Back to Journals » Cancer Management and Research » Volume 15
Enhanced Glycolysis Confers Resistance Against Photon but Not Carbon Ion Irradiation in Human Glioma Cell Lines
Authors Vashishta M, Kumar V, Guha C, Wu X , Dwarakanath BS
Received 18 August 2022
Accepted for publication 17 December 2022
Published 4 January 2023 Volume 2023:15 Pages 1—16
DOI https://doi.org/10.2147/CMAR.S385968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Mohit Vashishta,1– 4 Vivek Kumar,1– 3 Chandan Guha,5 Xiaodong Wu,1– 3 Bilikere S Dwarakanath1– 3,6,7
1R&D Department, Shanghai Proton and Heavy Ion Center (SPHIC), Shanghai, People’s Republic of China; 2Shanghai Key Laboratory of Radiation Oncology (20dz2261000), Shanghai, People’s Republic of China; 3Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai, People’s Republic of China; 4Rangel College of Pharmacy, Texas A&M University, College Station, TX, USA; 5Albert Einstein College of Medicine, The Bronx, NY, USA; 6Central Research Facility, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai India; 7Indian Academy Degree College Autonomous (IADC-A), Bengaluru, Karnataka, India
Correspondence: Bilikere S Dwarakanath, Indian Academy Degree College Autonomous (IADC-A), 230, Hennur Main Rd, Meganahalli, Kalyan Nagar, Bengaluru, Karnataka, 560043, India, Tel +91 9952081077, Email [email protected]
Purpose: Metabolic reprogramming is a key hallmark in various malignancies and poses a challenge in achieving success with various therapies. Enhanced glycolysis is known to confer resistance against photon irradiation while the tumor response to carbon ion irradiation (CII) has not been investigated. This study aimed to investigate the effects of enhanced glycolysis on the response of human glioma cell lines to CII compared to the response to X-rays.
Material and Methods: Glycolysis was stimulated using Dinitrophenol (DNP), a mild OXPHOS inhibitor, in three human glioma cell lines (U251, U87, and LN229) and assessed by monitoring glucose uptake and utilization as well as expression of regulators of glycolysis (glucose transporter protein type 1(Glut1), hexokinase-II (HKII), and Pyruvate Kinase-2 (PKM2). Radiation (X-rays and CII) induced loss of clonogenic survival growth inhibition and perturbations in cell cycle progression (G2+M block), cytogenetic damage (micronuclei formation), apoptosis, necrosis (reflecting interphase death), and cell migration (Scratch assay) were investigated as parameters of radiation response.
Results: DNP (1 mM) enhanced the expression levels of GLUT1, HKII, and PKM2 by 30– 60% and glucose uptake as well as usage by nearly 3 folds in U251 cells suggesting the stimulation of glycolysis. Enhanced glycolysis attenuated the loss of clonogenic survival with D10 doses increasing by 20% to 65% in these cell lines, while no significant changes were noted following CII. Concomitantly, dose-dependent growth inhibition, and cytogenetic damage as well as apoptosis and necrosis induced by X-rays were also reduced by elevated glycolysis in U251 and LN229 cells by 20– 50%. However, stimulation of glycolysis enhanced the X-ray-induced cell migration, while it had negligible effect on migration following CII.
Conclusion: Our results suggest that enhanced glycolysis confers resistance against X-ray-induced cell death and migration, while it may not significantly alter the cellular responses to carbon ion irradiation.
Keywords: carbon ion radiotherapy, metabolic-reprogramming, radio-resistance, Warburg effect, glucose metabolism, X-rays irradiation
Plain Language Summary
Radiotherapy is widely used for treating more than 50% of all malignancies. A major challenge in achieving success in radiotherapy is the development of radioresistant cancer cells partly linked to a reprogramming of the metabolism in the form of enhanced glucose dependency and utilization called the “Warburg effect” that occurs in most tumors. Treatment of tumors with a form of particle therapy using carbon ion- has been shown to be more effective against solid tumors.
Here, in this work, we have for the first time investigated the response of three human brain tumor (glioma) cell lines (U251, LN229, and U87) stimulated for enhanced glucose utilization (using OXPHOS modifier 2-dinitrophenol, DNP) to carbon ion irradiation (CII). Our results show that stimulation of glycolysis reduces radiation-induced cell death (mitotic, apoptotic and necrotic) growth inhibition in all three glioma cell lines, while it did not alter these responses significantly in carbon ion irradiated cells. Stimulation of glycolysis enhanced the X-ray-induced cell migration, while it did not alter the otherwise negligible migration induced by CII. Collectively, results of this study indicate that metabolic reprogramming associated with most tumors may not compromise the efficacy of carbon ions based radiotherapy of tumors unlike the well-established resistance it offers against photon-based radiotherapy.
