Back to Journals » Infection and Drug Resistance » Volume 16
Epidemiology and Mortality Analysis Related to Carbapenem-Resistant Enterobacterales in Patients After Admission to Intensive Care Units: An Observational Study
Authors Yoo EH, Hong HL , Kim EJ
Received 30 September 2022
Accepted for publication 17 December 2022
Published 7 January 2023 Volume 2023:16 Pages 189—200
DOI https://doi.org/10.2147/IDR.S391409
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Eun Hyung Yoo,1 Hyo-Lim Hong,2 Eun Jin Kim2
1Department of Laboratory Medicine, Daegu Catholic University School of Medicine, Daegu, Korea; 2Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
Correspondence: Eun Jin Kim, Department of Internal Medicine, Daegu Catholic University School of Medicine, 33, Duryugongwon-ro 17gil, Namgu, Daegu, 42472, Korea, Tel +82-53-650-4274, Fax +82-53-650-4942, Email [email protected]
Purpose: The prevalence of carbapenem-resistant Enterobacterales (CRE) is rapidly increasing worldwide. Patients in the intensive care unit (ICU) are susceptible to CRE infections, and the related mortality rate is increased. It is necessary to understand CRE strains and risk factors for CRE infection in the ICU, to facilitate development of effective prophylactic strategies and treatments for ICU patients.
Patients and Methods: This observational study was conducted in a tertiary hospital between 2016 and 2021. The subjects were patients with CRE cultured from specimens obtained after ICU admission. Genotypes of strains of CRE and carbapenemase-producing Enterobacterales (CPE) were identified, CRE infection was distinguished from mere colonization, and the clinical course of these patients was investigated.
Results: Among 327 CRE cases, 84 (25.7%) showed infection and 243 (74.3%) showed colonization. Of these patients, 138 (42.2%) died. The CRE strains were Klebsiella pneumoniae (253 cases, 77.4%), Enterobacter cloacae (44 cases, 13.5%), and Escherichia coli (15 cases, 4.6%). Among CRE cases, CPE was found in 249 (76.1%), including Klebsiella pneumoniae carbapenemase (KPC) in 164 (65.9%), and Guiana extended-spectrum (GES) in 64 (25.7%). A bedridden state, longer ICU stay, chronic kidney disease, malignancy, connective tissue disease, ICU admission for cardiac arrest, and CRE infection were associated with higher mortality, but cerebrovascular disease and ICU admission for trauma were associated with lower mortality. GES outbreak was caused by person-to-person transmission and was controlled through active surveillance.
Conclusion: The frequency of K. pneumoniae and KPC was the highest, but E. cloacae and GES was characteristically high in this study. Active CRE surveillance can be helpful for controlling outbreak.
Keywords: carbapenemase-producing Enterobacterales, genotypes, intensive care unit, Klebsiella pneumoniae carbapenemase, Guiana extended spectrum
Introduction
Carbapenems are recommended for the treatment of patients infected with Enterobacterales species producing extended-spectrum beta-lactamases. However, over time, carbapenem-resistant Enterobacterales (CRE) developed, greatly limiting the choice of antimicrobial treatments.1 The incidence of CRE is rapidly increasing worldwide. CRE infection is associated with high mortality and limited therapy options.1,2 In South Korea, since CRE were first reported in 2008, the incidence has increased annually, with 45,436 cases of CRE infection reported from 2017 to 2020.3
Several combined mechanisms underlie carbapenem resistance in CRE. These mechanisms include production of carbapenemase (carbapenemase-producing Enterobacterales [CPE]), modification of outer membrane permeability, and upregulation of efflux pump systems. In CPE, genes, including those encoding carbapenemases, are located on plasmids, by which the resistance gene is easily transmitted to other bacteria, contributing to the spread of CRE.4 The incidence rate of CRE is markedly higher than that of non-CPE.5 CRE, especially CPE, are usually spread from person to person through contact with an infected or colonized person, especially through contact with wounds or feces. This contact can occur through the hands of healthcare workers or medical equipment and devices that have not been properly cleaned.6 CRE also occurs endogenously through antibiotic selective pressure on the gut microbiome.7 In the intensive care unit (ICU) there is a lot of contact between patients and medical staff, and many antibiotics are used because of the high levels of disease severity. Therefore, since CRE can cause an outbreak of resistant bacteria, active monitoring and rapid identification of CRE, and appropriate infection control measures, are crucial. Currently, CRE is designated as a legal communicable disease in South Korea and active surveillance for CRE by means of culturing has been implemented since 2020.3
CRE affects severely ill patients with multiple comorbidities. Patients in intensive care units (ICUs) are particularly susceptible to CRE infection. The mortality rate among these patients is increased due to the high number and severity of underlying diseases, which leads to the use of antibiotics.8–10 Moreover, if CRE colonization is present in the ICU, the likelihood of CRE infection from colonization is at least twice as high as when it is absent.11 However, few studies have investigated how CRE colonization or infection is associated with mortality of CRE patients after ICU admission.
