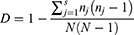Back to Journals » Infection and Drug Resistance » Volume 16
Epidemiology of Clinically Significant Aspergillus Species from a Large Tertiary Hospital in Shanghai, China, for the Period of Two Years
Authors Zhang Y, Wang S, Zhou C, Zhang Y, Pan J, Pan B, Wang B, Hu B, Guo W
Received 19 April 2023
Accepted for publication 12 July 2023
Published 17 July 2023 Volume 2023:16 Pages 4645—4657
DOI https://doi.org/10.2147/IDR.S417840
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yuyi Zhang,1 Suzhen Wang,1 Chunmei Zhou,1 Yao Zhang,2 Jue Pan,2 Baishen Pan,1 Beili Wang,1 Bijie Hu,2 Wei Guo1
1Department of Laboratory Medicine, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Department of Infectious Disease, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
Correspondence: Wei Guo, Department of Laboratory Medicine, Zhongshan Hospital, Fudan University, No. 180 Fenglin Road, Shanghai, 200032, People’s Republic of China, Tel/Fax +86-21-64041990-2376, Email [email protected] Bijie Hu, Department of Infectious Disease, Zhongshan Hospital, Fudan University, No. 180 Fenglin Road, Shanghai, 200032, People’s Republic of China, Tel/Fax +86-21-64041990-2328, Email [email protected]
Background: Aspergillus species are becoming a major public health concern worldwide due to the increase in the incidence of aspergillosis and emergence of antifungal resistance. In this study, we surveyed all Aspergillus species isolated from aspergillosis patients in Zhongshan Hospital Fudan University, Shanghai, China, from 2019 to 2021.
Methods: We characterized the susceptibility profiles of these Aspergillus species to medical azoles (voriconazole, itraconazole and posaconazole) using YeastOneTM broth microdilution system. To determine the underlying antifungal resistance mechanisms in azole-resistant A. fumigatus (ARAf) isolates, we characterized mutations in the cyp51A gene. Genotypic diversity of sampled A. fumigatus was investigated using CSP-typing.
Results: A total of 112 Aspergillus isolates (81 A. fumigatus, 17 A. flavus, 5 A. niger, 2 A. terreus, 2 A. lentulus, 2 A. oryzae, 1 A. nidulans, 1 A. versicolor and 1 A. sydowii) from 105 patients diagnosed with aspergillosis (including proven or probable invasive aspergillosis, chronic pulmonary aspergillosis, allergic bronchopulmonary aspergillosis and cutaneous aspergillosis) were obtained. Eight isolates (7 A. fumigatus and 1 A. niger) from seven patients were either azole non-susceptible or non-wild type. Azole non-susceptible or non-wild type rate was 7.1%/isolate and 6.7%/patient analysed. Four ARAf harbored TR34/L98H mutation, whereas one carried TR46/Y121F/T289A allele. The 81 A. fumigatus isolates were spread across 8 CSP types with t01 to be the predominant type (53.1%). ARAf isolates were distributed over CSP types t01, t02, t04A and t11.
Conclusion: Results from this study provided us with an understanding of the antifungal resistance and related characteristics of Aspergillus species in Eastern China. Further comparisons of our results with those in other countries reflect potential clonal expansion of A. fumigatus in our region. Further surveillance study is warranted to guide antifungal therapy and for epidemiological purposes.
Keywords: Aspergillus fumigatus, aspergillosis, antifungal susceptibility profile, non-susceptible, non-wild type, CSP-typing
Introduction
Aspergillus species are a group of globally distributed filamentous ascomycete molds which grow ubiquitously in nature.1 Over 800 Aspergillus species have been recorded and described.2 Infections with Aspergillus species lead to aspergillosis which contains a broad spectrum of illnesses including allergic bronchopulmonary aspergillosis (ABPA), chronic pulmonary aspergillosis (CPA) to life-threatening invasive aspergillosis (IA).3 The annual incidence of CPA and IA in the world is estimated to be 3 million cases and over 250,000 cases, respectively.4 The case-fatality rate associated with aspergillosis has been reported to be 20%–72% depending on the underlying conditions of the patients.5,6 Aspergillosis has become a public health concern worldwide, while routine antifungal susceptibility testing for Aspergillus species has not been a common practice in clinical microbiology laboratories in many places including China. In addition, the susceptibility data of Aspergillus species to commonly used antifungals as well as the association to clinical outcomes are still lacking, especially for infections with azole-resistant A. fumigatus (ARAf). In 2022, WHO developed its first fungal priority pathogens list with A. fumigatus ranked the highest for perceived public health importance.7
Azole agents target and inhibit the biosynthesis of cytochrome P450 sterol 14α-demethylase (CYP51A), which in turn inhibits the cell growth.8 Multiple azole resistance mechanisms have been characterized in A. fumigatus with the most well-described mechanism, mutations in the cyp51A gene. Mechanisms with tandem repeats (TR) in the promoter region of cyp51A gene, including TR34/L98H and TR46/Y121F/T289A were considered to be of environmental origin, whereas azole-resistance development after long-term medical azole therapy was considered as patient route.9 Currently, aspergillosis and the causal pathogens, Aspergillus species, have been investigated in different regions from many countries with a focus on their antifungal resistance against empirically used medical azoles and genotypic diversities.10–22 These reports suggested a high diversity in these fungal characteristics, which differ across geographical regions. For example, across countries in Europe, the detected azole-resistance frequencies have been found a 28-fold difference, ranging from 1.1% to 28%.14,15 A similar large difference was also observed in other continents including America, Asia, Africa and Oceania, ranging from 0.6% to 13.9%.10,16,17 The surveillance studies conducted in China reported the resistant rate to be lower than 10% with ARAf concentrated in the eastern and southeastern provinces.18–22 Even within the same city, the prevalence of ARAf varies between individual hospitals.18,19 With these highly diverse regional characteristics in Aspergillus species, it is necessary to develop surveillance programs on the antifungal resistance and genetic diversity of Aspergillus species at the hospital level and between different patient populations. Zhongshan Hospital Fudan University is one of the largest tertiary referral hospital in Eastern China with about 2005 beds and 104 ICU beds. Over 150,000 patients were admitted and around 4 million outpatient visits to this hospital per year. This study could provide a snapshot of the clinically significant Aspergillus species profile from Eastern China.
