Back to Journals » Journal of Inflammation Research » Volume 14
Endothelin-1/Endothelin Receptor Type A-Angiopoietins/Tie-2 Pathway in Regulating the Cross Talk Between Glomerular Endothelial Cells and Podocytes in Trichloroethylene-Induced Renal Immune Injury
Authors Xie H, Wang H, Wu Q, Peng J, Huang H, Wang Y, Huang M, Jiang W, Yang Y, Zhang X, Zhang J, Zhu Q
Received 8 January 2021
Accepted for publication 17 February 2021
Published 9 March 2021 Volume 2021:14 Pages 761—776
DOI https://doi.org/10.2147/JIR.S301104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Haibo Xie,1,2,* Hui Wang,1,2,* Qifeng Wu,3 Jiale Peng,4 Hua Huang,4 Yican Wang,4 Meng Huang,4 Wei Jiang,4 Yi Yang,4 Xuesong Zhang,4 Jiaxiang Zhang,2,4 Qixing Zhu1,2
1Department of Dermatology, First Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province, People’s Republic of China; 2Key Laboratory of Dermatology (Anhui Medical University), Ministry of Education, Hefei, People’s Republic of China; 3Guangdong Province Hospital for Occupational Disease Prevention and Treatment, Guangzhou, Guangdong Province, People’s Republic of China; 4Department of Occupational Health and Environmental Health, School of Public Health, Anhui Medical University, Hefei, Anhui Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiaxiang Zhang
Department of Occupational Health and Environmental Health, School of Public Health, Anhui Medical University, Hefei, Anhui, People’s Republic of China
Tel +86-055163869267
Email [email protected]
Qixing Zhu
Department of Dermatology, First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China
Email [email protected]
Introduction: This study aimed to investigate the mechanism in regulating the cross talk between glomerular endothelial cells and podocytes in “occupational medicamentosa-like dermatitis induced by trichloroethylene (OMLDT)” patients.
Methods: Totally 6 OMLDT patients, 18 controls, and 102 BALB/c female mice were involved in this study. Patient’s serum endothelin-1 (ET-1), angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2), blood urea nitrogen (BUN), and podocalyxin (PCX) were detected. All the mice were used to establish the trichloroethylene (TCE) sensitized mouse model. Transmission electron microscope results were used to reflect renal glomerulus injury. Protein levels were detected by Western blot. Ang-1/Ang-2 gene level was reflected by RT-PCR. Cell apoptosis level was detected by using TUNEL assay kit.
Results: We found that in OMLDT patients, ET-1, Ang-2, BUN, and PCX were highly expressed but Ang-1 was inhibited. In TCE sensitized positive mouse, the downregulation of Ang-1, pTie-2 and the upregulation of Ang-2 were mediated by ET-1/ETAR but not ET-1/ETBR. The promotor of apoptosis proteins was downregulated and the inhibitor of apoptosis proteins was upregulated by treating with BQ123.
Discussion: ET-1/ETAR-Angs/Tie-2 pathway mediated the cross talk between glomerular endothelial cells and podocytes. BQ123 can alleviate glomerulus immune injury.
