Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Epidemiology of Gastrointestinal Parasites of Cattle in and Around Hosanna Town, Southern Ethiopia
Authors Tiele D, Sebro E, H/Meskel D, Mathewos M
Received 26 September 2022
Accepted for publication 9 January 2023
Published 17 January 2023 Volume 2023:14 Pages 1—9
DOI https://doi.org/10.2147/VMRR.S389787
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Dembelo Tiele,1 Ephrem Sebro,2 Deginet H/Meskel,3 Mesfin Mathewos4
1Department of Veterinary Epidemiology, Wachemo University, Hosanna, Southern Region, Ethiopia; 2Department of Veterinary Gynaecology and Obstetrics, Wachemo University, Hosanna, Southern Region, Ethiopia; 3Department of Animal Science, Wachemo University, Hosanna, Southern Region, Ethiopia; 4Department of Veterinary Pathology, Wolaita Sodo University, Wolaita Sodo, Southern Region, Ethiopia
Correspondence: Mesfin Mathewos, Wolaita Sodo University, School of Veterinary Medicine, P. O. Box 138, Wolaita Sodo, Ethiopia, Email [email protected]
Introduction: Gastrointestinal parasites are ubiquitous parasitic agents of cattle all over the world, and cause both clinical and subclinical parasitism that results in significant financial losses. The purpose of the study was to evaluate the prevalence and the risk variables related to gastrointestinal tract (GIT) parasites in cattle in Hossana town and the nearby area.
Methods: On a total of 400 faecal samples, a cross-sectional investigation with a random sampling technique was carried out utilizing a coprologic parasitological examination.
Results: Two hundred and sixty-nine (67.2%) of the 400 faecal samples analyzed had one or more gastrointestinal parasites. Of this, 163 (40.75%) cattle had two or more parasites while a single infection was recorded in 106 (26.5%) cattle. Mixed infection of Strongyle+Fasciola (14%) was found a higher prevalence followed by Strongyle+Paramphistomum (7.75%) as compared to other GIT parasite combination. Major classes of parasites recorded include Trematodes, Nematodes, Cestodes, and Protozoa. The major parasites observed were Strongyle type (18.25%), Paramphistomum (9.5%), Fasciola (8.25%), Toxocara (3.25%) and Eimeria (2.75%). Strongyle type eggs were the most predominant type of eggs identified while Trichuris (2.25%) and Moniezia (1.5%) were observed to have relatively lowprevalence. There was a high relationship between risk factors such as age, body condition, and management system with the prevalence of GIT parasites.
Conclusion: The high frequency of GIT parasite infection in cattle in the research area necessitates the strategic deworming and effective management practices necessary for gastrointestinal parasite eradication.
Keywords: cattle, coprology, epidemiology, gastrointestinal parasites, hossana
Introduction
Ethiopia has a significant population of cattle, but the output is low because of poor diet, ineffective reproduction, management problems, and livestock diseases.1 One of the primary reasons for a decline in livestock productivity and output is internal parasite infection in cattle.2 Nematodes, cestodes, trematodes, and protozoa in domestic animals are the culprits. Due to decreased food intake, reduced fertility, decreased capacity for work, decreased production of meat and milk, and increased mortality rates during involuntary culling, parasitic illnesses typically result in decreased production and productivity.3,4
The cattle’s digestive system (GIT) is home to a wide range of parasites that can induce both clinical and subclinical effects.5 GIT parasites are the most severe source of production losses in farm ruminants. Nematodes are unquestionably the cause of major production losses to ruminants, as shown in various studies.4 Even while nematode parasites are rather common in ruminants all over the world, trematodes, cestodes, and coccidian parasites have also shown higher prevalence rates in the most of those specific jurisdictions.6,7 The most important GIT parasites in cattle include Strongyle, Toxocara, Trichuris, Fasciola, Paramphistomum, Monezia, and Eimeria.4,8 These parasites hurt an animal’s health and result in significant financial losses for the cattle sector in sub-Saharan Africa, and indeed worldwide.4,9 The consequences of gastrointestinal parasite infection vary depending on the type of parasite, the severity of the infestation, and other risk factors like species, age, and season.9,10
The most frequent means of diagnosis is based on fecal investigations of internal parasites in addition to clinical symptoms. Most frequently, fecal floatation, sedimentation, and fecal culture are used to diagnose the GIT parasites.11,12
The occurrence of GIT parasites, which ranges from 27.57% to 61%, has been previously reported by6,13–15 from different agroecologies in Ethiopia. However, there are few reports on the identification of gastrointestinal parasites from Hossana and its surroundings. Internal parasites continue to be a serious issue that lowers the efficiency of cattle in Hossana and the neighboring areas because of the large population of livestock. As a result, the current study sought to identify the main parasite genera in Hosanna town and its surroundings as well as the overall epidemiology of GIT parasites.