In this study, we surveyed all Aspergillus species isolated from aspergillosis patients in Zhongshan Hospital Fudan University from 2019 to 2021. We aimed to evaluate their susceptibility profiles to medical azoles and determine the potential underlying antifungal resistance mechanisms in ARAf. Furthermore, we characterized the genotypic diversity of sampled A. fumigatus using CSP-typing.
Materials and Methods
Clinical Data Collection and Definitions Used
One hundred and twelve Aspergillus species were isolated and collected from one hundred and five patients diagnosed with aspergillosis at Zhongshan Hospital Fudan University, Shanghai, China, from July 2019 to June 2021. Among these patients, seven patients were coinfected by two different isolates. Repeatedly isolated Aspergillus strains from the same patients were ruled out. The following data were collected for each patient: age, sex, underlying diseases, potential risk factors for fungal infections, previous exposures to azole antifungals, antifungal therapy received, and clinical outcome. Proven/probable invasive aspergillosis (IA), chronic pulmonary aspergillosis (CPA), and allergic bronchopulmonary aspergillosis (ABPA) were classified according to the European organization for research and treatment of cancer and the mycoses study group (EORTC/MSG) definitions proposed in 2019, the European Society for Clinical Microbiology and Infectious Diseases, the European Confederation of Medical Mycology and the European Respiratory Society Joint Clinical Guidelines (ESMID-ECMM-ERS) proposed in 2017 and new clinical diagnostic criteria for ABPA/Mycosis published in 2020, respectively.23–25 Prior antifungal exposure was defined as having received an azole agent in the 3 months prior to Aspergillus species detection.
Isolates Identification
All specimens and Aspergillus isolates were processed and cultured according to routine clinical mycological procedures.26 Briefly, all specimens were processed and inoculated on Sabouraud Dextrose Agar (Oxoid, UK). The inoculated plates were incubated at 35°C for at least 7 days. We first used Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-ToF-MS) (bioMerieux, France) to identify Aspergillus species, followed by molecular identification for azole non-susceptible or non-wild type A. fumigatus isolates. Partial β-tubulin genes were amplified and sequenced as previously described.27
In vitro Susceptibility Testing
Antifungal drug susceptibility was evaluated on all Aspergillus isolates against three commonly used medical azoles, itraconazole, posaconazole, and voriconazole, using SensititreTM YeastOneTM broth microdilution system (YO10 panel, Thermo Fisher Scientific, USA) according to the manufacturer’s instruction. Briefly, well-isolated colonies (over three different colonies per sample) from a 7-day culture of Aspergillus isolate were inoculated into the sterile saline water. The conidial suspension was then adjusted to 0.5 McFarland (0.6–5 × 106 CFU/mL) and 100uL of the suspension was added to 11mL YeastOneTM inoculum broth (Thermo Fisher Scientific, USA) and mixed thoroughly. A 100uL of the mixture was further inoculated into each well of the YeastOneTM microdilutions 96-well plate (Thermo Fisher Scientific, USA). The reference A. fumigatus isolate ATCC 204305 was used as the control. The minimal inhibitory concentration (MIC) values of isolate P53-27660 were further determined by the broth microdilution method according to the criteria of M38-A3 established by Clinical and Laboratory Standards Institute (CLSI).28 The antifungal drugs were purchased from Sigma-Aldrich, USA. The reference Candida parapsilosis isolate ATCC 22019 was used as the control. The results of susceptibility tests were compared with the MIC breakpoints and epidemiological cutoff values (ECVs) published by the CLSI.29,30 A. fumigatus isolates were regarded as resistant, intermediate and susceptible when the MIC was ≥2 ug/mL, 1 ug/mL and ≤0.5 ug/mL for voriconazole, respectively. On the other hand, A. fumigatus isolates with the MIC values above 1 ug/mL were considered to be non-wild type for itraconazole. For A. niger, isolates were classified as non-wild type if they had itraconazole, posaconazole and voriconazole MIC values above 4 ug/mL, 2 ug/mL and 2 ug/mL, respectively. A. flavus isolates were regarded as non-wild type if they had itraconazole, posaconazole and voriconazole MIC values above 1 ug/mL, 0.5 ug/mL and 2 ug/mL, respectively. For A. terreus, isolates were classified as non-wild type if they had itraconazole, posaconazole and voriconazole MIC values above 2 ug/mL, 1 ug/mL and 2 ug/mL, respectively. In this study, ARAf was referred to voriconazole non-susceptible (intermediate and resistant) and/or itraconazole non-wild type A. fumigatus isolates.
Detection of cyp51A Mutations
To determine the potential genetic mechanisms underlying antifungal resistance in the seven azole non-susceptible or non-wild type A. fumigatus isolates, we sequenced both the promoter region and the entire coding region of the cyp51A gene using Sanger sequencing following the protocols described previously.31,32 The sequences were aligned with the cyp51A gene in the azole-susceptible strain (GenBank accession number AF38659) using CLC Main Workbench (QIAGEN bioinformatics, Germany).