Keywords: trichloroethylene, glomerular, endothelin-1, endothelin receptor type A, angiopoietin-1, angiopoietin-2
Introduction
Trichloroethylene (TCE), a typical chlorinated organic compounds (COCs), is widely used in industrial processes, metal parts and electronic components cleaning due to its excellent degreasing property and non-combustible nature.1 In the United States, the imported or manufactured TCE has reached 172 million tons in 2015.2 Because of the widespread use, TCE is frequently released into groundwater. Archer et al found that TCE concentrations were 0.04 μg/L in the tap water and (0.17–0.61) μg/m3 in outdoor air in Grand Prairie.3 In China, TCE ranks the 3rd in the list of toxic water pollutants.4 TCE puts a wide range of toxicological effects on human life, like acute renal failure, Parkinson’s disease, neuropathy, nephral necrosis and cranial nerve palsies.5 TCE also has been classified as group I carcinogen.6 Although the production of TCE has declined over the past years, TCE is still widely used in China.7 In China, the use of TCE is concentrated in Guangdong Province, which leads to hypersensitivity syndrome induced by TCE which has gradually become one of the major occupational toxins. This occupational disease caused by trichloroethylene was named “occupational medicamentosa-like dermatitis induced by TCE (OMLDT)”.1 OMLDT is characterized by generalized rash, superficial lymphadenopathy, fever, kidney and liver dysfunction,8 which is also defined as TCE hypersensitivity syndrome (THS). Renal immune injury usually occurs at the early stage. Acute renal failure is one of the leading causes of death from OMLDT. In 2010, China, there still are at least 20 thousand new works exposed to TCE.9 Our previous studies found that the TCE sensitized mice got severe renal glomerular inflammation,10,11 but the mechanism in regulating the cross talk between glomerular endothelial cells and podocytes still have not been investigated.
The endothelin family includes three vasoactive peptides (endothelin-1, ET-1; endothelin-2, ET-2; endothelin-3, ET-3) and two G protein-coupled receptors (endothelin receptor-type A, ETAR; endothelin receptor-type B, ETBR) expressed on the cell surface. ET-1, which is mainly produced by endothelial cells, is the most well studied and the most powerful intrinsic vasoconstrictor and plays an important role in regulating blood vessel stability.12 ET-1 exerts different biological functions by binding to these two receptors. In the vascular system, ETAR act as growth-promoting and a primary vasoconstrictor by irreversible binding with ET-1, whereas ETBR is more important in clearing the circulating ET-1 which functions as a “clearance receptor”. Furthermore, ETAR is found to be involved in the development of many diseases, such as atherosclerosis, heart failure, arterial hypertension, cancer, diabetes and renal disease.13 Studies found that ET-1 stimulates renal inflammation and fibrosis by activating ETAR.14 ETA blockade could slow the progression of diabetic nephropathy by the anti-inflammatory mechanisms.15 Federica et al found that the ET-1 produced from endothelial glomerular cells could induce the loss of nephrin from podocytes, while ET-1 receptor antagonists abrogate the nephrin shedding.16
Angiopoietins are another family involved in the blood vessel remodeling, maturation and maintenance during both blood vessel pathological angiogenesis, and development.17 The angiopoietin family includes angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), angiopoietin-3 (Ang-3) and angiopoietin-4 (Ang-4), and the tyrosine kinase receptors (Tie-1 and Tie-2). Only the Ang/Tie2 signaling axis is the main regulator of angiogenesis. The Tie-2 receptor is highly expressed on endothelial cells. All four kinds of Angs were secreted factors that exert their biological effects by binding to Tie-2 receptor.18 Studies found that Ang-1 and Ang-4 function as agonists of Tie-2 but Ang-2 and Ang-3 function as antagonists. Tie-2 becomes phosphorylated on several cytoplasmic tyrosine residues and results in the PI3-kinase/protein kinase B (PI3k/AKT) and endothelial nitric oxide synthase 3 (eNOS) activation, which shows anti-apoptotic activity and vascular protective functions.19 In the kidney, Ang-1 was predominantly expressed in podocytes and plays an important role in the maintenance of the glomerular permeability.20 Ang-1 also shows anti-inflammatory activity by inhibiting tumor necrosis factor-α (TNF-α)-induced leukocyte capillary transmigration. On the contrary, Ang-2 is rapidly release from endothelial vesicles under pro-inflammatory signaling, where it competes with Ang-1 in an autocrine manner, thereby down-regulating the phosphorylation level of Tie-2, finally results in the activation of apoptotic proteins.21
Both endothelin-1 and angiopoietins are important factors in regulating the physiological functions of vascular endothelial cells; is there any relationship between these two proteins? In recent years, there are several studies involved in the investigation referred to endothelin-1 and angiopoietins. But they played different roles in different diseases.22,23 We know that angiopoietins are associated with endothelin-1 in kidney diseases, but how angiopoietins regulated by endothelin-1 have still not been well investigated. The newest study found that after traumatic brain injury, administered with ETBR antagonist BQ788 promoted the recovery of blood-brain barrier function through activation of the Ang-1/Tie-2 signal.24 In this TCE-sensitized mouse model, TCE-sensitized mice got glomerular endothelial dysfunction and podocyte damage. The Ang-1/Ang-2 expression level in TCE-sensitized mouse podocytes is unknown. Whether Ang-1/Ang-2 expression levels are affected by ET-1, and which endothelin receptor mediates the expression of Ang-1/Ang-2 needed further research.