Materials and Methods
Study Area
The study was conducted in and around Hosanna town, which is located approximately 232 kilometers south of Addis Ababa. Hosanna is the capital city of Hadiya zone, which is located at latitude of 7.58 (7° 34’ 60 N) and longitude of 37.88 (37° 52’ 60 E). The region lies at an altitude of between 1600 and 2240 masl and has a bimodal rainfall pattern (long and short rainy seasons). The short rainy season runs from March to April, whereas the long rainy season lasts from June to September. The average annual rainfall is between 950 and 1200 mm, and the highest and minimum temperatures are 23°C and 13°C, respectively.16
Study Population
The study included both male and female cattle of native and hybrid breeds. They were raised in intensive, semi-intensive, and extensive production systems.
Study Design
From February 2020 to September 2020, a cross-sectional study was carried out to gather information on the gastrointestinal parasites that affect cattle. Frandson and Spurgeon17 claim that in addition to the owners’ notes, the dental patterns of cattle were used to estimate the age and they were divided into three age groups: <1 year, 1–3 years, and >3 years. Based on Nicholson and Butterworth,18 the body condition score was recorded as classified as poor, medium, or fat.
Sample Size Determination
Study animals were chosen using a simple random sampling method. With a 50% expected prevalence, a 5% desired absolute precision, and a 95% confidence range, the sample size was calculated using the formula provided by Thrusfield et al.19
N = Z2×Pexp (1 – Pexp)/d2
where N is the required sample size, d is the desired absolute precision = 0.05, Z2 is the statistic for the level of confidence = 1.96, and Pexp is the anticipated prevalence. Therefore, a total of 384 cattle were needed for this study. However, 400 study animals were used to improve accuracy.
Sampling Collection and Examination
Plastic gloves and a sterile bottle were used to collect feces from rectum. Each fecal sample was put in a plastic jar with lids and labeled with animal identification records, including age, sex, and body condition (thin, moderate, and good). The specimen were then shipped to Wolaita Sodo Regional Veterinary Parasitology Laboratory utilizing an ice box for further analysis. The parasitological examination was conducted in accordance with recognized standards using direct smear, sedimentation, and flotation procedures.20,21
Data Management and Analysis
Each animal’s raw data was coded, entered, and filtered into a Microsoft Office Excel spreadsheet before being analyzed with SPSS version 20 software. The proportion of animals with a particular parasite that was investigated at the time was divided by the total number of animalsfor determining the prevalence of each parasite infection.19 The variables (sex, age, breed, body condition, and management) were examined for their relationships with the occurrence of GIT parasite using Chi-square statistics (χ2). At a 95% confidence level, the p-value was regarded as statistically significant if it was less than 0.05 and statistically not significant if it was more than 0.05.