Therefore, we conducted this study to confirm the types of CRE strains and genotypes, clinical course of ICU patients with CRE acquisition, and how the course differs depending on infection or colonization. An improved understanding of the types of CRE and the risk factors of CRE-related mortality in the ICU may help in the development of effective prevention strategies and treatment of ICU patients.
Materials and Methods
Study Design
This observational cohort study was conducted at a tertiary teaching hospital in South Korea between January 2016 and December 2021. This hospital has 874 beds and four ICUs (medical ICU [MICU], surgical ICU [SICU], neurosurgery ICU [NSICU], and neonatal ICU). It is an affiliated community hospital in our area that receives many patients from nearby nursing homes and long-term care facilities.
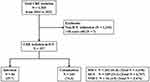
We enrolled patients aged ≥18 years from whom CRE were isolated from rectal swabs, blood, urine, sputum, or body fluid after ICU admission between January 2016 and December 2021 (Figure 1). The patients were admitted to the ICU from the emergency room or a ward according to the clinical judgment of each clinician. The MICU, SICU, and NSICU are composed of separate wards and closed ICU systems. The MICU and SICU are on the same floor and building, but the NSICU is located in the different floor and building. The total number of beds in all ICUs is 48 (MICU: 18 beds, SICU: 15 beds, and NSICU: 15 beds), of which 11 are separated single isolation beds (MICU: 3 beds, SICU: 6 beds, and NSICU: 2 beds).
At our hospital, when a patient is admitted to the ICU, CRE surveillance is routinely performed, using culture from rectal swabs. If the CRE culture was positive at the time of admission, the case was excluded from this study as it implied that the patient had previously harbored CRE and we wanted to monitor the prevalence of CRE acquisition after ICU admission and during ICU care.
After admission to the ICU, patients’ rectal swab samples were collected every Monday for follow-up CRE culture tests. In addition, according to the clinical condition of the patient, cultures were performed on sputum, blood, urine, and body fluids, and CRE were diagnosed according to the results. The type of carbapenemase was confirmed through polymerase chain reaction (PCR) testing for cases where CRE were cultured from specimens. For CRE culture-positive patients, medical records, including age, sex, comorbidities, prior exposure to healthcare facilities, Acute Physiology and Chronic Health Evaluation II (APACHE II) score on ICU admission, and carbapenem-use history were collected. In addition, data on the clinical course, such as length of stay in the ICU, time from ICU admission to positive CRE culture results, and in-hospital mortality, were recorded.
Definition of Terms
To define CRE, bacteria that were cultured from clinical specimens were identified, and antimicrobial susceptibility tests were performed. Among the identified Enterobacterales, the sample was defined as CRE-positive if the minimum inhibitory concentration was ≥4 µg/mL for imipenem or meropenem, or ≥2 µg/mL for ertapenem. The susceptibility breakpoints were interpreted according to recommendations by the Clinical and Laboratory Standards Institute (CLSI) M10012. The same MIC breakpoints for carbapenem were used throughout the entire study period.