Method
Reagents
Freund’s complete adjuvant (FCA) and TCE were bought from Sigma-Aldrich (St. Louis, MO, USA). Olive oil and acetone were purchased from the Shanghai Chemical Reagent Company (Shanghai, China). Selective endothelin A receptor (ETAR) antagonist BQ 123, selective endothelin B receptor (ETBR) antagonist BQ 788 and phosphatase inhibitors were bought from Absin (Shanghai, China). Anti-rabbit monoclonal GAPDH antibody, Anti-mouse monoclonal ET-1 antibody, anti-rabbit monoclonal NPHS-2 antibody, anti-rabbit monoclonal podocin antibody, anti-rabbit monoclonal Bax antibody, anti-rabbit monoclonal caspase-3 antibody, anti-rabbit polyclonal ETBR antibody, anti-rabbit polyclonal CD31 antibody, anti-rabbit monoclonal Ang-2 antibody were purchased from Abcam (Cambridge, UK). Anti-goat polyclonal Tie-2 antibody, anti-goat polyclonal nephrin antibody, anti-goat polyclonal Ang-1 antibody, anti-rabbit polyclonal phosphor-Tie-2 (pTie-2) antibody and human ET-1 ELISA kit were acquired from R&D Systems (Minnesota, USA). Anti-rabbit polyclonal eNOS antibody and anti-rabbit polyclonal Ang-1 antibody were acquired from Bioss (Beijing, China). Anti-mouse monoclonal ETAR antibody, anti-mouse monoclonal PI3K antibody, anti-mouse monoclonal pAKT antibody, anti-mouse monoclonal Fas antibody, anti-mouse monoclonal Fas-L antibody, anti-mouse monoclonal phosphorylation of Bad (pBad) antibody, anti-mouse monoclonal Bcl-2 antibody, anti-mouse monoclonal CD31 antibody, anti-rat monoclonal monocyte chemoattractant protein-1 (MCP-1) antibody were bought from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-rabbit monoclonal Bad antibody was bought from Boster biological technology (Wuhan, China). Dynabeads M-450 Tosylactivated (diameter 4.5 µm) was purchased from Dynal AS (Oslo, Norway). Type I collagenase was from Sigma-Aldrich (St. Louis, MO, USA). Phenylmethylsulfonylfluoride (PMSF) was bought from Cell Signaling Technology (Danvers, USA). BCA Kit, radio-immunoprecipitation lysis buffer (RIPA), human Ang1 and human Ang-2 ELISA kit were obtained from Beyotime Biotechnology (Shanghai, China). Mouse cystatin C (Cys-c) ELISA Kit, mouse podocalyxin (PCX) ELISA Kit, HumanCys-c ELISA Kit, Human PCX ELISA Kit and TUNEL Assay Kit (HRP-DAB) were acquired from Elabscience Biotechnology Co., Ltd (Wuhan, China). Mouse Creatinine (Cre) Assay kit and Urea Assay Kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Immunohistochemistry kit and DAB substrate kit were obtained from ZSJQ-BIO (Beijing, China); Thermo Scientific RevertAid First Strand cDNA Synthesis Kit was purchased from Thermo (Waltham, USA). LightCycler 480 SYBR Green I Master was purchased from Roche (Basel, Switzerland).