Result
Overall Prevalence and Associated Risk Factors of GIT Parasites in Cattle
Out of 400 cattle examined, 269 (67.2%) were found to be infected with one or more gastrointestinal parasites. Out of the 269 cattle (67.2%) identified as positive, 163 (40.75%) cattle had two or more parasites while a single infection was recorded in 106 (26.5%) cattle. Anorexia, diarrhea, emaciation, and anemia were among the typical clinical symptoms of gastrointestinal parasite infection in the current investigation. Age groups, body condition scores, and management approaches showed a statistically significant variation (p < 0.05) in terms of the prevalence of gastrointestinal parasites. However, no statistically significant variation (p > 0.05) was seen with the prevalence of GIT parasites between sex and breed (Table 1).
 |
Table 1 Prevalence and Variation of Gastrointestinal Parasites Among the Categories of the Considered Risk Factors |
Among mixed parasites of cattle, mixed infection of Strongyle + Fasciola (14%) was high followed by Strongyle + Paramphistomum (7.75%), Fasciola + Paramphistomum (6.25%), Strongyle+Fasciola+Paramphistomum (4.25%), and Strongyle+Toxocara (4%). However, mixed infection of Strongyle + Trichuris (3%) and Strongyle + Monezia (1.5%) revealed the least prevalence as compared to other mixed GIT parasites (Figure 1).
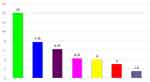 |
Figure 1 The prevalence of mixed parasite infection of cattle. |
Major classes of parasites recorded include Nematodes (23.8%), Trematodes (17.7%), Cestodes (1.5%), and Protozoa (2.8%) as indicated in Table 2. The major parasites observed were Strongyle type (18.25%), Paramphistomum (9.5%), Fasciola (8.25%), Toxocara (3.25%) and Eimeria (2.75%). Strongyle type eggs were the most predominant type of eggs identified while Trichuris (2.25%) and Moniezia (1.5%) were observed to have relatively low prevalence (Figure 2).
 |
Table 2 The Prevalence of Different Gastrointestinal Parasite Categories Among Age Groups, Sex, Management Conditions, and Breeds of Cattle, N = 400 |
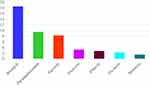 |
Figure 2 The major gastrointestinal parasites of cattle identified and their prevalence. |
Unlike other parasites, the prevalence of Toxocara was higher in young animals and its prevalence decreased as the age of the animals increased. All parasites are more prevalent in local zebu cattle compared to the cross breeds, and animals with poor body conditions were highly affected by all parasite types compared to those with medium and fat body conditions. Similarly, animals managed in an extensive management harbored a high number of all parasite eggs compared to the intensive and semi-intensive management systems (Table 2).
Discussion
One of the biggest challenges to the global production of cattle is parasitic diseases.22,23 The highest prevalence of parasitic infections has been reported in tropical and subtropical regions, especially in Africa, Asia, Australia, Eastern Europe, and Russia,24 particularly those related to climate, nutrition, and poor sanitation.25 Nematodes, Trematodes, Cestodes, and Protozoa are some of the parasitic worms that cause major economic loss due to, anemia, diarrhea, poor growth, low weight gain, decreased reproductive effectiveness, condemnation of affected organs, and mortality of infected animals.26,27 They are parasites that dwell inside their hosts’ bodies, including the blood, liver, lungs, gallbladder, and intestinal tissues or cells. Most of the time, the infectious eggs or oocyst are conveyed along with the feces when an animal defecates; succeeding animals would become infected if they grazed in the contaminated habitats; and humans might become infected through ingestion of contaminated food and water, close contact with the diseased animals, or other means.25
In the current study, cattle that was infected with gastrointestinal parasites were manifested by different clinical signs including anorexia, diarrhea, emaciation, and anaemia. This results in line with previous reports.28,29 The overall frequency of 67.2% of GIT parasite infection explored in this study was in line with previous studies15,30,31 that reported a prevalence of 61%, 59.5%, and 69.6% in East Showa Zone, Oromia Regional State, and around Bahir Dar. However, it was lower than the prevalences reported in previous studies6,8,14,32,33 that reported a prevalence of 50.08%, 41.5%, 95.5%, 77.6%, and 82.8% in Tulu district of west Harergae Zone, Diredawa, Southern Ghana, Jimma, and Holleta, respectively. The differences in the prevalence of GIT parasites that were observed in the current study could be due to poor management systems so that most of the cattle were infected while ingesting contaminated pasture as they graze on rangelands.