Introduction
Reprogramming of metabolism mainly in the form of enhanced aerobic glycolysis, referred to as the Warburg phenotype,1 is observed across many, if not all, malignancies and is now recognized as one of the hallmarks of cancer.2,3 In cancer cells, the Warburg effect allows for a higher cell proliferation rate, pro-survival in hypoxic conditions, and avoiding immune surveillance with altered metabolic shift.4 Glyco-metabolic reprogramming driven by both intrinsic and extrinsic factors facilitates tumor progression, and metastasis, and confers resistance against many therapies including photon (X-rays, gamma rays, and electron beam) based radiotherapy.4–6 Consequently, the glycolytic inhibitor and glucose analog 2-deoxy-D-glucose (2-DG) have been shown to enhance the efficacy of gamma-ray-induced cell death and growth inhibition in a variety of human tumor cell lines and animal tumors.7,8 Inhibition of DNA repair enhanced metabolic oxidative stress and induced mitotic as well as interphase death, besides alterations in unfolded protein response have been shown to mainly contribute to the enhanced radiation and chemotherapeutic drug-induced tumor cell death.9,10 Moreover, 2-DG has been shown to enhance antitumor immunity by attenuating the immune suppressive network and macrophage polarization thereby contributing to the local tumor control in murine tumors.11,12 A combination of systemically administered 2-DG with hypo-fractionated radiotherapy has not only been found to be well tolerated by patients with malignant glioma but is also associated with minimal acute and late toxicity, besides providing improved quality of life.13–17
Local recurrence after RT is a major challenge in the treatment of solid tumors.18 Radioresistant tumor cells exhibit altered metabolic modulation, such as enhanced glycolytic flux, overexpression of glycolytic markers, diversion of glycolytic intermediates, high ATP production, and accumulation of lactate.19 Furthermore, these metabolic changes contribute to increased cell proliferation and therapy-resistant cancer progression.20
Photon radiotherapy together with temozolomide is widely employed in the treatment of high-grade gliomas. However, intrinsic and therapy-induced radioresistance limits the efficacy.21–23
The relative biological efficacy of radiotherapy using charged particles, especially using heavy ions (typically carbon) is higher than photon-based radiotherapy.24 The clinical superiority of carbon ion radiotherapy (CIRT) has been mainly attributed to its physical property and ballistic advantages when compared to photons, thus leading to favourable dose distributions in the tissue (in the spread-out Bragg peak; SOBP) and sparing of organs at risk (OAR).25–27
However, the potential differences in biological responses to CIRT as compared to photon-based radiotherapy require a great deal of understanding to derive maximum benefit from this form of radiotherapy.
Recent studies have shown that transient stimulation of glycolysis using mild uncouplers of oxidative phosphorylation (using dinitrophenol; DNP) confers resistance against photon irradiation in human glioma and squamous carcinoma cell lines,28 which is in line with the metabolic-reprogramming linked resistance of tumors to photon-based radiotherapy.29,31
However, the impact of enhanced glycolysis on the tumor cell responses to CIRT has not been investigated in much detail, although limited studies were carried out in Hela cell line.32 Therefore, in the present studies, we investigated the impact of enhanced glycolysis on the effects of transient stimulation of glycolysis (using DNP) on the response of three human glioma cell lines to CII by analyzing the radiation-induced cell death and migration that are important in determining the local tumor control and invasive behavior of gliomas. Our results show that elevation of glycolysis confers resistance against photon, but not carbon ion-irradiation induced cell death. Moreover, enhanced glycolysis accentuated photon-induced cell migration, while this effect was not observed by carbon ion irradiation.
Materials and Methods
Materials
Primary antibodies like Lactate dehydrogenase (LDHA Cat# 2012S), PKM-2 (Cat# 3198S), and hexokinase-2 (cat # 2867S) were purchased from Cell signaling technologies, Massachusetts, USA, while anti-actin (sc-47778), was purchased from Santa Cruz Biotechnology Inc., Dallas, TX, USA. Secondary antibodies like anti-rabbit HRP conjugated monoclonal antibody (cat# sc-2357) was purchased from Santa Cruz, Dallas, TX, USA, and goat anti-mouse IgG2b HRP conjugated antibody (cat# 43593) was purchased from cell signaling technology, Danvers, MA, USA. 2,4-Dinitrophenol (DNP) was a kind gift from Prof. Anant Bhatt. Propidium iodide (cat#ST511), Bradford reagent-G250 (cat#P0006C-1), PCR-based mycoplasma detection (cat# D7228) kit, Hoechst 33258 dye (cat#C1017), crystal violet staining solution (cat# C0121), trypsin with 0.25% EDTA (cat# C0201), TEMED (cat#ST728), Pen-strep (100x) (cat#C0224), and Annexin-V FITC apoptosis kit (cat#C1062S, Beyotime Biotechnology, Shanghai, China) were purchased from (Beyotime-Biotechnology, Shanghai, China). Cytochalasin B (cat#abs820868-1mg), was purchased from Absin Bioscience Inc. Shanghai, China. Dulbecco’s modified essential medium (DMEM) with high glucose (cat# 11965–084), fetal bovine serum (FBS) (cat# SV30087, Gibco, New York, USA), Glucose colorimetric assay kit (cat# 10009582, Cayman Chemical, Michigan, USA), ClarityTM Western ECL Substrate, 200 mL (cat#1705060 BioRad Laboratories Inc., California, USA), polyvinylidene fluoride PVDF Blotting membrane (Cat# H10600029) Amersham Hybond P-0.45 PVDF (GE healthcare Life Sciences, Freiburg, Germany). Bovine serum albumin (cat# B2064-100G) of Australian origin was purchased from Sigma-Aldrich, Burlington, MA, USA.
Cell Culture
Human glioma cell lines (U87 (ATCC # HTB-14), and LN229 (ATCC # CRL-2611)) were purchased from the American Type Culture Collection, while the U251 cell line was purchased from Procell (Wuhan, China). All the cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% (v/v) fetal bovine serum and 1% antibiotic solution (penicillin and streptomycin). All cells were plated at a density of 8000 to 12,000 cells/cm2 in 25 cm2 culture flasks, incubated at 37°C, with 5% CO2 in a humidified incubator, and were passaged every 3–4 days. Cell lines were cultured in this study for less than 6 months after resuscitation and were deemed free of Mycoplasma contamination by in-house testing using the PCR-based mycoplasma detection kit (cat#C0301S, Beyotime-Biotechnology, China).