Determination of CSP1 Types and Genetic Diversity
We also sequenced the csp1 region and determined the genetic diversity of all sampled A. fumigatus isolates.33 The CSP types of these isolates were assigned according to the CSP-typing nomenclature described by Klaasen et al in 2009. Simpson’s index of diversity (D) was used to analyze the genetic diversity between these isolates, where nj is the total number of A. fumigatus isolates of the particular jth type, N is the total number of A. fumigatus isolates in the test population, and s is the total number of different CSP types described.34 MEGA 11.0 software was used to construct the maximum likelihood phylogenetic tree based on csp gene sequences to illiterate the distribution of ARAf isolates.
Statistical Analysis
The prevalence of CSP types between different hospital wards and departments was compared using the Fisher's exact test. Two-sided p-values of <0.05 were considered statistically significant. R studio version 4.2.1 software was used for statistical analysis.
Ethics Statement
The study protocol was approved by the Ethics Committee of Zhongshan Hospital Fudan University (approval number: B2022-376R).
Results
Specimen Origin and Characteristics of Aspergillosis Patients
In total, 112 Aspergillus isolates from 105 patients diagnosed with aspergillosis were obtained from July 2019 to June 2021. They were recovered from sputum (90.2%), bronchoalveolar lavage (7.1%), lung biopsy (0.9%), fluid from the surgical wound (0.9%), and peritoneal dialysis fluid (0.9%). The majority of the Aspergillus isolates were recovered from the Department of Respiratory Medicine (32.1%), Surgical ICU (22.3%), and Department of Infectious Diseases (16.1%). In addition, twelve (10.7%) and seven (6.3%) isolates were obtained from outpatients and patients who went to the emergency room, respectively.
All patients aged from 53 to 72 years old (median = 65) and the majority were males (75.2%). Out of 105 patients, 102 of them received antifungal therapy and azoles were administered as the empirical treatment for 91 patients. Fifteen patients had azole exposure before Aspergillus species detection. The most prevalent underlying diseases were chronic lung diseases (48.6%), hematological or solid malignancies (23.8%), and hematopoietic cell or solid organ transplantation (19.0%) (Table 1). Fifty-five (52.4%) patients were diagnosed with IA (2 proven, 53 probable). Thirty-seven (35.2%) and twelve (11.4%) patients were diagnosed with CPA and ABPA, respectively. The remaining patient was diagnosed with post-operative cutaneous aspergillosis. The mortality rate of patients with IA was 7.6 folds higher than those with CPA. Among patients who had hematopoietic cell or solid organ transplantation, the rate of mortality within the observation period was 65.0%.
 |
Table 1 Demographic Data and Clinical Characteristics of the Patients |
Distribution of Aspergillus Isolates
Using MALDI-ToF-MS, we have identified 9 different Aspergillus species. The most frequent species was A. fumigatus sensu stricto (72.3%), followed by A. flavus (15.2%), A. niger (4.5%), and A. terreus (1.8%). For azole non-susceptible or non-wild type A. fumigatus isolates, we further used β-tubulin sequencing to confirm the species identification. Our sequencing results showed 100% consistency and all species were identified as A. fumigatus sensu stricto. These β-tubulin gene sequence data have been submitted to the GenBank databases under accession number OR136835 to OR136841. Several cryptic species were identified, including two isolates of A. lentulus, and one A. sydowii. In addition, two A. oryzae, one A. nidulans and one A. versicolor were identified. Co-infection with multiple Aspergillus species was not very often observed in our hospital. Only five patients were observed coinfecting with A. fumigatus and A. flavus, whereas one patient was coinfected by A. niger and A. flavus. Two A. fumigatus strains (P13-27328-1 and P13-27328-2) with different morphological appearance were isolated from the same patient.
Susceptibility to Azoles
The antifungal susceptibility testing results of 112 Aspergillus species for medical azoles are represented in the MIC range, MIC50, and MIC90 (Table 2). The overall prevalence of azole non-susceptible or non-wild type isolates was 7.1% (8 out of 112). Seven patients (6.7%) were infected with azole non-susceptible or non-wild type isolates. Among A. fumigatus isolates, four were found resistant to voriconazole and non-wild type for itraconazole. Isolate P81-27727 was resistant to voriconazole only, whereas isolate P3-27224 was non-wild type for itraconazole and intermediate to voriconazole (Figure 1). For isolate P53-27660, the MIC values of posaconazole, voriconazole and itraconazole were determined as 0.5 ug/mL, 2 ug/mL and 1 ug/mL using YeastOneTM broth microdilution system, respectively. The MIC value of voriconazole by the CLSI method was one dilution lower than this from YeastOneTM (1 ug/mL, intermediate). For posaconazole and itraconazole, the results from YeastOneTM and CLSI methods were identical. We also detected non-wild type isolate in the non-fumigatus species. One A. niger isolate had voriconazole and itraconazole MIC values above the ECVs. The azole non-wild type rate of A. niger was 20%. For the other Aspergillus sections species identified in our study, since ECVs or clinical breakpoints have not been established by CLSIs or European Committee on Antimicrobial Susceptibility Testing (EUCAST), we only reported measured MICs without determining whether they were resistant to the three azoles. One isolate of A. oryzae (O20-27329) recovered from a CPA patient had MIC of 4 ug/mL towards voriconazole. Two A. lentulus isolates had MICs of 1 ug/mL towards voriconazole.