Participants
Six newly diagnosed OMLDT patients recruited from January 2016 to January 2020 at Guangdong Province Hospital for Occupational Disease Prevention and Treatment were involved in this study. The age range of the patients was from 16–29 years of age (3 males and 3 females). All the patients’ serum and urine were collected on the first day in hospital (before treatment) and one month after treatment but not discharged (after treatment) respectively. Eighteen controls aged from 18 years old to 30 years old were recruited from health examining center. OMLDT patients were matched (1:3 ratio) to controls without OMLDT (based on age±3 years, the same gender). Patients aged over 60 years old, suffered from mental disease, accompanied by primary kidney disease, chronic diseases and other serious organic diseases caused by other reasons were excluded. This work received approval for research ethics from Anhui Medical University and a proof/certificate of approval is available upon request (Ethical approval number: 20,131,419). Participants under the age of 18 had a legal guardian provide informed consent, while participants over the age of 18 provided their own informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Animals
Pathogen-free BALB/c female mice weighted (18–20) g and were 7–8 weeks of age when used in this study were all purchased from the Experimental Animal Center of Anhui (Anhui, China). All mice were fed with standard lab chow, eggs and drinking water, with a 12 h/12 h light and dark cycle. Padding was replaced every day to remain the dry living environment. All the mice were housed and bred following the institutional guidelines for animal use and care. All animal studies were conducted in accordance with the Regulations on the Administration of Laboratory Animals published by the Ministry of Science and Technology of the People’s Republic of China and was approved by Anhui Medical University (animal ethical committee number: SCXK 2017–001). One hundred and two female mice were randomly divided into the blank control group (with 8 replicates), solvent control group (with 8 replicates), BQ123 control group (with 8 replicates), BQ788 control group (with 8 replicates), TCE treatment group (with 30 replicates), TCE + BQ123 treatment group (with 20 replicates), TCE + BQ788 treatment group (with 20 replicates). The TCE-sensitized mouse model was established according to the previous study.25 Before the model was established, all animals’ 2 × 2 cm2 dorsal hair was removed. On the first day of the experiment, TCE treatment group mice, TCE + BQ123 treatment group mice and TCE + BQ788 treatment group mice were received a dorsal hypodermic injection of a mixture of 50 μL FCA and 50 μL 50% TCE (TCE: olive oil: acetone = 5:2:3, volume ratio). On days 4, 7, and 10, these mice received topical application of 100 μL 50% TCE. On days 17 and 19, they were treated with 30% TCE (TCE: olive oil: acetone = 3:2:5, volume ratio). On days 17 and 19, 2 h before the challenge, BQ123 control group mice and TCE + BQ123 treatment group mice received BQ123 6.7 mg/kg; BQ788 control group mice and TCE + BQ788 treatment group mice were received BQ788 3 mg/kg. The blank control group mice received no treatment. BQ123 control and BQ788 control group mice received no treatment before the challenge. The solvent control group mice received identical volume ratio of olive oil and acetone. On the 20th day, the cutaneous reactions were evaluated.25 According to the cutaneous reactions, TCE treatment group mice were further divided into TCE sensitized negative group (TCE− group) (n=20), TCE sensitized positive group (TCE+ group) (n=10), the sensitization rate was 33.3%; TCE + BQ123 treatment group were further divided into TCE + BQ123 sensitized negative group (TCE + BQ123− group) (n=11), TCE + BQ123 sensitized positive group (TCE + BQ123+ group) (n=9), the sensitization rate was 45.0%; TCE + BQ788 treatment group mice were further divided into TCE + BQ788 sensitized negative group (TCE + BQ788− group) (n=14), TCE + BQ788 sensitized positive group (TCE + BQ788+ group) (n=6), the sensitization rate was 30.0%. All the mice 24 h urine was collected by using metabolism cage before sacrifice. Part of each mouse’s left kidney was taken and fixed in 4% neutral buffered formaldehyde for 48 hours. The left mice were anesthetized and then perfused with dynabeads via abdominal aorta. After perfusion, the two kidneys were removed and then digested with type I collagenase for 30 mins. The tissue was then filtered through a 100 um cell strainer and centrifuged at 200×g at 4°C for 5 min. The pure glomeruli were obtained and after three washes with ice-cold sterile PBS using a magnetic particle concentrator.