Irradiation (Carbon and X-Ray)
Irradiation of cell lines was performed in standard 25 cm2culture flasks (for CII) or 60mm culture dish (for X-rays). A 225 kVp X-ray (13.30 mA) beam filtered with 2 mm AI by a XRAD225 from PXI Precision irradiator (Ge Inspection Technologies Shimadzu, Japan) at a dose rate of 3.2 Gy/min ± 0.02 was used for X-ray irradiation. Carbon Ion Irradiation was done using heavy ion synchrotron accelerator (Siemens, AG) (IONTRIS intensity modulated raster scan system) at SPHIC as described before.33 Briefly, CII was delivered as a homogeneous extended Bragg peak with energy of 333.82 MeV/u. An advanced Markus chamber (TM34045, PTW, Germany) was used to verify the delivered dose at the cell layer. The delivered doses at the cell layer were verified by using an advanced Markus chamber. TRS-398 was used to calibrate the chamber. The dose averaged LET at the cell layer was calculated by using in-house software. The dose averaged linear energy transfer; (LETd) was 56.37 keV/μm on the target. The irradiation was done at room temperature. It has to be emphasized that the accelerator beam time was very limited which restricted the number of independent experiments.
Clonogenic Survival Assay
Exponentially growing cells were irradiated and incubated for 4 h with low glucose DMEM without serum. Cells were then plated followed by trypsinization with 200, 600, and 1800 cells in 60 mm diameter Petri-dishes 4 h post-irradiation. Plated cells were allowed to form colonies for 8–12 days at 37°C in humidified 5% CO2 atmosphere after the treatment. Colonies were fixed in PBS: methanol (1:1) and stained with 1% crystal violet for 10 minutes at room temperature followed by washing with running distilled water. Colonies containing more than 50 cells were counted. Images of each group were captured by a colony counting machine (Gel-Count, Oxford Optronix Ltd.).
Cell Lysate and Western Blotting
Briefly, after irradiation, cells were washed off with ice cold PBS and scrapped off the culture flask. The cell suspension was centrifuged at 1000 x g at 4°C for 15 minutes. Cell pellet was collected and the supernatant was discarded. The cell pellet was again washed twice with ice-cold PBS and chilled on ice for 30 minutes with RIPA cell lysis (cat#A32955) buffer supplemented with protease inhibitors (cat#A32955, Thermo Scientific, USA). The supernatant (whole cell extract) was collected and stored at −80°C followed by centrifugation at 10,000 x g for 15min at 4°C. The whole cell extract (30μg) was resolved on 10% SDS-PAGE and transferred onto a PVDF membrane (Hybond C pure, Amersham Biosciences, USA). The blots were blocked with 5% bovine serum albumin (BSA) for 1 hr at room temperature. The primary antibody (1:1000) with 3% BSA in TBST was incubated at 4°C overnight, followed by washing with TBST (TBS with 0.1% Tween 20). A secondary antibody tagged with horseradish peroxidase (1:2000) was incubated at room temperature for 90 minutes. Furthermore, for loading control β-actin was probed. The blots were developed by chemiluminescence using the luminol reagent (ClarityTM Western ECL Substrate, 200 mL (cat#1705060 BioRad Laboratories Inc., USA) with Chemidoc Image system, Bio-RAD gel doc system).
Estimation of Glucose
The levels of glucose were estimated with the Glucose colorimetric assay kit (cat#10009582, Cayman Chemical, Michigan, USA). U251 cells were incubated with the serum free medium (low glucose DMEM) with DNP-1 µM for 4 h. The spent medium was collected and centrifuged at 11,200 x g for 10 minutes at 4°C and stored in a −80°C deep freezer. The glucose level was estimated, as per the manufacturer’s instructions.
2-NBDG Uptake Assay
2-NBDG is a fluorescent derivative of glucose whose uptake is inhibited by D-glucose. Cells were plated in 25cm2 culture flasks and allowed to attach and grow for 36–44 h. Cells were then incubated in a medium containing 1% serum and 10 µM of 2-NBDG for 5 minutes at 37°C in an incubator with 5% CO2. Cells were then washed with PBS twice and trypsinized to a single-cell suspension in flow buffer (1% BSA in PBS). The fluorescence was measured by a Flow cytometer with an absorption wavelength of 488nm. The data were analyzed with the Flow Jo_10 version.
Micronuclei Analysis
Irradiated cells were incubated with Cytochalasin-B (2.5 µM/L) for 36 h and were fixed with 70% ethanol overnight. Fixed cells were washed with ice-cold PBS and incubated in Carnoy’s solution (methanol and acetic acid solution (3:1)) for 15 minutes at room temperature, and air-dried overnight. Acetic acid-methanol fixed cells were stained with a DNA-specific fluorochrome, Hoechst 33258 dye (Beyotime Biotechnology, China), followed by PBS wash (twice) at room temperature, and approximately 1000 cells were analyzed from duplicate slides to count the micronuclei. Data were analyzed by obtaining integrated values of micronuclei frequency and normalizing the values with respect to cell numbers as described earlier.34 The frequency of cells with micronuclei called the M-fraction (MF) was calculated as MF (%) = Nm/Nt x 100, where Nm is the number of cells with micronuclei and Nt is the total number of cells analyzed.
Cell Growth Inhibition and Cell Cycle Distribution Analysis
Cells were cultured for 36–40 h before irradiation, and were harvested by trypsinization at 48 h following irradiation. The cell number was enumerated with the help of a cell hemocytometer. The extent of proliferation was assessed by calculating the proliferation index (Px) as follows:
Proliferation index Px = Nt / N0;
where Nt is the cell number at different post-irradiation times and N0 is the number at the time of plating. Cells were fixed with ice-cold 80% ethanol and stored at 4°C for at-least 24 h before analyzing the DNA content by flow cytometry. Ethanol fixed cells were washed twice with PBS and incubated with RNAase-A (cat# 10109142001, Sigma, USA) for 45 minutes at 37°C. Cellular DNA was stained with propidium iodide (25 µg/mL) (0.1 x 106 cells/100µL of PBS) and incubated in dark at room temperature for 30 minutes. PI-stained cells were then subjected to flow cytometry with CytoflexS (Beckman Coulter, California, USA). All the data was acquired with CytExpert software and was provided with a cytometer. Acquired data were analyzed with the FlowJo_10.4 version. The percentages of cells in G0/G1-, S-, and G2+M phases were determined, after filtering for doublets and aggregates using the inbuilt software.