 |
Table 2 Susceptibility Profiles of Aspergillus Species Isolated from Aspergillosis Patients |
 |
Figure 1 Distribution of posaconazole (POS), voriconazole (VOR) and itraconazole (IZ) MICs for 81 clinical A. fumigatus isolates. |
Molecular Determination of Azole-Resistance Mechanism, ARAf Prevalence and Patient Outcomes
Five different mutations were detected in the cyp51A gene in six ARAf isolates. Four of them harbored TR34/L98H mutations, one carried TR46/Y121F/T289A mutations, and one with N248K mutation. In addition, in voriconazole-intermediate isolate P53-27660, we did not detect any mutations on the cyp51A gene. These cyp51A gene sequence data have been submitted to the GenBank databases under accession number OQ679715 to OQ679721. According to the number of patients included in this study, the overall prevalence of ARAf in all patients was 6.7%, which is lower than the prevalence in ICU patients (9.7%) and in the patients who had received prior azole therapy (16.7%) (Supplement Table 1). The mortality rate of patients infected with ARAf was 50% which is higher than the mortality rate of patients infected with azole-susceptible A. fumigatus (36.5%). For IA patients, patients with non-susceptible/non-wild type isolates had mortality rate of 75%, whereas patients with azole susceptible isolates had mortality rate of 60.8%. However, all CPA and ABPA patients with non-susceptible/non-wild isolates survived over the observation period. Hence, ARAf were not associated with a higher mortality than azole susceptible isolates in CPA and ABPA patients.
Molecular Characterization of A. fumigatus
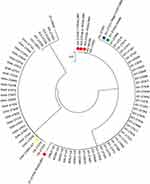
The 81 A. fumigatus isolates that recovered in our study were categorized into 8 CSP types, t01 (53.1%), t02 (6.2%), t03 (6.2%), t04A (28.4%), t10 (1.2%), t11 (1.2%), t16 (2.5%), and t21 (1.2%). The Simpson’s index of diversity was calculated at 0.64. Besides that, we observed no significant differences in the prevalence of CSP types between different hospital wards and departments (Table 3). We also compared the distribution of resistant alleles in different CSP types. We showed that isolates carrying TR34/L98H allele were mainly distributed over CSP types t02 and t11. Remarkably, the t01 type contained both azole-susceptible and azole-resistant isolates that harbored TR46/Y121F/T289A or N248K mutations. Isolate P53-27660 (intermediate to voriconazole without mutation in cyp51A gene) belonged to t04A (Figure 2).
 |
Table 3 Prevalence of CSP Types Between Different Hospital Wards and Departments |
Discussion
In this study, we reported findings from the survey on 112 Aspergillus species isolated from patients diagnosed with aspergillosis in Zhongshan Hospital Fudan University in Shanghai, China. We characterized the species distribution as well as their susceptibility to three medical azoles. For the major species in our survey, A. fumigatus, we further determined its azole non-susceptible or non-wild type mechanisms by focusing on the mutations in the cyp51A gene and characterized the genetic diversity using CSP-typing. Our results provided a regional perspective on the management of aspergillosis patients as well as pathogen characterization in China.
The Second Abundant Aspergillus Species Differs Across Geographical Regions
Similar to previous epidemiological reports in China and other countries, the most frequently isolated species from aspergillosis patients is A. fumigatus.18–21 However, the second abundant Aspergillus species differed across geographical regions. In our study, A. flavus was the most common non-fumigatus Aspergillus species. This is consistent with several other reports in Asia, Africa, and the Middle East.12,35,36 By contrast, data from Switzerland and Korea described A. niger as the most common non-fumigatus Aspergillus species.14,37 This difference could be caused by many factors such as climatic conditions and characteristics of patient populations. For example, in tropical and subtropical regions such as Northeastern Iran, A. flavus has been reported to be the leading cause of invasive pulmonary aspergillosis.38 It has higher fitness than the other non-fumigatus Aspergillus species because it is able to survive in hot and dry conditions.35 This would lead to a higher prevalence of A. flavus in these regions, which is supported by aspergillosis surveys in these countries.35,37–40 Besides, the second dominant Aspergillus species also depends on patient populations. In a Danish cystic fibrosis patient study, A. terreus was reported as the second most common species.41 While 60% of non-fumigatus Aspergillus causing invasive aspergillosis in children and adults after hematopoietic stem cell transplantation (HSCT) and chemotherapy in a Russian medical center was A. niger.42 This suggests that interactions between different Aspergillus species and patient characteristics could be important determinants of the distribution and spreading of Aspergillus species.43
Aspergillus Susceptibilities to Azoles are Highly Geographically Variable
The prevalence of azole-resistant Aspergillus species is geographically variable. Our study showed that 7.1% of Aspergillus species were either azole non-susceptible or non-wild type. This is similar to the reports in many other Asian countries including China, Japan, Korea, Pakistan, and Taiwan, where the prevalence of azole-resistant Aspergillus species was lower than 10%.18–22,43–46 However, the prevalence of azole resistance in Asian countries is much lower than that in European countries.10 ARAf has been reported in almost all European countries with the highest rate described in the United Kingdom to be 28%.15 Even within the same country, the prevalence of ARAf is geographically variable. A Chinese surveillance study observed ARAf was concentrated in the eastern and southeastern areas of China.22
This variation between different studies may mainly result from the patient’s underlying conditions, prior azole exposure, and/or selective pressure from localized azole fungicide use. Resistance can develop after exposure to antifungal drugs either after long-term medical azole therapy (patient route) or after exposure to agriculture azole fungicides (environmental route).9 In our study, the overall azole non-susceptible or non-wild type rate in patients who had received prior azole therapy was 20% (2 patients infected with A. fumigatus and 1 infected with A. niger). Similar high levels of azole-resistance have also been reported in chronic pulmonary aspergillosis patients and cystic fibrosis patients with long-term azole treatment.47 Furthermore, localized resistance selection following exposures to agricultural fungicide uses may contribute to the variation in resistance against azoles. In Europe, the extensive application of agricultural azole has been associated with the emergence of ARAf in agricultural fields and ARAf detected in hospitals.48 In China, we do have surveillance on determining sources of Aspergillus resistance.49 Four patients infected with ARAf in our study had no prior azole exposure. Based on a recent population genomic study which suggests azole-naïve patients acquire ARAf from the environment, we hypothesize that some of the clinical ARAf isolates in our study are environmental origin.50