We classified the presence of CRE as colonization or infection, according to the clinical situation in which CRE-positivity was identified. “Colonization” was defined in the following cases: confirmed as CRE in a sample that was not sterile (mainly when CRE were identified from rectal swabs only), absence of interaction between the host and the organism, like being found in or on the body without causing any symptoms or disease, lack of infection signs (fever, vital signs, inflammatory laboratory results) in the patient, and where no anti-CRE antibiotics were used. “Infection” was defined as cases in which bacteria were cultured from a sterile specimen (including blood, peritoneal fluid, bile fluid) or when the patient showed clinical signs and symptoms of infection, based on a combination of imaging, clinical, and laboratory criteria. In such cases, the patients were classified as having CRE infection based on the opinions of two experts, and antibiotics were used to treat the CRE infection.13,14
Infection Prevention and Control
As the number of CRE isolation cases increased, when CRE was confirmed, the patient was moved to an isolation bed in each ICU. If there were more CRE cases than prepared isolation rooms in the ICU, the patients were moved to the cohort beds area in the ICU. When contact with the patient was required, the medical staff wore gowns and gloves mandatorily. This was monitored and supervised by the infection control team. Handwashing by medical staff was further recommended and monitored. In addition, if an outbreak occurred, environmental culture was performed on walls, beds, and equipment near beds like ventilators, suction bottles, infusion pumps, and desks, to confirm the isolation of CRE from the environment. High- and low- touched surfaces and floors were cleaned and disinfected. Chlorhexidine patient bath of were administered at the onset of the outbreak, and medical staff tried to minimize invasive procedures and monitored antibiotics use. The infection control team educated medical staff, performed regular CRE surveillance, collected microbiological data, and monitored CRE outbreaks.
Ethics
The study protocol was approved by the institutional review board of Daegu Catholic University Medical Center (IRB No. CR-22-097). This study was conducted in accordance with the tenets of the Declaration of Helsinki. The requirement for obtaining informed patient consent was waived because of the retrospective study design. We have maintained confidentiality to prevent leakage of personal information.
Microbiological Definitions and Methods
Bacterial Culture and Antimicrobial Susceptibility Testing
We performed routine bacterial culture using clinical specimens and CRE screening culture from rectal swabs on ChromID CARBA agar (bioMérieux, Marcy l’Etoile, France). Bacterial identification was performed by using the Vitek 2 system (bioMérieux, Hazelwood, MO, USA), MicroScan (Beckman Coulter, Brea, CA, USA), or Vitek MS (bioMérieux, France) matrix-assisted laser desorption ionization-time of flight mass spectrometry systems according to the manufacturer’s instructions. Antimicrobial susceptibility tests were performed using modified broth microdilution tests with Vitek 2 AST-N224 cards or a MicroScan GN combo-72 panel. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used for quality control in antimicrobial susceptibility testing.
Rapid Carbapenemase Gene Assay
To detect carbapenemase genes in rectal swab specimens, PCR was performed using the Xpert® Carba-R assay (Cepheid, Sunnyvale, CA, USA). This cartridge-based real-time PCR assay detects and identifies the most prevalent carbapenemase genes (blaNDM, blaKPC, blaVIM, blaIMP-1, and blaOXA-48). For detecting Guiana extended-spectrum beta-lactamase (GES) type, genetic analyses (blaNDM, blaKPC, blaVIM, blaIMP-1, blaOXA-48, and blaGES) were conducted in the Institute.
Statistical Analysis
Normally distributed continuous variables are expressed as numbers and percentages, or as means, or as medians and interquartile ranges (IQRs), whereas non-normally distributed continuous variables are expressed as medians and IQRs. Quantitative results in the survival and death patient groups were compared using Mann–Whitney U-tests. Quantitative results in the MICU, SICU, and NSICU groups were compared using the Kruskal–Wallis test. Qualitative variables were compared between two or three groups using the chi-square (χ2) test. To identify the risk factors affecting survival among CRE acquisition cases in the ICU, univariate and multivariate logistic regression models were constructed and results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). All statistical analyses were performed using SPSS version 25 (SPSS, Inc., Chicago, IL, USA), and statistical significance was set at P <0.05.
Results
We identified 327 CRE-positive cases after admission to ICU, from January 2016 to December 2021. Among them, 84 cases (25.7%) were classified as CRE infection, and 243 cases (74.3%) were classified as CRE colonization. The highest rate of CRE-positivity occurred in the MICU (202 cases; 61.8%), followed by the SICU (109 cases; 33.3%), and the NSICU (16 cases; 4.9%) (Figure 1).
MICU patients had significantly higher age and APACHE-II scores than SICU or NSICU patients (P = 0.001 and <0.001, respectively). MICU patients had more carbapenem usage, longer hospital duration, and more cases of bedridden state than SICU or NSICU patients (P <0.001, <0.001 and <0.001, respectively) (Table S1).