Scratch Assay
Migration of the cells was assessed by the in vitro scratch assay of the irradiated. In brief, a total of 0.2×106 cells were seeded with DMEM with 1% of serum in 24-well plates and allowed to form a 70–80% confluent monolayer for 8–10 h. Scratch was made using a sterile 1000 µL pipette tip followed by washing the monolayer cells with sterile PBS removing the disloged cells. The cells were allowed to grow for 18 h with DMEM with 1% of serum. Bright-field microphotographs were taken at 0 h and 18 h after scratching. The percentage of migration of irradiated cells was quantitated by measuring the surface area of the cell-free zone immediately after making the scratch at 0 h and at 18 h later using image analysis software (Image J2). The difference was calculated as rate of migration and normalised it with area covered by untreated group from the 5 microphotographs captured for each group by Image J2 software. The readings of distance of each sample were measured and repeated three times. Rate of migration relative to unirradiated control groups were assessed and quantified by GraphPad Prism 8.0 software.
Apoptosis Assay
Radiation induced apoptosis was evaluated with Annexin-V/Pi staining (Beyotime Biotechnology, Shanghai, China). Following Irradiation, cells were trypsinized and plated for 48 h, as 50,000 cells per cm2 in a 35mm culture dishes. After incubation, cells were trypsinized and suspended in flow buffer (0.5% BSA in 1x PBS) and washed with PBS twice at 1000 x g for 5 minutes. Annexin-V staining was done as per the manufacturer’s instructions. Briefly, 0.1×106 cells were resuspended in the 195µL binding buffer with 10µL of Annexin-V and 5µL of PI (propidium iodide), mixed gently. Cells were incubated at room temperature in dark followed by gentle tapping. After incubation cells were examined with flow cytometer (CytoFlex, Beckman coulter, IN, USA). Acquired data were analyzed with the FlowJo_10.4 version.
Statistical Analysis
Statistical analysis was conducted by GraphPad Prism (version 8.0, GraphPad Software). Unpaired Student’s t-test was used to test the significant difference between two or three independent samples. P-value <0.05 was considered statistically significant. Significance between the groups for Student’s unpaired two-tailed t-test are denoted as follows: *p ≤ 0.05; **p < 0.01 and ***p < 0.001.
Results
Mitochondrial Modifier, DNP, Stimulates Glycolysis
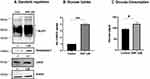
Non-toxic concentrations of dinitrophenol (DNP), the chemical uncoupler of oxidative phosphorylation, have been earlier shown to stimulate glycolysis as a compensatory response to the reduction in ATP production in human tumor cell lines (20). Exposure of U251 cells to DNP (1 µM) enhanced the protein levels of glucose transporter GLUT1, Hexokinase II (HKII), and lactate dehydrogenase (LDHA) by 30–70% (Figure 1A), which was accompanied by a four-fold increase in the glucose uptake (Figure 1B), as well as a significant increase in glucose usage (Figure 1C) as reported earlier,20 thus suggesting enhanced glycolysis under these conditions.
Transient Elevation of Glycolysis Attenuates X-Ray Induced, but Not Carbon Ion-Induced Loss of Clonogenic Survival
Transient elevation of glycolysis has been shown to confer resistance against photon radiation-induced cell death.20 To investigate if transient induction of glycolysis offers resistance against CIRT, we irradiated DNP (1 µM) stimulated U251, LN229, and U87 cells, and analyzed the clonogenicity using clonogenic survival assay by plating cells after 4 h of incubation in DNP. A shouldered dose response was seen following X-ray irradiation in all the three cell lines, while an exponential dose response without a shoulder (as widely reported earlier) was seen following carbon ion irradiation (Figure 2). The RBE (relative biological effectiveness) values at 10% survival (ie, at D10 dose) were 2.9, 1.5, and 2.1 for LN229, U251 and U87 cells, respectively (as shown in Table 1). While stimulation of glycolysis (with DNP) enhanced the survival following X-ray irradiation in all three cell lines, no significant change was noted in the survival following carbon ion irradiation in these cell lines (Figure 2). The values of dose modifying factor (DMF)32 varied between 1.2 and 1.65 for X-rays in these cell lines (Table 2) suggestive of glycolysis-induced radio-resistance. However, for CIRT the DMF value was nearly 1 in all three cell lines suggestive of a lack of glycolysis-induced resistance against carbon ion irradiation.
 |
Table 1 Relative Biological Effectiveness (RBE) of Carbon Ion Radiation Calculated at 10% Survival in Human Glioma Cell Lines at 10% Survival |
 |
Table 2 Dose Modifying Factor (DMF) Following Stimulation of Glycolysis with DNP in Human Glioma Cell Lines |
Enhanced Glycolysis Reduces X-Ray-Induced Growth Inhibition
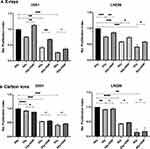
Next, we investigated the effects of enhanced glycolysis on radiation-induced growth inhibition in U251 and LN229 cell lines by analyzing the extent of proliferation at the end of 48 h post-irradiation by calculating the proliferation index from the cell numbers enumerated. The relative proliferation indices values showed that stimulation of glycolysis reduced the extent of dose-dependent growth inhibition induced by X-ray in both the cell lines, with the effect relatively more pronounced at lower doses (2 and 4 Gy), particularly in U251 cells (Figure 3A). However, no significant changes could be observed in the CI-induced growth inhibition in both the cell lines (Figure 3B).