Immunofluorescence
Immunofluorescence was used for ET-1, Tie-2, Ang-2, ETAR, Ang-1 location. The kidneys were taken and fixed in 4% neutral buffered formaldehyde for 48 hours, then embedded in paraffin. Finally, a 3 μm thick section for assessment of pathology was collected. The slices were then dewaxed in xylene solution, rehydrated in ethyl alcohol, processed with 0.3% Triton-100 for 30 min, incubated in goat serum for 30 min at 37°C and then incubated with CD 31 and ET-1, CD 31 and Tie-2, CD 31 and Ang-2, NPHS2 and ETAR, NPHS2 and Ang-1 antibodies overnight at 4 °C, respectively. The slices were washed with phosphate buffer saline (PBS) and incubated with fluorogenic secondary antibodies for 30 min and DAPI for 3 min. Finally, the immuno-binding products were analyzed with a fluorescent microscope.
Renal Injury Detection
Mouse renal function index urea creatinine (Cre) and urea nitrogen(UN) were detected by using creatinine assay kit and urea assay kit according to manufacturer’s instructions, respectively. Human Cre and blood urea nitrogen (BUN) were detected by using automatic biochemical analyzer.
Enzyme-Linked Immunosorbent Assay (ELISA)
The mouse and human 24 h urine were diluted and then used for detecting the levels of Cys-c and PCX to reflect glomerular filtration function and podocyte injury by using mouse or human Cys-c ELISA kit and PCX ELISA kit, respectively. Human serum ET-1, Ang-1 and Ang-2 levels were also detected by using human ET-1, Ang-1 and Ang-2 ELISA kit.
Transmission Electron Microscope (TEM)
A little part of renal cortex was fixed in glutaraldehyde-paraformaldehyde for 10 hours, then post-fixed with 1% osmium tetroxide (OsO4) in PBS for 1 hour and dehydrated through a graded series of ethanol (30, 50, 70, 80, 90, 95, 100%) for 15 min at each step, then transferred into absolute acetone for 20 min. Tissue was placed into 1:1 mixture of absolute acetone-spurr resin for 1 hour, then into 1:3 mixture of absolute acetone-spurr resin for 3 hours at room temperature, incubated in resin mixture overnight. The next day, tissue was transferred into capsules containing spurr resin and heated for 9 hours at 70°C. Finally, they were stained with uranyl acetate/alkaline lead citrate for 15 min and examined with a Hitachi H-7650 transmission electron microscope.
Western Blot
Glomeruli total protein was extracted by using RIPA, PMSF and phosphatase inhibitors (100:1:1) mixture. After centrifuged, BCA Kit was used for measuring protein concentration. The proteins were denatured by heating to 100 °C, then equal amounts of 40 μg were loaded and separated by SDS-PAGE. Proteins were transferred into PVDF membrane and blocked in 5% no fat milk for 2 h and then incubated with ET-1, ETAR, ETBR, Ang-1, Ang-2, Tie-2, pTie-2, PI3K, pAKT, eNOS, Bcl-2, Bad, pBad, Fas, Fas-L, Bax, caspase-3, MCP-1, TNF-α, nephrin, podocin, GAPDH antibody overnight at 4 °C, respectively. On the second day, membranes were washed and then incubated in goat anti-rabbit IgG or goat anti-mouse IgG or rabbit anti-goat IgG at room temperature for 2 hours. Finally, membranes were washed and signals were detected by using enhanced chemiluminescence (ECL).
