH, Grootjans J, Dejong CH, Olde Damink SW. New insights in intestinal ischemia-reperfusion injury: implications for intestinal transplantation. Curr Opin Organ Transplant. 2013;18(3):298–303. doi:10.1097/MOT.0b013e32835ef1eb
25. Fukudome I, Kobayashi M, Dabanaka K, et al. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med Mol Morphol. 2014;47(2):100–107. doi:10.1007/s00795-013-0055-7
26. Zhang L, Xie Q, Li X. Esculetin: a review of its pharmacology and pharmacokinetics. Phytother Res. 2022;36(1):279–298. doi:10.1002/ptr.7311
27. Yu Y, Klemann C, Feng X, et al. Increased inflammatory reaction to intestinal ischemia-reperfusion in neonatal versus adult mice. Eur J Pediatr Surg. 2015;25(1):46–50. doi:10.1055/s-0034-1387945
28. Zhou Y, Zhang M, Zhao X, Feng J. Ammonia exposure induced intestinal inflammation injury mediated by intestinal microbiota in broiler chickens via TLR4/TNF-α signaling pathway. Ecotoxicol Environ Saf. 2021;226:112832. doi:10.1016/j.ecoenv.2021.112832
29. Liu XH, Yang YW, Dai HT, Cai SW, Chen RH, Ye ZQ. Protective role of adiponectin in a rat model of intestinal ischemia reperfusion injury. World J Gastroenterol. 2015;21(47):13250–13258. doi:10.3748/wjg.v21.i47.13250
30. Chai S, Liu K, Feng W, et al. Activation of G protein-coupled estrogen receptor protects intestine from ischemia/reperfusion injury in mice by protecting the crypt cell proliferation. Clin Sci. 2019;133(3):449–464. doi:10.1042/cs20180919
31. Dong H, Qiang Z, Chai D, et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging. 2020;12(13):12943–12959. doi:10.18632/aging.103378
32. Fan Z, Jing H, Yao J, et al. The protective effects of curcumin on experimental acute liver lesion induced by intestinal ischemia-reperfusion through inhibiting the pathway of NF-κB in a rat model. Oxid Med Cell Longev. 2014;2014:191624. doi:10.1155/2014/191624
33. Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2018;14(2):207–215. doi:10.1080/15548627.2017.1378838
34. Wang Z, Li Z, Feng D, et al. Autophagy induction ameliorates inflammatory responses in intestinal ischemia-reperfusion through inhibiting NLRP3 inflammasome activation. Shock. 2019;52(3):387–395. doi:10.1097/shk.0000000000001259
35. Wen J, Xu B, Sun Y, et al. Paeoniflorin protects against intestinal ischemia/reperfusion by activating LKB1/AMPK and promoting autophagy. Pharmacol Res. 2019;146:104308. doi:10.1016/j.phrs.2019.104308
36. Hebert AS, Dittenhafer-Reed KE, Yu W, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013;49(1):186–199. doi:10.1016/j.molcel.2012.10.024
37. Zhai M, Li B, Duan W, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63(2):e12419. doi:10.1111/jpi.12419
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.