Resistant isolates that recovered from ICU patients, hematopoietic cells or solid organ transplantation recipients usually receive more clinical attention because they often lead to severe complications and high mortality.51,52 According to the number of A. fumigatus isolates recovered in this study, the rate of ARAf was 12.5% in ICU patients which is 2 folds higher than in those who have not been admitted to ICU. We also showed that hematopoietic cell or solid organ transplantation recipients are also more likely to harbor ARAf with a prevalence to be 10%. A higher prevalence of ARAf in similar patient populations was also observed in the Netherland and Germany, which was suggested to be due to the prophylactic and empiric prescribing of antifungals in these patients.52,53 This also partially explains our observation of a much higher ARAf rate than another hospital in Shanghai (2.6%), which has a distinct patient population.19
Since our main Aspergillus species is A. fumigatus, we were able to further infer the genetic mechanism underlying its azole resistance surveyed mutations in both the promoter region and the protein-coding of the cyp51A genes in all resistant A. fumigatus. We found tandem repeats (TR) in the promoter region including TR34/L98H and TR46/Y121F/T289A, which were also considered to be of environmental origin.9,50 This further supports our previous hypothesis that some ARAf in our survey could result from broad application of agricultural azole fungicides. In addition to the TR in promoter region, point mutations in the cyp51A gene are another main mechanism responsible for the increase in MICs to azoles.9 In our study, we also noticed a special case, isolate P3-27224 harbored N248K mutation in cyp51A gene. It was obtained from IA patient who had severe hepatitis and liver failure with no prior azole exposure. This isolate exhibited high MIC towards itraconazole (>16 ug/mL) but relatively lower MIC to posaconazole and voriconazole (1ug/mL). N248K was found in a Belgian lung transplant patient who had prior voriconazole treatment.54 This isolate was resistant to voriconazole only. More recently, this polymorphism was detected in ARAf isolated from the environment of Vietnam.55 However, N248K amino acid substitutions have also been reported in many azole-susceptible strains, particularly in Asian countries.44,56 These make it difficult to confirm whether N248K mutation confers azole resistance. Hence, further investigations on this polymorphism are required.
The infection with azole non-wild type non-fumigatus species continues to emerge in recent years and started causing high morbidity and mortality in many countries.57 In our study, an isolate of A. niger (O15-27838) was found to be resistant to both voriconazole and itraconazole. It was isolated from a CPA patient with bronchiectasis. Besides that, A. oryzae O20-27329 recovered from another CPA patient was found resistant to voriconazole. A potential contributor to this resistance found in both patients could be their azole therapies prior to Aspergillus species detection. We also identified three other cryptic species which contribute to 2.7% of all isolated Aspergillus species. A. lentulus is a cryptic species of A. fumigatus complex. It was observed to exhibit high MICs for all triazoles in several studies and was considered to have intrinsic azole resistance.58 In this study, two A. lentulus isolates had MICs of 0.5 ug/mL, 1 ug/mL and 0.5 ug/mL to posaconazole, voriconazole and itraconazole, respectively. As neither ECVs nor clinical breakpoints have not been established for A. lentulus, applying clinical breakpoint of voriconazole for A. fumigatus suggests these two A. lentulus isolates were intermediate voriconazole resistant. This suggests the necessity of a surveillance program on azole resistance in non-fumigatus Aspergillus species as well as their genetic mechanisms.59
Genetic Diversity Survey Suggests a Potential Clonal Expansion of A. fumigatus
Genetic typing of A. fumigatus isolates yielded from both environmental and clinical sources in different geographic regions has been done to demonstrate the clonality between these isolates.60 Various genotyping methods were introduced, including multi-locus length polymorphism analysis (STRAf) and sequencing of the repeat region of the csp1 gene (CSP typing). Although STRAf typing has high discriminatory, it may over interpret fast-changing loci.61 CSP typing method is highly interlaboratory reproducible and easy handling which made it widely adopted.33,34 Although there are a total of 30 different CSP types of A. fumigatus described,60 we only observed eight types in our study, with t01 being the dominant CSP type (53.1%) and t04 (28.4%) the second. This pattern was also observed previously in China and clinical isolates in the Netherland, where t01 dominates surveyed A. fumigatus (37.3% and 34.5%, respectively).33,62 The second dominant CSP type in our study, t04A, was reported to dominate in several other countries including Iran (45.6%), Argentina (41.3%), Mexico (38.8%), Australia (28.7%), Spain (22.9%) and another study conducted in Beijing, China (31.5%), suggesting t04 could have the potential to dominate in China as well.34,63–66 One report in a German survey showed that t03 was the most common type among clinical isolates,60 whereas another Dutch survey showed that t04B was the more prevalent one in the environmental and clinical isolates.67 The fact that CSP types t01, t03, and t04A and t04B of A. fumigatus have become the most prevalent types worldwide which suggests their ability to better survive in diverse and adverse conditions.64,68
We used Simpson’s index of diversity to quantify the genetic diversity of A. fumigatus recovered in our study. We calculated Simpson’s index based on the data reported in other CSP typing studies and included the results in Supplement Table 2. The Simpson’s index in our study was 0.64, which is lower than the average of indices in the other studies (0.81, ranging from 0.76 to 0.86). This suggests a potential occurrence of clonal expansion in our hospital. We hypothesize that A. fumigatus may undergo microevolutionary processes during the infection in human hosts and further adaptation to specific niches.69 Although our current evidence is not enough to confirm the clonal spread of CSP type t01 in Chinese patient populations or our hospital, we propose to include more environmental samplings and phylogenetic comparisons to determine the causes of limited CSP type diversity. This will allow us to understand the spread of A. fumigatus in the local population and healthcare facilities which may result in novel pathogenetic mechanisms.