Of the 327 patients, 138 (42.2%) died during hospital stay. The median age was 69 years, and males accounted for 66.4%. Of all the patients, 22.0% were referred from other hospitals, nursing homes, or long-term care facilities. Carbapenem use was recorded in 60.9% of all CRE cases, the median hospital stay was 54 days, and the median ICU stay was 23 days. The median time to the first CRE isolation after admission to the ICU was 17 days. At the time of admission to the ICU, 119 patients (36.5%) were bedridden. Hypertension was the most common comorbidity, followed by diabetes mellitus, heart disease, and cerebrovascular disease. Pneumonia or acute respiratory distress syndrome was the most common reason for admission to the ICU, followed by neuromuscular disease, cardiac arrest, trauma-related injuries and sepsis. In a comparison according to survival, the APACHE-II score was significantly higher (P = 0.005), and the bedridden state was more common (P <0.001) in the death group than in the survival group. The length of ICU stay was longer in the death than in the survival patients (P = 0.038), but the overall hospital length of stay was shorter in patients who died (P = 0.015). In terms of comorbidity, cerebrovascular disease was significantly more common in the survival than in the death group (P <0.001), while chronic kidney disease (CKD), malignancy, connective tissue disease, and immunocompromised conditions were significantly more common in the death than in the survival group (P = 0.033, 0.015, 0.019, and 0.028, respectively). When inspecting the reasons for ICU admission, cardiac arrest was more common among the death than among the survival group (P = 0.004), while trauma-related injury was significantly more common among the survival than among the death group (P = 0.004). Mortality rate was significantly higher among the cases of CRE infection than among those with colonization (P <0.001) (Table 1); 94.5% of all CRE-positive cases were over 50 years of age. There was no difference in the frequency of CRE isolation between the sexes (Table S2).
 |
Table 1 Clinical Characteristics of Total CRE Isolates and According to Survival in the Intensive Care Units |
Klebsiella pneumoniae was found in 253 cases (77.4%), Enterobacter cloacae in 44 cases (13.5%), and Escherichia coli in 15 cases (4.6%). There was no statistically significant difference in strain distribution between those who died and those who survived. When CRE were classified in terms of the specimen type from which they were isolated, rectal swab was the most common (172 cases; 52.6%), followed by sputum (110 cases; 33.6%), and blood (25 cases; 7.6%). In addition, CRE were isolated from peritoneal fluid, urine, catheter-related samples, and bile. There was a statistically significant difference in the positive specimen distribution between the survival and death groups (P = 0.046) (Table 2).
 |
Table 2 Distribution of Species and Specimen Types in Total CRE Isolates and According to Survival |
When the CRE strains were classified by the ICU, 90% were K. pneumoniae, 6% E. cloacae, and 3% E. coli in the MICU. However, in the SICU, K. pneumoniae was 56%, E. cloacae was 29%, and E. coli was 6%. In the NSICU, K. pneumoniae was 69%, Citrobacter freundii, and E. coli was 6%, respectively, showing a difference in the CRE strain distribution between the three units (P <0.001) (Figure 2).
 |
Figure 2 The prevalence of CRE according to intensive care units. Abbreviation: CRE, Carbapenem-resistant Enterobacterales. |
In terms of survival according to the CRE strain, K. pneumoniae and E. cloacae were associated with survival more than with death, whereas E. coli was associated more with death than with survival. However, there was no statistically significant difference in survival according to the type of CRE strain (P = 0.067) (Figure S1).
Among all isolated CRE, strains isolated from 249 individuals (76.1%) produced carbapenemase. In terms of CPE genotypes, K. pneumoniae carbapenemase (KPC) accounted for 65.9%, GES accounted for 25.7%, New Delhi metallo-β-lactamase (NDM) for 5.2%, and other genotypes for 3.2%. There was no difference in survival according to the genotype (Table 3). When CPE genotypes were classified by the ICU, 71% were KPC, 22% GES, and 4% NDM in the MICU. However, in the SICU, KPC was 55%, GES was 36%, and mixed genotypes were 4%. In the NSICU, KPC was 75%, NDM was 17%, and VIM was 8%, showing a difference in the CPE genotypes distribution between the three units (P <0.001) (Figure S2). The incidence of GES was also higher in the SICU than in other ICU types, according to the location of occurrence. GES accounted for 41.9% (13/31) of CRE in patients with chronic liver disease, which was higher than that of the other genotypes (P = 0.023). (Data not shown).