This study showed a high overall prevalence of Nematodes infection (23.8%) than Trematodes (17.7%), Cestodes (1.5%) and Protozoa (2.8%). This result corroborates with that of Tulu et al and Cheru et al6,15 but differed to Etsehiwot and Derb8,34 who reported that trematodes were the most prevalent parasite than other parasites including nematodes, cestodes and protozoa. The fact that worms do not require intermediate hosts and that the parasite is contagious in both its larval and adult phases may account for the great incidence of nematodes.
Strongyles (18.3%) were the predominant Nematodes followed by Toxocara (3.25%) and Trichuris sp. (2.3%). This was in agreement with the previous findings of.35 This may be attributed to the temperature and climatic condition of the area, which favor the growth of these parasites.
Toxocara is the common nematode of canine and feline hosts that was recovered in this study from cattle. Similarly,35,36 have been previously reported a high prevalence of Toxocara. The existence of such parasite in the current study might be the consequence of their close association with dogs, and probable dog-faecal contamination to the cattle food.
Trichuris has been observed less frequently in cattle of the current study as compared with many previous studies who reported a high occurrence of the parasite worldwide.35 The high prevalence may be due to feeding of grasses directly from the ground. It is possible that the high incidence such parasite might be due to ingesting of grasses directly from the ground.
Infections with Fasciola and Paramphistomum in cattle farming upshot in severe liver and rumen flukes, which can lead to emaciation, decreased milk output, reduced reproductive rates, and sometimes even fatality. Evidently, many scholars have discovered that the abundance of flukes rely on the population of intermediate snail hosts mainly of Bulinus and Planorbis.37 The spread of snail-borne Trematode infections depends heavily on these intermediate snail hosts. Additionally, the grazing system, nutritional status, and environment all influences incidence of fluke.38 Trematodes were present in this study with an overall prevalence of 17.7% (9.5% for Paramphistomum and 8.25% for Fasciola). A low occurrence of Fasciola that has been observed in the current study was not in agreement with the findings of Dorny et al.39 In this investigation, the occurrence of Paramphistomum was much lower than the reports of Ayalew et al, Haridy et al and Manaye.40–42 This heterogeneity in the incidence of trematodes in cattle could be brought on by agro-ecological circumstances, animal husbandry techniques, breeds, and the predominance of intermediate snail hosts.
Moniezia was the only Cestodes that was observed in the current study with an overall prevalence of 1.5%. This prevalence was lower than the reports of.8 The presence of this parasite in the current investigation was linked to the intake of oribatid mites carrying Moniezia cysts.43
According to Das et al,44 Eimeria is the most serious intestinal diseases of domestic animals that causes high morbidity. Across the world, cattle and other ruminants are infected by this protozoan. Anemia, electrolyte deficits, and diarrhea can result from it via invading the small and large intestine of the host.45 A prevalence of 2.8% was discovered in the cattle of Hosanna, and that was lower than all previous findings including25,35,46–49 that also stated an overall prevalence of 3–94.7%. The significant occurrence of Eimeria in the current study may be caused by unsanitary yards and the dung of sick cattle contaminating feed and drinking water. Protozoa have a lengthy lifespan inside oocysts and may adapt to a variety of settings and climatic situations. Finally, adult protozoans formed in cattle can be contaminated by oocysts, which can also infect pastures and water where protozoa will grow to sporulate.50 Overcrowding, adult and calf cohabitation, poor farm management, lack of sanitation, and untreated cattle are all contributing factors to the high prevalence of protozoa.