Quantitative Real-Time PCR (qRT-PCR)
Total glomerulus RNA was extracted by using Trizol Reagents according to the manufacturer’s instructions. Revert Aid First Strand cDNA Synthesis Kit was used to yield cDNA. cDNA was amplified by PCR using Light Cycler 480 SYBR Green I Master kit and respective primers. Primer sequences see in Table 1. The amplification program was as follows: initial denaturation at 95 °C for 10 minutes, then started 45 cycles, which include 95°C for 15 seconds, 60°C for 15 seconds and 72°C for 20 seconds. Gene expression was analyzed by the 2−ΔΔCt method.
 |
Table 1 Primers for RT-PCR |
TUNEL
The level of apoptosis in renal glomeruli was detected by using TUNEL assay kit. The 3 μm thickness cortex paraffin sections were dewaxed in xylene solution, rehydrated in ethyl alcohol, incubated in protease K for 30 min and then blocked with H2O2 for 20 min at room temperature. The samples were incubated in TdT protease and then in Streptavidin-HRP for 30 min at 37 °C, then processed with DAB and hematoxylin. Finally, the immuno-binding products were detected by using a light microscope.
Statistical Analysis
Data in this study are shown as mean ± standard deviation (x̄ ± s) or P50 (P25, P75). In mouse, the difference between groups was tested by one-way analysis of variance (ANOVA) with the least significant difference (LSD) test. Related samples Friedman’s two-way analysis of variance by ranks was used for human serum ET-1, Ang-1, Ang-2, Ang-1/Ang-2 analyzing, and human urine PCX, Cre and BUN testing. Linear correlation analysis was used for investigating the relationship between ET-1, Ang-1 and BUN. SPSS V.23.0 (SPSS Inc., Chicago, USA) software was used to perform the statistical analyses. P< 0.05 was considered statistically significant.
Results
OMLDT Patients Renal Injury Indexes and Serum ET-1, Ang-1, Ang-2 Levels
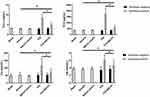
Results from Table 2 showed that on admission, OMLDT patients’ serum level of ET-1, Ang-2, BUN and urine level of PCX were 60.69 (45.35, 85.86) pg/mL, 932.26 (725.67, 1214.97) pg/mL, 5.20 (4.53,7.38) mmol/l and 2884.53 (2169.36, 5173.75) pg/mL, respectively, significantly higher than those after one month treatment and control group (P<0.05). The Ang-1 level detected on admission was lower than those after treatment and control group (P<0.05). The Ang-1/Ang-2 level was also changed after treatment (P<0.05). Further, before treatment, OMLDT patients' serum ET-1 was negatively correlated with Ang-1 (P<0.05) but not significantly associated with BUN. Ang-1 was negatively correlated with BUN (P<0.05). Results see in Table 2 and Figure 1.
 |
Table 2 OMLDT Patients Serum ET-1, Ang-1, Ang-2, Cre, BUN and Urine PCX Levels (P50 (P25, P75)) |
ET-1 but Not Ang-1 Elevated in TCE Sensitized Positive Mouse
In TCE sensitization mouse model, compared to blank control group, solvent control group, and TCE sensitized negative group, ET-1 significantly increased in TCE sensitized positive group. Ang-1 as the promotor of blood vessel repairing did not increase or even decrease in TCE sensitized positive group mice (Figure 2). There is good agreement between OMLDT patients and TCE sensitization mouse models.
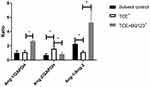
BQ123 Reduced the Level of Urinary Cys-c, PCX and Protected Renal Function
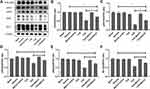
Both Cys-c and PCX are the two main factors that more sensitive for detecting mild to moderate changes in the estimated glomerular filtration rate.26 Compared to blank control groups, urine levels of Cre, UN, Cys-c and PCX were significantly increased in TCE sensitized positive group but not in solvent control group, BQ123 control group, TCE sensitized negative group and TCE+BQ123 sensitized negative group. The injection of BQ123 promoted the repairing of podocytes and the recovery of renal function (Cre and UN). Results are in Figure 3.