 |
Table 3 The Carbapenemase Genotypes by Carbapenemase-Producing Enterobacterales Isolates (N = 238, 75.5% of Total CRE Cases) |
Among cases with K. pneumoniae, 76.7% of strains produced carbapenemase, of which 83.0% were KPC, and 13.9% were GES. Among cases with E. cloacae, 79.5% of strains produced carbapenemase, all of which were of the GES type. Among cases with E. coli, 53.3% produced carbapenemase, of which 62.5% were mixed types (three cases of NDM and oxacillinase [OXA]-181 and two cases of NDM-5 and OXA-48). There was a significant difference in the distribution of these CRE strains and types of carbapenemase (P <0.001) (Table S3).
When the direct causes of death were investigated, CRE infection accounted for 27 cases (19.6%), and other causes for 111 cases (80.4%). The most common causes of death were sepsis caused by multidrug-resistant (MDR) pathogens or fungal infections (33 cases [23.9%]), respiratory failure (27 cases [19.6%]), cardiac failure or arrest (17 cases [12.3%]), multiorgan failure or suspension of life-sustaining treatment (9 cases [6.5%]), brain hemorrhage or death (8 cases [5.8%]), hepatic failure (6 cases [4.3%]), and cancer per se (4 cases [2.9%]) (Table 4).
 |
Table 4 Classification of the Main Cause of Death (N= 132 Cases, 41.9% of Total CRE Cases) |
In a multivariate analysis of survival, a bedridden state, length of ICU stay, CKD, malignancy, connective tissue disease, hospitalization for cardiac arrest, and CRE infection significantly increased the risk of death. Hospitalization for cerebrovascular disease or trauma was associated with a significantly lower risk of death (Table 5).
 |
Table 5 Univariate and Multivariate Analysis of Risk Factors Associated with Survival in Intensive Care Unit Patients with Carbapenemase-Producing Enterobacterales Isolates |
When checking the prevalence of the CPE genotype according to the month of occurrence and the ICUs, GES was characteristically high in this study, occurred and peaked from 2016, and then disappeared until early 2018. After that, KPC gradually increased and reached a peak. In addition, for both types, MICU had an outbreak first, followed by SICU (Figure 3). Active surveillance was implemented during the GES and KPC outbreaks. At the GES outbreak, environmental cultures were all negative. At the KPC outbreak, there were two cases of KPC CRE culture in 20 environmental cultures.
Discussion
In this study, we investigated the epidemiology, strains, and carbapenemase genotypes of CRE that occurred in patients after being admitted to the ICU over a 6-year period, from 2016 to 2021.
We found that the prevalent CRE strains and genotypes were different in each ICU. In particular, it was confirmed that GES genotype was prevalent in one ICU. The overall mortality rate from infection was high, with 38.4% of patients dying after acquiring CRE in the ICU. The development of CRE into infection itself affects ICU mortality.
In this study, compared to other ICUs, the frequency of CRE isolates was the highest in MICU which had patients with higher age and APACHE-II scores, higher number of patients in a bedridden state, and more carbapenem exposure. In a previous study,15 the APACHE-II score, the need for additional antibiotic exposure, and the requirement for invasive treatment were higher in cases with “CRE acquisition” than in those with “no CRE acquisition.”
In our study, cases classified as CRE infections accounted for 25.7% of all CRE cases in ICUs. This was higher than the overall risk of systemic CRE infection over colonization in a meta-analysis of 16.5%,10 which may be because our study was ICU-based. In other ICU-based studies, carbapenem-resistant K. pneumoniae carriers had a 29–73% chance of developing an infection.8,9,16 In our study, 94.5% of all CRE cases occurred in people >50 years of age, which is consistent with other domestic studies showing that the frequency of CRE is high in older individuals (>60 years of age).15,17
In our study, the death rate among CRE cases was 42.2%, which was similar to that reported in other ICU studies.9,11 It was also similar to the crude mortality rate of diverse infections caused by carbapenem-resistant K. pneumoniae (30.1–44%) in other studies of hospitalized patients.13,18,19 Our study confirmed the mortality associated with all strains of CRE. Other studies differed from ours in that they examined the mortality associated with carbapenem-resistant K. pneumoniae, the most common type of CRE. In our study, the mortality rate associated with “infection” was 63.1% (53/84), but that associated with colonization was 35.0% (85/243). A pooled analysis from three studies10 found that mortality in colonized or infected patients reached 10%; however, in those who developed infection, mortality was very high, ranging from 30% to 75%. These points indicate the risk of developing an infection, and the importance of not only preventing colonization (CRE acquisition), but also preventing this from developing into an infection.