In addition, the existence of mixed infection such as Strongyle+Fasciola, Strongyle+Paramphistomum, Fasciola+Paramphistomum, Strongyle+Fasciola+Paramphistomum, Strongyle+Toxocara, Strongyle+Trichuris and Strongyle+Moniezia was inline with the earlier reports.7,15,51 The presence of two or more GIT parasites indicated a mixed infection. A significant contributor to morbidity and decreased productivity in cattle has been identified as the mixed infection phenomenon.52 Consequently, the immune system of the host is suppressed by mixed infections, which makes the host more vulnerable to other illnesses or parasites.53 Additionally, a prevalence of 40.75% for mixed parasite infection was found in this investigation. It is possible that the high prevalence of mixed infections is the result of inadequate control tactics, such as ignoring subclinical forms, persistent chronic nutritional stress, and the climate’s favorable conditions for parasite survival and growth.54
Putative risk factors including the age groups, body condition score, and management systems have shown a statistically substantial variation (p < 0.05) with the occurrence of GIT parasites and was found to be concurred with other studies.55,56
According to Pfukenyi et al57 and Regassa et al,31 the exposure and pathogenicity of GIT infections are greater in young animals than the matured ones, which was in line with the present study that observed a high prevalence of GIT parasites young animals. On the other hand, numerous researchers have also noted that the incidence of GIT parasites rises with age.15,58 Although the reasons for changes in parasite prevalence at various age groups are difficult to pinpoint, they may be related to the animals’ immunological status, variations in the grazing area, and management practices.31
GIT parasites are more common in cattle with poor body conditions than in animals with favorable body conditions. This result was consistent with the earlier reports.8,15,31,32 Due to their weak immunity, cattle were more susceptible to GIT parasites, which may have contributed to the high prevalence of the parasite in animals with poor body condition.59
As previously stated by Martins et al,60 the production system may be a risk factor for gastrointestinal illnesses. The present finding has also revealed that cattle managed in the extensive management system had a markedly high prevalence of GIT parasites than semi-intensive and intensive production systems. It is possible that consuming contaminated grass with parasite is the cause of the high prevalence of GIT parasites in extensive management systems.61 Additionally, the majority of farmers discharged their cattle into neighboring rivers for watering and feeding or providing chopped grass from rangelands for grazing. Based on inquiries, each owner did not deworm their cattle because the cattle had to be moved a considerable distance for grazing. Controlling gastrointestinal parasite infections in livestock necessitates knowledge of the epidemiology of the illness, pasture management, deworming, agricultural practices, and agroclimatic variables like temperature and rainfall.25,59
Conclusion and Recommendations
The overall high incidence of gastrointestinal parasites in the current study suggests that it was a serious animal health issue that impair the productivity cattle in and around Hossana. Age, body condition of animal, and management approaches showed a statistically significant variation with the occurrence of gastrointestinal parasites. Therefore, to mitigate these problems, awareness should be created for farmers to manage their cattle intensively and to keep an environmental sanitation in addition to strategic deworming of cattle with effective broad spectrum anthelmintic to reduce the prevalence of the GIT parasites of cattle.
Data Sharing Statement
Upon the respective author’s request, the information will be made available.
Ethics Approval and Consent to Participate
The Wachemo University of Research Ethics and Review Committee offered ethical clearance with a minute number of WU/REC/20/05/746 for this research. The study’s objective was conceded out following recommendations for best practices in animal care. Cattle owners had to give their verbal consent before samples could be taken from their animals under strict hygiene guidelines. The process of oral informed consent was approved by the Wachemo University of Research Ethics and Review Committee.
Acknowledgments
The authors acknowledge Wachemo University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was not supported by any funding source or institution.
Disclosure
All authors declared that there is no conflict of interest in this work.
References
1. Alsan M. The Effect of the TseTse Fly on African Development (Job Market Paper). Available from: http://web.mit.edu/posner/www/WGAPE/tsetse_29october2012.pdf; 2012.
2. Wadhawa A, Tanwar RK, Singla LD, et al. Prevalence of gastrointestinal helminths in cattle and buffaloes in Bikaner, Rajasthan, India. Veterinary World. 2011;4:417–419. doi:10.5455/vetworld.2011.417-419
3. Getachew H, Guadu T, Fentahun T, et al. Small ruminant Hydatidosis: occurrence and economic importance in Addis Ababa abattoir. Global Veterinaria. 2012;8:160–167.
4. Rafiullah T, Sajid A, Shah SR, et al. Prevalence of gastrointestinal tract parasites in cattle of Khyber Pakhtunkhwa. J Agri Biol Sci. 2011;9:6.