Elevated Glycolysis Reduces X-Ray but Not CII Induced G2+M Delay
Ionizing radiation induces perturbations in the progression of cells through the cell cycle manifesting in the form of delay in entering the M phase from the G2 phase of the cell cycle (linked to G2+M checkpoint blockade) even at low doses as well as the transition from G1 phase to S-phase at higher doses34 contributing to the growth inhibition. In view of our results on growth inhibition (Figure 3), we investigated the effect of glycolysis stimulation on G2+M block induced by X-rays and CII by analyzing the time-dependent changes in the cell cycle distribution obtained from flow cytometric. DNP stimulation reduced the excess G2+M fraction of cells induced by X-rays at 2 Gy and 4 Gy in both U251 and LN229 cells, while a significant reduction was not observed in the CII induced excess G2+M fraction in LN229 cell line but with U251 at 2 Gy there is minor G2+M reduction (Figure 4).
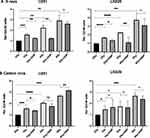
Stimulation of Glycolysis Does Not Alter CII Induced Micronuclei Expression While Reducing X-Ray Induced Micronuclei Expression
Ionizing radiation-induced mitotic catastrophe (cell death during mitosis) contributes to cell death at moderate doses.20 Therefore, we investigated radiation-induced micronuclei, a form of cytogenetic damage linked to the mitotic catastrophe that is found in the daughter cells from chromosome aberrations that arise due to unrepaired/miss-repaired DNA double-strand breaks (DSBs), as well as aberrant mitosis following irradiation and correlates with changes in survival.30 Micronuclei were assessed in binucleated cells obtained using the arrest of cytokinesis with cytochalasin B at post 36 h irradiation.3,10 Dose-dependent induction of micronuclei was evident in both the U251 and LN229 cell lines following X-rays as well as CII, with the level of induction nearly two or more folds higher. Further, a decrease in the micronuclei induction was observed at an X-ray dose of 8 Gy as compared to 4 Gy in LN229 cells suggestive of interphase death. Stimulation of glycolysis reduced the X-ray-induced micronuclei in both the cell lines, while such a decrease was not observed following CII (Figure 5).
CII Induced Apoptosis is Not Influenced by Enhanced Glycolysis
Both mitotic (linked to cytogenetic damage) and interphase death contribute to the radiation-induced loss of clonogenicity.35–37 Therefore, we sought to investigate the effects of stimulated glycolysis on CII and X-Ray induced interphase death comprising apoptosis and necrosis using flow cytometric measurement of cell surface Annexin-V binding (representing predominantly apoptosis) and PI uptake (reflective of necrosis). DNP stimulated glycolysis significantly reduced the X-ray-induced apoptosis (total annexin-V positive cells), while no significant changes were noted in the CII-induced apoptosis (Figure 6). Similar observations were made for the necroptosis fraction (total PI-positive cells) under these conditions (data not shown).
Radiation-Induced Migration of Tumor Cells
Radiation-induced dissemination of tumor cells contributes to the re-growth of tumors outside the planned (irradiated) tumor volume thereby compromising the efficacy of radiotherapy.38,39 Therefore, we investigated the effect of DNP stimulated glycolysis on X-ray and CII-induced migration using the scratch assay in U251 cells. A dose-dependent increase in migration was evident following X-ray irradiation (Figure 7A) as reported earlier38 and interestingly, a decrease in the migration was observed at all three doses (1, 2, and 4 Gy) following CII (Figure 7B). Stimulation of glycolysis accentuated X-ray-induced migration, while it did not significantly alter the migration of U251 cells following CIRT at all the doses investigated (Figure 7).
Earlier studies demonstrated low-dose irradiation induced EMT with gain in malignant behaviour induces migration and invasion promoting tumor progression which contributes to development of resistant cells.40,41 Our results indicate that CII mediated migration inhibition may restrict the development of radiation mediated EMT transition. Our data show that DNP stimulation has no effect in altering the response to CII. Hence, CII response to tumors with altered metabolic status is equally effective to control tumor progression.
Discussion
Metabolic reprogramming, one of the hallmarks of cancer enhances the resistance against all forms of photon-based therapies both in terms of reduced local tumor control (linked to therapy-induced cell death) and induced resistance as well as metastasis.41,42 While radiotherapy using carbon ion irradiation (CII) is a promising modality with enhanced local tumor control (linked to cell death) as well as potential reduced stem cell generation than photons, the influence of metabolic reprogramming on the efficacy of CIRT has not been investigated.26,27,43,44 Results of the present studies show that elevated glycolysis (using DNP) does not significantly influence the CII-induced cell death in three human glioma cell lines and migration in two cell lines investigated. On the contrary, elevated glycolysis reduced the X-ray-induced cell death and enhanced the migration suggestive of compromised efficacy of photon irradiation as reported earlier.11,45 This differential effect of enhanced glycolysis on cell death induced by X-rays and CII was contributed by commensurate changes in the interphase (apoptotic and necrotic) and mitotic death as well as growth inhibition. These observations suggest that CIRT could be as effective in tumors with high rate of glycolysis (the Warburg phenotype) as tumors with low to moderate degree of glycolysis. Further mechanistic studies probing the DNA damage response (DDR) as well as epithelial to mesenchymal transfer (EMT) and induction of stem phenotype are required to unravel the underlying reasons for the lack of significant effects of enhanced glycolysis on the response of tumor cells to CIRT.
Facilitation of DNA repair and reduced ROS levels and oxidative stress have been suggested to contribute to the glycolysis linked resistance against photon (gamma rays) irradiation.20 The underlying mechanisms responsible for the lack of significant effects of enhanced glycolysis on the response of all three tumor cells to CII- observed here - need to be investigated in the future with particular attention to the DDR since the DNA damage induced by CII is complex in nature with clustering of different types of damage46 which is also observed in clinical samples following CIRT.47 The lack of significant changes in the CII-induced accumulation of cells in the G2+M phase of the cell cycle linked to perturbation in the cell cycle progression also suggested that the activation of checkpoints related to the complex DNA damage induced by CII is less susceptible to glycolytic status. However, both these suggestions need to be systematically investigated using genetic (use of genetically manipulated cells) and pharmacological approaches (using modifiers that target specific regulators of radiation response).