The length of ICU stay was longer in patients who died, but the overall hospital length of stay was shorter in patients who died. When CRE was acquired and then patients died, it often led to death of the patient while in the ICU. In contrast, patients who survived were transferred to the general ward where they remained for a long time. Thus, CRE infection eventually increased hospital costs among survivors.
Compared to the distribution of CRE strains (E. coli: 18%) isolated from 2017 to 2020 in our country,3 the frequency of E. coli in our hospital was low, at 4.6%. When the prevalence of CRE strains was classified by ICUs, the MICU and SICU showed a difference in the distribution of CRE strains, indicating that the prevalence of different strains may vary across hospitals and even across units in one hospital. CRE were most often isolated from rectal swabs, because of CRE surveillance based on rectal swab cultures. The next most common specimens from which CRE were isolated were sputum 33.6% and blood (7.6%). There was a difference in mortality depending on the specimen in which the CRE were identified. The CRE-positivity rate in cultures from blood was high among those who died, whereas that in urine cultures was high among those who survived. Among the CRE strains, E. coli was associated with a high mortality rate. However, as only 15 cases were identified with E. coli CRE, this result should be interpreted with caution.
Similar to the finding that CPE constituted 73.9% of CRE in the Korea Centers for Disease Control and Prevention Epidemiologic Management Report,3 in our hospital, CPE constituted 76.1% of CRE. The acquisition of either KPC or GES carbapenemase genes, was responsible for phenotypic resistance in 91.6% of the CPE strains tested (65.9% and 25.7%, respectively) in our hospital. We found higher GES frequencies than previous studies.15,20–22 GES was the second most common type of CPE, found in 64 cases (25.7%). According to a 2016 report23 in Korea, KPC was the most common type (70.7%), followed by NDM (13.5%), OXA-48 (9.6%), and GES (3.1%). In a report published in Korea between 2017 and 2020,3 KPC was the most common type (75.4%), followed by NDM (18.0%), mixed type (3.6%), and OXA (2.4%), whereas GES was found in only 0.2% of cases. In a CRE epidemiologic study conducted in a single large city, GES was found in only three (0.2%) of 1468 CRE isolates.17 This suggests that, since the CPE genotypes prevalent in each hospital may be different, it is necessary to identify the relevant genotypes in each locale. In our study, 96.9% of GES types were detected in E. cloacae and K. pneumoniae. To date, no studies have elucidated the clinical significance of GES. In one study,24 multispecies clusters of GES-5 cases occurred for 18 weeks 1 year after K. oxytoca, a GES-5 positive CRE, was first discovered. Detection of GES-5 was initially delayed because of its low carbapenem minimum inhibitory concentration. Most commercial molecular assays do not include the evaluation of GES. This suggests a possible under-diagnosis of GES CRE. In our study, GES CRE was evaluated by genetic analysis at the Health and Environmental Research Institute. This suggests the need for continued use of routine culture and follow-up investigations to discover novel or uncommon resistance mechanisms. In our study, at 41.9% (13/31) (P = 0.023), GES was significantly more common in patients with chronic liver disease than were other genotypes and was significantly more common in the SICU than in other ICUs (P <0.001). We found that GES was prevalent in liver transplantation patients in the SICU.
In our study, CRE infection was directly related to 19.6% of all deaths. However, death by other concomitant MDR bacteria or fungal infections in the blood accounted for 23.9% of all deaths, indicating that deaths from infection in the ICU accounted for 43.5% of all deaths. The risk of death was higher in bedridden patients, those with a longer ICU stay, with CKD, malignancy, or connective tissue disease, with ICU admission for cardiac arrest, and among those classified with CRE infection. Conversely, patients with cerebrovascular disease and those admitted to the ICU for trauma had a lower mortality risk. In one study, disease severity and previous hospitalization, as well as admission to the MICU only, predicted 90-day mortality.16 Taken together with our findings, surgical problems, such as trauma and cerebrovascular disease, seemed to have little impact on mortality.