5. Yonas GH, Meron D, Solomon ME. Prevalence of gastrointestinal helminth parasites and identification of major nematodes of cattle in and around Bishoftu, Oromia Region, Ethiopia. J Veterinary Med Animal Health. 2018;10:165–172. doi:10.5897/JVMAH2018.0690
6. Tulu D, Lelisa K. A study on major gastro-intestinal Helminths parasites of cattle in Tulo District, West Hararghe Zone, South-Eastern Ethiopia. Austin J Veterinary Sci Animal Husbandry. 2016;3:3–6.
7. Regea G. Prevalence of major gastrointestinal tract parasite of cattle at municipal abattoir of Jimma Town, Oromia, South Western Ethiopia. Vet Med Open J. 2019;4:36–44. doi:10.17140/VMOJ-4-134
8. Etsehiwot W Study on bovine gastrointestinal helminthes in dairy cows in and around Holetta DVM thesis, Debre zeit, Ethiopia; 2004.
9. Singh S, Malhotra P, Singla L. Fatal Natural Infection with Microfilariae of Setaria Species in a Cattle Bull, Progressive Research. 2014; 9 (1): 355-356.
10. Perry BD. Investing in Animal Health Research to Alleviate Poverty. ILRI (aka ILCA and ILRAD); 2002.
11. Hendrix C. Diagnostic Veterinary Parasitology.
12. Gupta S, Singla L, Gupta S. Diagnostic trends in parasitic diseases of animals: Satish Serial Publishing House, Delhi. Cancer Causes & Control: CCC. 2012;23 Suppl 1:81–112. doi:10.1007/s10552-012-9903-3
13. Tigist A, Bogale B, Chanie M. Occurrence of gastro intestinal nematodes of cattle in and around Gondar town, Amhara regional state, Ethiopia. Acta Parasitologica Globalis. 2012;3:28–33.
14. Muktar Y, Belina D, Alemu M, et al. Prevalence of gastrointestinal nematode of cattle in selected Kebeles of Dire Dawa districts eastern Ethiopia. Adv Biol Res. 2015;9:418–423.
15. Cheru T, Birhanu A, Diriba L, et al. Prevalence of gastrointestinal parasitism of cattle in East Showa Zone, Oromia regional state, Central Ethiopia. J Veterinary Med Animal Health. 2014;6(2):54–62. doi:10.5897/JVMAH2013.0260
16. CSA. Summary and statistical report of the 2007 population and Housing Census, Addis Ababa. Ethiopia: Population and Housing Census Commission. 2008.
17. Frandson R, Spurgeon T. Physiology of Male Reproduction. In: Anatomy and Physiology of Farm Animals.
18. Nicholson M, Butterworth MH. A Guide to Condition Scoring of Zebu Cattle. ILRI (aka ILCA and ILRAD); 1986.
19. Thrusfield M. Veterinary Epidemiology.
20. Dunn AM. Veterinary Helminthology. William Heinemann Medical Books Ltd; 1978.
21. Urquhart G, Armour J, Duncan J, et al. Veterinary helminthology. Vet Parasitol. 1996;2:35–38.
22. Hamid P, Kristianingrum Y, Prastowo J, et al. Gastrointestinal parasites of cattle in Central Java. Am J Animal Veterinary Sci. 2016;11:119–124. doi:10.3844/ajavsp.2016.119.124
23. Obi C, Akata M, Ezubelu O. Prevalence of gastrointestinal helminth parasites of trade cattle in Aguata and Orumba South Local Government Areas, Southeastern Nigeria. J Parasitic Dis. 2020;44:546–552. doi:10.1007/s12639-020-01227-3
24. Sintayehu M, Mekonnen A. Prevalence and intensity of Paramphistomum in ruminants slaughtered at Debre Zeit industrial abattoir, Ethiopia. Global Veterinaria. 2012;8:315–319.
25. Thanasuwan S, Piratae S, Tankrathok A. Prevalence of gastrointestinal parasites in cattle in Kalasin Province, Thailand. Veterinary World. 2021;14:2091. doi:10.14202/vetworld.2021.2091-2096
26. Jittapalapong S, Sangwaranond A, Nimsuphan B, et al. Prevalence of gastro-intestinal parasites of dairy cows in Thailand. Agr Natural Res. 2011;45:40–45.