X-ray induced migration of glioma cells is linked to ROS generation followed by the activation of antioxidant enzymes,48–50 and induction of EMT.51 ROS generated by CII is more localised and elicits a weak oxidative stress response as compared to photon (X-rays) irradiation which is more diffused and elicits a strong oxidative stress response,50,52 which is in line with the negligible cell migration induced by CII observed here. Therefore, the results of the present study indirectly suggest that the bulk of the damage leading to cytotoxicity and other responses following CIRT may not be necessarily mediated through the generation of oxidative stress, which is in line with the existing notion regarding damage caused by high LET (particle) radiation.25 Elevation of glycolysis has been shown to reduce (low LET) gamma-ray-induced oxidative stress.45 Therefore, it appears that the enhanced X-ray induced cell migration seen here could come from the higher number of surviving cells linked to the reduced interphase death observed under these conditions, which is also in line with the lack of any significant effect of enhanced glycolysis on cell migration following CIRT.
It is pertinent to note that the elevated glycolysis was transient in nature prevailing for a few hours post-irradiation as DNP (used for glycolysis stimulation) was removed at 4 h after irradiation and cells plated for observing the manifestation of various endpoints related to the damage. Yet, the transient elevation of glycolysis significantly modified the cellular responses to X-ray damage as reported earlier,20 but had little influence on the response to CII suggesting that molecular events initiated immediately after damage induction by X-rays are strongly linked to the metabolic status. This notion is supported by our earlier studies with the transient inhibition of glycolysis using the glycolytic inhibitor 2-DG in several tumor cell lines, where inhibition of DNA repair and an increase in both mitotic and interphase death were observed.20,53–55 Earlier, radioresistance mediated by high glycolysis was reported with radioresistant Hela cells where increased killing by carbon ion irradiation was observed.32 However, the exact mechanism underlying for response to high glycolytic glioma to irradiation still needs to be studied in detail. One possible explanation could be the ROS mediated DNA damage by photon-based therapy or carbon ion radiotherapy. The DNA damage by photon or X-rays irradiation is mainly through an indirect mechanism (by producing reactive oxygen species (ROS)), while the carbon ion irradiation directly mediates the DNA damage and is less dependent on the ROS.55,56 Glioma cells with high glycolysis generate radio-resistance via ROS generation to X-rays irradiation while CII could not. As glycolytic flux is a consequence of metabolic reprogramming in cancer cells, certain intermediates of glycolysis efficiently scavenge radiation-induced ROS that may lead to resistance to X-rays or photons.57 Since carbon ions damage DNA directly with minimal contribution from ROS in damage induction, this defensive mechanism is relatively less effective with CIRT. Therefore, glioma cells with high glycolysis flux can display resistance to X-rays or photon. The clinical implication of this concept is that inhibitors of glycolysis can be used as the adjuvant to photon radiotherapy to enhance its efficacy.
Though our work has limitations including the lack of studies using isogenic cells with reduced mitochondrial DNA (that leads to a compensatory increase in glycolysis) to support the observations made using DNP (a pharmacologic) stimulated glycolysis, it appears that the molecular events that follow CII-induced lesions are relatively less susceptible to modification by metabolic status. It will be interesting to see if inhibitors of glycolysis (like 2-deoxy-D-glucose) alone or in combination with anti-mitochondrial agents could further enhance the effects of CII (or CIRT).
Conclusion
Collectively, our data indicate that the presence of high glycolytic cells in the tumor could compromise the efficacy of photon-based radiotherapy by attenuating the cytotoxic (cell death) and cytostatic (inhibition of cell proliferation) effects as well as by enhancing the induced cell migration leading to disease dissemination. On the other hand, it may not significantly alter tumor responses (cell death and cell migration) to therapy using Carbon ion beam. Since, MRP is a general phenomenon seen in most, if not all tumors, studies employing cells from other tumor types need to be carried out to validate the generality of these findings.
Abbreviations
2-NBDG, 2-Deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-glucose; CIRT, Carbon ion radiation therapy; DNP, Dinitrophenol; Glut1, Glucose transporter 1; HKII, hexokinase 2; PKM2, Pyruvate kinase isoform 2; LDHA, Lactate Dehydrogenase; RT, Radiotherapy; IMRT, Intensity-modulated radiotherapy.
Ethics Statement
No animal work or clinical studies were involved in this study and all cell line work was approved as per the institutional grant.
Acknowledgments
We thank Dr. Xiaomao Guo, Dean, Shanghai Proton and Heavy Ion Centre (SPHIC), Shanghai, China for the wholehearted support and encouragement provided in carrying out this study. We are thankful to Mr. Weiwei Wang and Mr. Zhijie Huang (Medical Physicists) for providing valuable support in carbon ion irradiation. We thank Dr. Y. Sun, Ms. Li Bing, and Miss Zhou Ying, SPHIC, Shanghai Engineering Research Centre of Proton and Heavy Ion Radiation Therapy, Shanghai, China for providing technical and administrative support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by a grant (#RD2018001) from Shanghai Proton and Heavy Ion Center (SPHIC), Shanghai, China.
Disclosure
Dr Chandan Guha reports grants from NIH/NCI R01CA257509, during the conduct of the study; grants from Janssen, personal fees from FUSF, Siemens/Varian, outside the submitted work. The authors report no conflicts of interest in this work.