When looking at the prevalence graph of GES or KPC in our study (Figure 3), it can be seen that an outbreak first occurs in MICU with high severity and carbapenem usage, followed by an outbreak in SICU which is on the same floor in the same building. Although the onset of CRE occurrence may be endogenous, the eventual transmission of CRE suggests the possibility that it has become transmissible through person-to-person contact or environmental contamination. More specifically, the fact that environmental culture was negative at the time of the GES outbreak suggests person-to-person transmission. During the study period, we performed active CRE surveillance, and considered hand hygiene to be the most important factor to transmit CRE. The GES outbreak ended in early 2018, and active CRE surveillance was a useful strategy for controlling the spread of CRE in the ICU. In contrast, KPC is the most frequently occurring CRE worldwide and appears to have been introduced to our hospital from other hospitals and continues to be transmitted within the hospital.
Our study had several limitations. Because many factors are involved in ICU patients’ condition, it was impossible to analyze all relevant factors. We therefore selected and investigated factors that could affect CRE based on several previous studies. Thus, selection bias may have occurred in this process. Our study was a retrospective single-center cohort study, and there may be limitations associated with the retrospective study design and those associated with a single-center study. Since the CPE genotypes prevalent during the study period were differed across different ICUs, it was not possible to generalize the risk according to the occurrence of the genotypes, and there are limitations to this.
Conclusion
In conclusion, CRE can be a major source of infection in critically ill patients, and the epidemiology may differ across hospitals and across units in a single hospital. Unlike other studies in Korea3,15 and those in other countries,21,22 our study differs in that the frequency of E. coli was low, that of E. cloacae was high, and that of the GES type of carbapenemases was high, suggesting that different hospitals showed different epidemiology. Looking at the transmission patterns of GES, person-to-person transmission is considered to be the most important in the CRE transmission in the ICU environment. Active surveillance can be helpful strategy to control the transmission of CRE in the ICU. In addition, we found that if colonization developed into an infection, the risk of death increased. This implies that preventive measures are required to avoid this transition.
Our findings emphasize the need for each hospital and unit to understand the prevalent CRE epidemiology and to conduct infection control activities to address risk.
Abbreviations
APACHE II, Acute Physiology and Chronic Health Evaluation II; CI, confidence interval; CKD, chronic kidney disease; CPE, carbapenemase-producing Enterobacterales; CRE, carbapenem-resistant Enterobacterales; GES, Guiana extended-spectrum beta-lactamase; ICU, intensive care unit; IQRs, interquartile ranges ; KPC, Klebsiella pneumoniae carbapenemase; MDR, multi-drug resistant; MICU, medical intensive care unit; NDM, New Delhi metallo-β-lactamase; NSICU, neurosurgery intensive care unit; OR, odds ratio; OXA, oxacillinase; PCR, polymerase chain reaction; SICU, surgical intensive care unit.
Author Contributions
Conceptualization: EH Yoo and EJ Kim. Data curation: EJ Kim. Formal analysis: EJ Kim. Funding acquisition: EJ Kim. Methodology: EH Yoo, H Hong, and EJ Kim. Project administration: EJ Kim. Visualization: EJ Kim. Writing – original draft: EJ Kim. Writing – review & editing: EH Yoo, H Hong, and EJ Kim.
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by a grant from the Research Institute of Medical Science of Daegu Catholic University (2021).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9(4):228–236. doi:10.1016/S1473-3099(09)70054-4
2. Zhang Y, Wang Q, Yin Y, et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: report from the China CRE Network. Antimicrob Agents Chemother. 2018;62(2):e01882–17. doi:10.1128/AAC.01882-17
3. Joo S, Kim M, Shin E, Kim J, Yoo J. Molecular characteristic analysis and antimicrobial resistance of carbapenem-resistant Enterobacteriaceae (CRE) isolates in the Republic of Korea, 2017-2020. Public Health Weekly Rep. 2021;14(53):3799–3804.
4. Sawa T, Kooguchi K, Moriyama K. Molecular diversity of extended-spectrum β-lactamases and carbapenemases, and antimicrobial resistance. J Intensive Care. 2020;8:13. doi:10.1186/s40560-020-0429-6
5. Kopotsa K, Osei Sekyere J, Mbelle NM. Plasmid evolution in carbapenemase-producing Enterobacteriaceae: a review. Ann N Y Acad Sci. 2019;1457(1):61–91. doi:10.1111/nyas.14223
6. Collins AS. Preventing Health Care–Associated Infections. In: Patient Safety and Quality. An Evidence-Based Handbook for Nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008:chap 41.