ARAf isolates in this study were distributed over CSP types t01, t02, t04A and t11. ARAf with TR34/L98H were mainly found in CSP type t02 (3/4) which were genetically less diverse than azole-susceptible isolates. Similar observations were also noted in previous publications.60,63 These suggest that isolates with TR34/L98H polymorphism may develop from a common ancestor and emerged independently. Similar conclusions were drawn by pan-genome analysis.50 However, further work on global collections and analysis of A. fumigatus will be needed to confirm the hypothesis of TR34/L98H polymorphism origins.
CSP typing was also used to determine A. fumigatus nosocomial outbreaks.70 In our study, two CSP type t16 A. fumigatus (P11-27753 and P17-27219) recovered from two separate Respiratory ICU patients with probable invasive aspergillosis. It was a rare CSP type that has only been reported in a Dutch clinical study.33 P11-27753 was recovered from a patient with tracheotomy who was admitted to Respiratory ICU from January 2020 to March 2020. P17-27219 was isolated from a patient who had tracheal intubation during the hospital stay. This patient was admitted to Respiratory ICU in May 2020. Coincidentally, both patients were assigned to the same hospital bed. Although we did not detect A. fumigatus in the environment of our ICU wards through routine nosocomial infection monitoring, this coincidence suggests the possibility of the nosocomial spread of A. fumigatus in the hospital.
Conclusion
To conclude, results from this study provided us with a regional report on the antifungal resistance and related characteristics of Aspergillus species in Eastern China. Further comparisons of our results with those in other regions may reflect an environmental origin of resistant Aspergillus isolates and diverse antifungal resistance mechanisms in hospitals. Based on these data, we hypothesized that there could be a potential clonal expansion of A. fumigatus in Chinese patient populations as well as a potential nosocomial outbreak in our hospital, while further surveys and experiments are needed to test this hypothesis. Given that infection of azole-resistant Aspergillus species continues to emerge, further surveillance study is warranted to guide antifungal therapy and for epidemiological purposes.
Data Sharing Statement
The cyp51A gene sequence data in this study are openly available at the GenBank databases under accession number OQ679715 to OQ679721.
Ethics Approval and Informed Consent
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Zhongshan Hospital, Fudan University (approval number B2022-376R) obtained on 1 September 2022. Informed consent was obtained from all subjects involved in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by research grants from the Zhongshan Hospital, Fudan University (2021ZSQN41), to Yuyi Zhang.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mullins J, Harvey R, Seaton A. Sources and incidence of airborne Aspergillus fumigatus (Fres). Clin Exp Allergy. 1976;6(3):209–217. doi:10.1111/j.1365-2222.1976.tb01899.x
2. Species Fungorum [homepage on the Internet]. Aspergillus – search Page. Available from: www.speciesfungorum.org.
3. Latgé JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019;33(1):e00140–18.
4. Bongomin F, Gago S, Oladele RO, Denning DW. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi. 2017;3(4):57.
5. Hammond EE, McDonald CS, Vestbo J, Denning DW. The global impact of Aspergillus infection on COPD. BMC Pulm Med. 2020;20(1):241.
6. González-García P, Alonso-Sardón M, Rodríguez-Alonso B, et al. How Has the Aspergillosis Case Fatality Rate Changed over the Last Two Decades in Spain? J Fungi. 2022;8(6):576.
7. WHO. Fungal priority pathogens list to guide research, development and public health action [Online]. Geneva: World Health Organization; 2022. Available from: https://www.who.int/publications-detail-redirect/9789240060241.
8. Zhang J, Li L, Lv Q, Yan L, Wang Y, Jiang Y. The Fungal CYP51s: their Functions, Structures, Related Drug Resistance, and Inhibitors. Front Microbiol. 2019;10:691.
9. Verweij PE, Lucas JA, Arendrup MC, et al. The one health problem of azole resistance in Aspergillus fumigatus: current insights and future research agenda. Fungal Biol Rev. 2020;34(4):203–214.
10. Rivero-Menendez O, Alastruey-Izquierdo A, Mellado E, Cuenca-Estrella M. Triazole Resistance in Aspergillus spp.: a Worldwide Problem? J Fungi. 2016;2:21.
11. van der Linden JW, Arendrup MC, Warris A, et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis. 2015;21(6):1041–1044.
12. Rotjanapan P, Chen YC, Chakrabarti A, et al. Epidemiology and clinical characteristics of invasive mould infections: a multicenter, retrospective analysis in five Asian countries. Med Mycol. 2018;56:186–196.
13. Lestrade PPA, Bentvelsen R, Schauwvlieghe AFAD, et al. Voriconazole resistance and mortality in invasive aspergillosis: a multicentre retrospective cohort study. Clin Infect Dis. 2019;68:1463–1471.
14. Ragozzino S, Goldenberger D, Wright PR, et al. Distribution of Aspergillus Species and Prevalence of Azole Resistance in Respiratory Samples From Swiss Tertiary Care Hospitals. Open Forum Infect Dis. 2021;9(2):ofab638.