27. Marskole P, Verma Y, Dixit AK, et al. Prevalence and burden of gastrointestinal parasites in cattle and buffaloes in Jabalpur, India. Veterinary World. 2016;9(11):1214. doi:10.14202/vetworld.2016.1214-1217
28. Craig TM. Gastrointestinal nematodes, diagnosis and control. Veterinary Clin. 2018;34:185–199.
29. Hassan EAME. Gastrointestinal Helminths (Haemonchosis). In: Infectious Diseases of Dromedary Camels. Springer; 2021:245–255.
30. Teka M A study on prevalence of Gastrointestinal Helminthes in Cattle with Patent Natural Schistosoma Infection in and Around Bahir Dar DVM thesis, FVM, AAU, Debre Zeit Ethiopia; 2008.
31. Regassa F, Sori T, Dhuguma R, et al. Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. Int j Appl Res Veterinary Med. 2006;4:51.
32. Squire SA, Amafu-Dey H, Beyuo J. Epidemiology of gastrointestinal parasites of cattle from selected locations in Southern Ghana. Livestock Res Rural Dev. 2013;25:1–13.
33. Degefu H, Abera C, Yohannes M, et al. Gastrointestinal helminth infections in small-scale dairy cattle farms of Jimma town, Ethiopia. Ethiopian J Appl Sci Technol. 2011;2:31–37.
34. Derb Y Study on Endoparasites of Dairy Cattle in Bahir Dar and It’s Surrounding DVM Thesis, Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia; 2005.
35. Purja R. Gastro-Intestinal Parasites in Goat (Capra Hircus) of Puranchour Vdc, Pokhara: central Department of Zoology Institute of Science and Technology Tribhuvan University Kirtipur, Kathmandu. 2015;1–57.
36. Jelalu K, Yitagele T. Prevalence of gastrointestinal parasitism of cattle in Gedebano Gutazer Wolene district, Ethiopia. J Veterinary Med Animal Health. 2013;5:365–370.
37. Hambal M, Ayuni R, Vanda H, et al. Occurrence of Fasciola gigantica and Paramphistomum spp infection in Aceh cattle.
38. Shinggu P, Olufemi O, Nwuku J, et al. Liver flukes egg infection and associated risk factors in white Fulani cattle slaughtered in Wukari, southern Taraba State, Nigeria. Adv Prev Med. 2019;2019:1–5. doi:10.1155/2019/2671620
39. Dorny P, Stoliaroff V, Charlier J, et al. Infections with gastrointestinal nematodes, Fasciola and Paramphistomum in cattle in Cambodia and their association with morbidity parameters. Vet Parasitol. 2011;175:293–299. doi:10.1016/j.vetpar.2010.10.023
40. Ayalew G, Tilahun A, Aylate A, et al. A study on prevalence of Paramphistomum in cattle slaughtered in Gondar Elfora Abattoir, Ethiopia. J Veterinary Med Animal Health. 2016;8:107–111.
41. Haridy FM, El-Sherbiny GT, Morsy TA. Some parasitic flukes infecting farm animals in Al-Santa Center, Gharbia Governorate, Egypt. J Egypt Soc Parasitol. 2006;36:259–264.
42. Manaye M Study on bovine gastrointestinal helminthes in Asella and its surrounding highland areas in the Oromia regional state DVM Thesis DebreZeit, Ethiopia; 2002.
43. Ntonifor H, Shei S, Ndaleh N, et al. Epidemiological studies of gastrointestinal parasitic infections in ruminants in Jakiri, Bui Division, North West Region of Cameroon. J Veterinary Med Animal Health. 2013;5:344–352.
44. Das M, Deka D, Sarmah P, et al. Diversity of Eimeria spp. in dairy cattle of Guwahati, Assam, India. Veterinary World. 2015;8(8):941. doi:10.14202/vetworld.2015.941-945
45. Hassan NM, Farag TK. Prevalence assessment of gastrointestinal parasitic infections among goats in Giza Governorate, Egypt. Bulletin National Res Centre. 2019;43:1–7. doi:10.1186/s42269-019-0151-5
46. Heidari H, Sadeghi-Dehkordi Z, Moayedi R, et al. Occurrence and diversity of Eimeria species in cattle in Hamedan province, Iran. Veterinární med. 2014;1:59.