7. Goodman KE, Simner PJ, Tamma PD, Milstone AM. Infection control implications of heterogeneous resistance mechanisms in carbapenem-resistant Enterobacteriaceae (CRE). Expert Rev Anti Infect Ther. 2016;14(1):95–108. doi:10.1586/14787210.2016.1106940
8. Debby B, Ganor O, Yasmin M, et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31(8):1811–1817. doi:10.1007/s10096-011-1506-5
9. Papadimitriou-Olivgeris M, Marangos M, Fligou F, et al. Risk factors for KPC-producing Klebsiella pneumoniae enteric colonization upon ICU admission. J Antimicrob Chemother. 2012;67(12):2976–2981. doi:10.1093/jac/dks316
10. Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: a systematic review. Am J Infect Control. 2016;44(5):539–543. doi:10.1016/j.ajic.2015.12.005
11. Dickstein Y, Edelman R, Dror T, Hussein K, Bar-Lavie Y, Paul M. Carbapenem-resistant Enterobacteriaceae colonization and infection in critically ill patients: a retrospective matched cohort comparison with non-carriers. J Hosp Infect. 2016;94(1):54–59. doi:10.1016/j.jhin.2016.05.018
12. Lewis JS. Performance Standards for Antimicrobial Susceptibility Testing.
13. Borer A, Saidel-Odes L, Eskira S, et al. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J Infect Control. 2012;40(5):421–425. doi:10.1016/j.ajic.2011.05.022
14. CDC/NHSN. Surveillance Definitions for Specific Types of Infections. CDC/NHSN. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
15. Kang JS, Yi J, Ko MK, Lee SO, Lee JE, Kim KH. Prevalence and Risk Factors of Carbapenem-resistant Enterobacteriaceae Acquisition in an Emergency Intensive Care Unit in a Tertiary Hospital in Korea: a Case-Control Study. J Korean Med Sci. 2019;34(18):e140. doi:10.3346/jkms.2019.34.e140
16. McConville TH, Sullivan SB, Gomez-Simmonds A, Whittier S, Uhlemann A-C. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS One. 2017;12(10):e0186195. doi:10.1371/journal.pone.0186195
17. Park SH, Park SH, Kim JS, et al. Genetic Distribution of Carbapenem-Resistant Enterobacteriaceae in Seoul Korea, 2018~2020. J Bacteriol Virol. 2022;52(1):28–38. doi:10.4167/jbv.2022.52.1.028
18. Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52(3):1028–1033. doi:10.1128/AAC.01020-07
19. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Infection and the Impact of Antimicrobial and Adjunctive Therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099–1106. doi:10.1086/592412
20. Kim YA, Park YS. Epidemiology and treatment of antimicrobialresistant gram-negative bacteria in Korea. Korean J Intern Med. 2018;33(2):247–255. doi:10.3904/kjim.2018.028
21. Dautzenberg MJD, Wekesa AN, Gniadkowski M, et al. The association between colonization with carbapenemase-producing Enterobacteriaceae and overall ICU mortality: an observational cohort study. Crit Care Med. 2015;43(6):1170–1177. doi:10.1097/CCM.0000000000001028
22. Wang Q, Wang X, Wang J, et al. Phenotypic and Genotypic Characterization of Carbapenem-resistant Enterobacteriaceae: data From a Longitudinal Large-scale CRE Study in China (2012-2016). Clin Infect Dis. 2018;67(suppl_2):S196–S205. doi:10.1093/cid/ciy660
23. Lee HJ, Lee DG. Carbapenem-resistant Enterobacteriaceae: recent updates and treatment strategies. J Korean Med Assoc. 2018;61(4):281–289. doi:10.5124/jkma.2018.61.4.281
24. Ellington MJ, Davies F, Jauneikaite E, et al. A Multispecies Cluster of GES-5 Carbapenemase-Producing Enterobacterales Linked by a Geographically Disseminated Plasmid. Clin Infect Dis. 2020;71(10):2553–2560. doi:10.1093/cid/ciz1130
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.