References
1. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi:10.1038/nrc1478
2. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi:10.1038/nrc2981
3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi:10.1016/j.cell.2011.02.013
4. Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancerprogression. Science. 2020;368:6487. doi:10.1126/science.aaw5473
5. Hanahan D. Hallmarks of Cancer: new Dimensions. Cancer Discov. 2022;12:31–46. doi:10.1158/2159-8290.CD-21-1059
6. Miranda-Galvis M, Teng Y. Targeting hypoxia-driven metabolic reprogramming to constrain tumor progression and metastasis. Int J Mol Sci. 2020;21(15):5487. doi:10.3390/ijms21155487
7. Singh S, Pandey S, Chawla AS, et al. Dietary 2-deoxy-D-glucose impairs tumor growth and metastasis by inhibiting angiogenesis. Eur J Cancer. 2019;123:11–24. doi:10.1016/j.ejca.2019.09.005
8. Prasanna VK, Venkataramana NK, Dwarakanath BS, Santhosh V. Differential responses of tumors and normal brain to the combined treatment of 2-DG and radiation in glioblastoma. J Cancer Res Ther. 2009;1:S44–7.
9. Gupta S, Farooque A, Adhikari JS, Singh S, Dwarakanath BS. Enhancement of radiation and chemotherapeutic drug responses by 2-deoxy-D-glucose in animal tumors. J Cancer Res Ther. 2009;S16–20. doi:10.4103/0973-1482.55135
10. Dwarakanath BS. Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2-deoxy-D-glucose in vitro. J Cancer Res Ther. 2009;1:S27–31. doi:10.4103/0973-1482.55137
11. Farooque A, Singh N, Adhikari JS, Afrin F, Dwarakanath BS. Enhanced antitumor immunity contributes to the radio-sensitization of Ehrlich ascites tumor by the glycolytic inhibitor 2-deoxy-D-glucose in mice. PLoS One. 2014;9(9):e108131. doi:10.1371/journal.pone.0108131
12. Farooque A, Afrin F, Adhikari JS, Dwarakanath BS. Polarization of macrophages towards M1 phenotype by a combination of 2-deoxy-d-glucose and radiation: implications for tumor therapy. Immunobiology. 2016;221(2):269–281. doi:10.1016/j.imbio.2015.10.009
13. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68. doi:10.3389/fonc.2017.00068
14. Singh S, Pandey S, Chawla AS, et al. Dietary 2-deoxy-D-glucose impairs tumor growth and metastasis by inhibiting angiogenesis. Eur J Cancer. 2019;123:11–24.
15. Dwarakanath BS, Singh D, Banerji AK, et al. Clinical studies for improving radiotherapy with 2-deoxy-D-glucose: present status and future prospects. J Cancer Res Ther. 2009;1:S21–6. doi:10.4103/0973-1482.55136
16. Heminger K, Jain V, Kadakia M, Dwarakanath B, Berberich SJ. Altered gene expression induced by ionizing radiation and glycolytic inhibitor 2-deoxy-glucose in a human glioma cell line: implications for radiosensitization. Cancer Biol Ther. 2006;5(7):815–823. doi:10.4161/cbt.5.7.2812
17. Mohanti BK, Rath GK, Anantha N, et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: Phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35(1):103–111. doi:10.1016/S0360-3016(96)85017-6
18. Schulz A, Meyer F, Dubrovska A, Borgmann K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers. 2019;11(6):862. doi:10.3390/cancers11060862
19. Sertorio M, Perentesis JP, Vatner RE, Mascia AE, Zheng Y, Wells SI. Cancer Cell Metabolism: implications for X-ray and Particle Radiation Therapy. Int J Part Ther. 2018;5(1):40–48. doi:10.14338/IJPT-18-00023.1
20. Bhatt AN, Chauhan A, Khanna S, et al. Transient elevation of glycolysis confers radio-resistance by facilitating DNA repair in cells. BMC Cancer. 2015;1(15):335. doi:10.1186/s12885-015-1368-9
21. Cook KM, Shen H, McKelvey KJ, Gee HE, Hau E. Targeting glucose metabolism of cancer cells with dichloroacetate to radiosensitize high-grade gliomas. Int J Mol Sci. 2021;22(14):7265. doi:10.3390/ijms22147265
22. Shen H, Decollogne S, Dilda PJ, et al. Dual-targeting of aberrant glucose metabolism in glioblastoma. J Exp Clin Cancer Res. 2015;34(1):14. doi:10.1186/s13046-015-0130-0
23. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi:10.1056/NEJMoa043330
24. Loeffler JS, Durante M. Charged particle therapy--optimization, challenges and future directions. Nat Rev Clin Oncol. 2013;10(7):411–424. doi:10.1038/nrclinonc.2013.79
25. Durante M, Orecchia R, Loeffler JS. Charged particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol. 2017;14(8):483–495. doi:10.1038/nrclinonc.2017.30
26. Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7:37–43. doi:10.1038/nrclinonc.2009.183
27. Durante M, Flanz J. Charged particle beams to cure cancer: strengths and challenges. Semin Oncol. 2019;46(3):219–225. doi:10.1053/j.seminoncol.2019.07.007
28. Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. doi:10.1186/s13046-015-0221-y
29. Bhatt AN, Chauhan A, Khanna S, et al. Transient elevation of glycolysis confers radio-resistance by facilitating DNA repair in cells. BMC Cancer. 2015;15:335.
30. Bao C, Sun Y, Dwarakanath B, et al. Carbon ion triggered immunogenic necroptosis of nasopharyngeal carcinoma cells involving necroptotic inhibitor BCL-x. J Cancer. 2021;12(5):1520–1530. doi:10.7150/jca.46316
31. Bao C, Sun Y, Dong Y, Le Z, Lin L-C. The relative biological effectiveness of proton and carbon ion beams in photon-sensitive and resistant nasopharyngeal cancer cells. Translate Cancer Res. 2018;7:548.