47. Jiménez A, Montenegro V, Hernández J, et al. Dynamics of infections with gastrointestinal parasites and Dictyocaulus viviparus in dairy and beef cattle from Costa Rica. Vet Parasitol. 2007;148:262–271. doi:10.1016/j.vetpar.2007.06.015
48. Yuwajita C, Pruangka S, Sukwong T. Prevalence of gastrointestinal parasites of cattle in Udon Thani, Thailand. Khon Kaen Agric J. 2014;42:20–24.
49. Wongsawang W, Nakthong SSC. The Survey of Gastro-Intestinal Parasites in Beef, Sai-Yok District, Kanchanaburi Province. J Appl Animal Sci. 2014;7:33–42.
50. Ola-Fadunsin SD, Rabiku M, Hussain K, et al. Epidemiological studies of Eimeria species of cattle in Ilorin, North-Central Nigeria. Ann Parasitol. 2020;5:66.
51. Lemy E, Egwunyenga A. Prevalence of Parasitic Helminthes from Feacal Samples of Cattle at Various Abattoirs in Abraka, Delta State, Nigeria. J Animal Health Behav Sci. 2017;1:107.
52. Bersissa K, Tigist T, Teshale S, et al. Helminths of sheep and goats in central Oromia (Ethiopia) during the dry season. J Animal Veterinary Adv. 2011;10:1845–1849. doi:10.3923/javaa.2011.1845.1849
53. Wang C, Qiu J, Zhao JP, et al. Prevalence of helminthes in adult dogs in Heilongjiang Province, the People’s Republic of China. Parasitol Res. 2006;99(5):627–630. doi:10.1007/s00436-006-0219-7
54. Adedipe OD, Uwalaka EC, Akinseye VO, et al. Gastrointestinal helminths in slaughtered cattle in Ibadan, South-Western Nigeria. J Veterinary Med. 2014;2014:1–6. doi:10.1155/2014/923561
55. Olkeba WG, Sarba EJ, Deres BA, et al. Prevalence of major skin diseases of cattle and associated risk factors around Ambo town, Ethiopia. Animal Health Production. 2016;64:355–365.
56. Mideksa MGTMT, Duguma HAM, Gudeta T. Epidemiological study of gastro intestinal Helminthes parasites of calves in urban, peri urban and rural smallholder dairy farms of East Wollega Zone, Western Ethiopia. Biol Agric Health. 2015;6(17);29–34.
57. Pfukenyi DM, Willingham A, Mukaratirwa S, et al. Epidemiological studies of parasitic gastrointestinal nematodes, cestodes and coccidia infections in cattle in the highveld and lowveld communal grazing areas of Zimbabwe. Onderstepoort J Veterinary Res. 2007;74:129–142. doi:10.4102/ojvr.v74i2.132
58. Qureshi A, Tanveer A. Seroprevalence of fasciolosis in buffaloes and humans in some areas of Punjab, Pakistan. Pak J Sci. 2009;61:91–96.
59. Gunathilaka N, Niroshana D, Amarasinghe D, et al. Prevalence of gastrointestinal parasitic infections and assessment of deworming program among cattle and buffaloes in Gampaha District, Sri Lanka. Biomed Res Int. 2018;2018:1–10. doi:10.1155/2018/3048373
60. Martins NS, Dos Santos CC, da Motta SP, et al. Gastrointestinal Parasites in sheep from the Brazilian Pampa Biome: prevalence and associated factors. Br J Veterinary Med. 2022;2;44.
61. Keyyu J, Kassuku AA, Msalilwa L, et al. Cross-sectional prevalence of helminth infections in cattle on traditional, small-scale and large-scale dairy farms in Iringa district, Tanzania. Vet Res Commun. 2006;30(1):45–55. doi:10.1007/s11259-005-3176-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