15. Bueid A, Howard SJ, Moore CB, et al. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother. 2010;65:2116–2118.
16. Baddley JW, Marr KA, Andes DR, et al. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J Clin Microbiol. 2009;47(10):3271–3275.
17. Chowdhary A, Sharma C, van den Boom M, et al. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother. 2014;69(11):2979–2983.
18. Xu Y, Chen M, Zhu J, et al. Aspergillus Species in Lower Respiratory Tract of Hospitalized Patients from Shanghai, China: species Diversity and Emerging Azole Resistance. Infect Drug Resist. 2020;13:4663–4672.
19. Xiao C, Qiao D, Xiong L, et al. Clinical and Microbiological Characteristics of Aspergillosis at a Chinese Tertiary Teaching Hospital. Infect Drug Resist. 2022;15:7249–7257.
20. Yang X, Chen W, Liang T, et al. A 20-Year Antifungal Susceptibility Surveillance (From 1999 to 2019) for Aspergillus spp. and Proposed Epidemiological Cutoff Values for Aspergillus fumigatus and Aspergillus flavus: a Study in a Tertiary Hospital in China. Front Microbiol. 2021;12:680884.
21. Wang Y, Zhang L, Zhou L, Zhang M, Epidemiology XY. Drug Susceptibility, and Clinical Risk Factors in Patients With Invasive Aspergillosis. Front Public Health. 2022;10:835092.
22. Deng S, Zhang L, Ji Y, et al. Triazole phenotypes and genotypic characterization of clinical Aspergillus fumigatus isolates in China. Emerg Microbes Infect. 2017;6(12):e109.
23. Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–e38.
24. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376.
25. Asano K, Hebisawa A, Ishiguro T, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. 2021;147(4):1261–1268.e5.
26. Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS. Manual of Clinical Microbiology.
27. Samson RA, Visagie CM, Houbraken J, et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2008;78:141–173.
28. CLSI. 2017 Reference Method for Broth Dilution Antifungal Susceptibility Testing of fIlamentous Fungi. CLSI Standard M38.
29. CLSI. 2020 Performance Standards for Antifungal Susceptibility Testing of Filamentous Fungi. CLSI Standard M61.
30. CLSI. 2020. Epidemiological Cutoff Values for Antifungal Susceptibility Testing. CLSI Standard M59.
31. Sewell TR, Zhang Y, Brackin AP, Shelton JMG, Rhodes J, Fisher MC. Elevated prevalence of azole-resistant Aspergillus fumigatus in urban versus rural environments in the United Kingdom. Antimicrob Agents Chemother. 2019;63:e00548–19.
32. Ozmerdiven GE, Ak S, Ener B, et al. First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey. J Infect Chemother. 2015;21(8):581–586.
33. Klaassen CH, de Valk HA, Balajee SA, Meis JF. Utility of CSP typing to sub-type clinical Aspergillus fumigatus isolates and proposal for a new CSP type nomenclature. J Microbiol Methods. 2009;77:292–296.
34. Gao LJ, Sun Y, Wan Z, Li RY, Yu J. CSP typing of Chinese Aspergillus fumigatus isolates: identification of additional CSP types. Med Mycol. 2013;51:683–687.
35. Krishnan S, Manavathu EK, Chandrasekar PH. Aspergillus flavus: an emerging non- fumigatus Aspergillus species of significance. Mycoses. 2009;52:206–222.
36. Rudramurthy SM, Paul RA, Chakrabarti A, Mouton JW, Meis JF. Invasive Aspergillosis by Aspergillus flavus: epidemiology, Diagnosis, Antifungal Resistance, and Management. J Fungi. 2019;5(3):55.
37. Heo MS, Shin JH, Choi MJ, et al. Molecular identification and amphotericin B susceptibility testing of clinical isolates of Aspergillus from 11 hospitals in Korea. Ann Lab Med. 2015;35:602–610.
38. Zanganeh E, Zarrinfar H, Rezaeetalab F, et al. Predominance of non-fumigatus Aspergillus species among patients suspected to pulmonary aspergillosis in a tropical and subtropical region of the Middle East. Microb Pathog. 2018;116:296–300.
39. Al-Wathiqi F, Ahmad S, Khan Z. Molecular identification and antifungal susceptibility profile of Aspergillus flavus isolates recovered from clinical specimens in Kuwait. BMC Infect Dis. 2013;13:126.
40. Zarrinfar H, Saber S, Kordbacheh P, et al. Mycological microscopic and culture examination of 400 bronchoalveolar lavage (BAL) samples. Iran J Public Health. 2012;41:70–76.
41. Risum M, Hare RK, Gertsen JB, et al. Azole-Resistant Aspergillus fumigatus Among Danish Cystic Fibrosis Patients: increasing Prevalence and Dominance of TR34/L98H. Front Microbiol. 2020;11:1850.
42. Al-Bader N, Sheppard DC. Aspergillosis and stem cell transplantation: an overview of experimental pathogenesis studies. Virulence. 2016;7(8):950–966.
43. Takeda K, Suzuki J, Watanabe A, et al. High detection rate of azole-resistant Aspergillus fumigatus after treatment with azole antifungal drugs among patients with chronic pulmonary aspergillosis in a single hospital setting with low azole resistance. Med Mycol. 2021;59(4):327–334.
44. Cho SY, Lee DG, Kim WB, et al. Epidemiology and Antifungal Susceptibility Profile of Aspergillus Species: comparison between Environmental and Clinical Isolates from Patients with Hematologic Malignancies. J Clin Microbiol. 2019;57(7):e02023–18.
45. Moin S, Farooqi J, Jabeen K, Laiq S, Zafar A. Screening for triazole resistance in clinically significant Aspergillus species; report from Pakistan. Antimicrob Resist Infect Control. 2020;9(1):62.