32. Bing Z, Yang G, Zhang Y, et al. Proteomic analysis of effects by x-rays and heavy ion in HeLa cells. Radiol Oncol. 2014;48(2):142–154. doi:10.2478/raon-2013-0087
33. Kumar V, Vashishta M, Kong L, et al. Carbon Ion Irradiation Downregulates Notch Signaling in Glioma Cell Lines, Impacting Cell Migration and Spheroid Formation. Cells. 2022;11(21):3354. doi:10.3390/cells11213354
34. Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2(5):1084–1104. doi:10.1038/nprot.2007.77
35. Pawlik TM, Keyomathe K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–942. doi:10.1016/j.ijrobp.2004.03.005
36. Durante M, Formenti SC. Radiation-Induced Chromosomal Aberrations and Immunotherapy: micronuclei, Cytosolic DNA, and Interferon-Production Pathway. Front Oncol. 2018;8:192. doi:10.3389/fonc.2018.00192
37. Verma A, Venkateswaran K, Farooque A, et al. Cytotoxic and radio- sensitizingradio-sensitizing effects of polyphenolic acetates in a human glioma cell line (BMG-1). Curr Pharm Des. 2020;2020(7):1161–1169.
38. Dwarakanath BS. Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2-deoxy-D-glucose in vitro. J Cancer Res Ther. 2009;5(Suppl 1):S27–31.
39. Fei Y, Xiong Y, Shen X, et al. Cathepsin L promotes ionizing radiation-induced U251 glioma cell migration and invasion through regulating the GSK-3β/CUX1 pathway. Cell Signal. 2018;44:62–71. doi:10.1016/j.cellsig.2018.01.012
40. Rieken S, Habermehl D, Wuerth L, et al. Carbon ion irradiation inhibits glioma cell migration through downregulation of integrin expression. Int J Radiat Oncol Biol Phys. 2012;83(1):394–399. doi:10.1016/j.ijrobp.2011.06.2004
41. Yan S, Wang Y, Yang Q, et al. Low-dose radiation-induced epithelial-mesenchymal transition through NF-κB in cervical cancer cells. Int J Oncol. 2013;42(5):1801–1806. doi:10.3892/ijo.2013.1852
42. Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. doi:10.1038/nrc3038
43. Schwartz L, Supuran CT, Alfarouk KO, Warburg T. Effect and the Hallmarks of Cancer. Anticancer Agents Med Chem. 2017;17(2):164–170. doi:10.2174/1871520616666161031143301
44. Moncharmont C, Guy JB, Wozny AS, et al. Carbon ion irradiation withstands cancer stem cells’ migration/invasion process in Head and Neck Squamous Cell Carcinoma (HNSCC). Oncotarget. 2016;7(30):47738–47749. doi:10.18632/oncotarget.10281
45. Chiblak S, Tang Z, Lemke D, et al. Carbon irradiation overcomes glioma radioresistance by eradicating stem cells and forming an antiangiogenic and immunopermissive niche. JCI Insight. 2019;4(2):e123837. doi:10.1172/jci.insight.123837
46. Rai Y, Anita KN, Singh S, Kalra N, Soni R, Bhatt AN. Mild mitochondrial uncoupling protects from ionizing radiation-induced cell death by attenuating oxidative stress and mitochondrial damage. Biochem Biophys Acta Bioenerg. 2021;1862(1):148325. doi:10.1016/j.bbabio.2020.148325
47. Okayasu R. Repair of DNA damage induced by accelerated heavy ions--a mini review. Int J Cancer. 2012;130(5):991–1000. doi:10.1002/ijc.26445
48. Oike T, Niimi A, Okonogi N, et al. Visualization of complex DNA double-strand breaks in a tumor treated with carbon ion radiotherapy. Sci Rep. 2016;6:22275. doi:10.1038/srep22275
49. Kam WWY, Banati RB. Effects of ionizing radiation on mitochondria. Free Radic Biol Med. 2013;65:607–619. doi:10.1016/j.freeradbiomed.2013.07.024
50. Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1–2):48–60. doi:10.1016/j.canlet.2011.12.012
51. Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356(2Pt A):156–164. doi:10.1016/j.canlet.2014.04.001
52. Zhou YC, Liu JY, Li J, et al. Ionizing radiation promotes migration and invasion of cancer cells through transforming growth factor-beta-mediated epithelial-mesenchymal transition. Int J Radiat Oncol Biol Phys. 2011;81(5):1530–1537. doi:10.1016/j.ijrobp.2011.06.1956
53. Wozny AS, Vares G, Alphonse G, et al. ROS Production and Distribution: a New Paradigm to Explain the Differential Effects of X-ray and Carbon Ion Irradiation on Cancer Stem Cell Migration and Invasion. Cancers. 2019;11(4):468. doi:10.3390/cancers11040468
54. Khaitan D, Chandna S, Arya MB, Dwarakanath BS. Differential mechanisms of radiosensitization by 2-deoxy-D-glucose in the monolayers and multicellular spheroids of a human glioma cell line. Cancer Biol Ther. 2006;5(9):1142–1151. doi:10.4161/cbt.5.9.2986
55. Dwarakanath BS, Jain VK. Modification of the radiation-induced damage by 2-deoxy-D-glucose in organ cultures of human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1987;13(5):741–746. doi:10.1016/0360-3016(87)90293-8
56. Taghizadeh-Hesary F, Akbari H, Bahadori M, Behnam B. Targeted Anti-Mitochondrial Therapy: the Future of Oncology. Genes. 2022;13(10):1728. doi:10.3390/genes13101728
57. Ray S, Cekanaviciute E, Lima IP, Sørensen BS, Costes SV. Comparing Photon and Charged Particle Therapy Using DNA Damage Biomarkers. Int J Part Ther. 2018;5(1):15–24. doi:10.14338/IJPT-18-00018.1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.