46. Hsu TH, Huang PY, Fan YC, Sun PL. Azole Resistance and cyp51A Mutation of Aspergillus fumigatus in a Tertiary Referral Hospital in Taiwan. J Fungi. 2022;8(9):908.
47. Lv Q, Elders BBLJ, Warris A, Caudri D, Ciet P. Aspergillus-related lung disease in people with cystic fibrosis: can imaging help us to diagnose disease? Eur Respir Rev. 2021;30(162):210103.
48. Burks C, Darby A, Gómez Londoño L, Momany M, Brewer MT. Azole-resistant Aspergillus fumigatus in the environment: identifying key reservoirs and hotspots of antifungal resistance. PLoS Pathog. 2021;17(7):e1009711.
49. Cao D, Wang F, Yu S, et al. Prevalence of Azole-Resistant Aspergillus Fumigatus Is Highly Associated With Azole Fungicide Residues in the Fields. Environ Sci Technol. 2021;55(5):3041–3049.
50. Rhodes J, Abdolrasouli A, Dunne K, et al. Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat Microbiol. 2022;7(5):663–674.
51. Fuhren J, Voskuil WS, Boel CH, et al. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J Antimicrob Chemother. 2015;70(10):2894–2898.
52. Steinmann J, Hamprecht A, Vehreschild MJGT, et al. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother. 2015;70:1522–1526.
53. van Paassen J, Russcher A, In ‘t Veld-van Wingerden AW, Verweij PE, Kuijper EJ. Emerging aspergillosis by azole-resistant Aspergillus fumigatus at an intensive care unit in the Netherlands, 2010 to 2013. Euro Surveill. 2016;21(30):3456.
54. Montesinos I, Argudín MA, Hites M, et al. Culture-Based Methods and Molecular Tools for Azole-Resistant Aspergillus fumigatus Detection in a Belgian University Hospital. J Clin Microbiol. 2017;55(8):2391–2399.
55. Duong TN, Le TV, Tran KH, et al. Azole-resistant Aspergillus fumigatus is highly prevalent in the environment of Vietnam, with marked variability by land use type. Environ Microbiol. 2021;23(12):7632–7642.
56. Majima H, Arai T, Kusuya Y, et al. Genetic differences between Japan and other countries in cyp51A polymorphisms of Aspergillus fumigatus. Mycoses. 2021;64(11):1354–1365.
57. L R, Ninan MM, Kurien R, F NA, Sahni RD, Michael JS. Cryptic aspergillosis: a rare entity and a diagnostic challenge. Access Microbiol. 2022;4(4):000344.
58. Nematollahi S, Permpalung N, Zhang SX, Morales M, Marr KA. Aspergillus lentulus: an Under-recognized Cause of Antifungal Drug-Resistant Aspergillosis. Open Forum Infect Dis. 2021;8(8):ofab392.
59. Pérez-Cantero A, López-Fernández L, Guarro J, Capilla J. New Insights into the Cyp51 Contribution to Azole Resistance in Aspergillus Section Nigri. Antimicrob Agents Chemother. 2019;63(7):e00543–19.
60. Bader O. Phylogenetic Distribution of csp1 Types in Aspergillus fumigatus and Their Correlates to Azole Antifungal Drug Resistance. Microbiol Spectr. 2021;9(3):e0121421.
61. de Groot T, Meis JF. Microsatellite stability in STR analysis Aspergillus fumigatus depends on number of repeat units. Front Cell Infect Microbiol. 2019;9:82.
62. Chen Y, Lu Z, Zhao J, et al. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus isolates from China. Antimicrob Agents Chemother. 2016;60:5878–5884.
63. Falahatinejad M, Vaezi A, Fakhim H, et al. Use of cell surface protein typing for genotyping of azole-resistant and -susceptible Aspergillus fumigatus isolates in Iran. Mycoses. 2018;61:143–147.
64. Duarte-Escalante E, Frías-De-León MG, Martínez-Herrera E, et al. Identification of CSP Types and Genotypic Variability of Clinical and Environmental Isolates of Aspergillus fumigatus from Different Geographic Origins. Microorganisms. 2020;8(5):688.
65. Kidd SE, Nik Zulkepeli NA, Slavin MA, Morrissey CO. Utility of a proposed CSP typing nomenclature for Australian Aspergillus fumigatus isolates: identification of additional CSP types and suggested modifications. J Microbiol Methods. 2009;78:223–226.
66. Garcia-Rubio R, Gil H, Monteiro MC, Pelaez T, Mellado E. A New Aspergillus fumigatus Typing Method Based on Hypervariable Tandem Repeats Located within Exons of Surface Protein Coding Genes (TRESP). PLoS One. 2016;11(10):e0163869.
67. Camps SM, Rijs AJ, Klaassen CH, et al. Molecular epidemiology of Aspergillus fumigatus isolates harboring the TR34/L98H azole resistance mechanism. J Clin Microbiol. 2012;50(8):2674–2680.
68. Ashu EE, Korfanty GA, Xu J. Evidence of unique genetic diversity in Aspergillus fumigatus isolates from Cameroon. Mycoses. 2017;60:739–748.
69. Ballard E, Melchers WJG, Zoll J, Brown AJP, Verweij PE, Warris A. In-host microevolution of Aspergillus fumigatus: a phenotypic and genotypic analysis. Fungal Genet Biol. 2018;113:1–13.
70. Balajee SA, Tay ST, Lasker BA, Hurst SF, Rooney AP. Characterization of a novel gene for strain typing reveals substructuring of Aspergillus fumigatus across North America. Eukaryot Cell. 2007;6:1392–1399.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.