BQ123 Reduced the Protein Levels of TNF-α and MCP-1 in Glomeruli
Inflammation mediators such as MCP-1 and inflammatory factors such as TNF-α were overexpressed in TCE sensitized positive mice, but BQ123 reduced the expression of MCP-1 and TNF-α in TCE+BQ123 sensitized positive group mice (P<0.05). Results see in Figure 4.
BQ123 Alleviated Endothelial Cell Injury
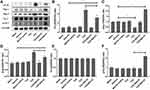
Figures 5 and 6 show that compared to TCE sensitized positive group, BQ123 downregulated promotor of apoptosis proteins (Fas, Fas/L, Bax, Bad, pro-caspase-3 and cleaved-caspase-3) and upregulated inhibitor of apoptosis proteins (PI3K, pAKT, eNOS, pBad and Bcl-2) in TCE+BQ123 sensitized positive group (P<0.05).
Under TEM observation (Figure 7), foot processes of the podocytes appeared fused (red arrow), vacuolate degeneration of massive mitochondria (yellow arrow) and dilation of Golgi’s apparatus (green arrow) were also observed in TCE sensitized positive mice glomerular endothelial cells. But in TCE+BQ123 sensitized positive mice, glomerular basement membrane slightly thickened (black arrow), occasional vacuolate degeneration of mitochondria (yellow arrow) was seen.
Glomerular Apoptosis Rate
We detected the glomerular apoptosis rate by using TUNEL assay kit (Figures 8 and 9). Blank control group, solvent control group, BQ123 control group, TCE sensitized negative group and TCE+BQ123 sensitized negative group were rarely showed apoptotic cells, the cell apoptosis rate was about 3%. In TCE sensitized positive group, the cell apoptosis rate was (16.96±7.17)%, significantly higher than the solvent control group (2.95%±0.84%). But the cell apoptosis rate was significantly decreased with the treatment of BQ123 (apoptosis rate: 8.85%±2.53%; P<0.05).
BQ123 Alleviated Podocyte Injury
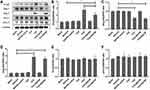
The mice glomeruli podocyte injury was reflected by detecting the protein levels of podocin and nephrin (Figure 10) and transmission electron microscope observation (Figure 11). Results indicated that in TCE sensitized positive mice, podocin and nephrin were significantly reduced (P<0.05), the podocytes had light swelling (blue arrow) with mitochondria vacuolate degeneration (yellow arrow). Mitochondrial injury reduced (yellow arrow), podocin and nephrin increased in TCE+BQ123 sensitized positive mice by injecting with BQ123.
BQ123 Downregulated ETAR, Ang-2 and Upregulated Ang-1, pTie-2
We gave the mice BQ123 or BQ788 to investigate whether Ang-1 was inhibited by ET-1. Western blot results (Figure 12) showed that compared to the solvent control group, ETAR and Ang-2 significantly increased while Ang-1 decreased in TCE sensitized positive group. The ETAR inhibitor BQ123 downregulated ETAR and Ang-2 expression levels and upregulated Ang-1 and pTie-2 expression levels significantly (P<0.05).
BQ788 Downregulated ETBR but Not Upregulated Ang-1, pTie-2
Figure 13 shows that the expression level of ETBR was inhibited by BQ788. ETBR and Ang-2 increased in TCE sensitized positive group, Ang-1 decreased in TCE sensitized positive group, but Ang-2 was not inhibited by BQ788, Ang-1 and pTie-2 were not promoted by BQ788.
The Location of ET-1, ETAR, ETBR, Ang-1, Ang-2 and Tie-2
We further identified the localization of ET-1, ETAR, Ang-1, Ang-2 and Tie-2 by immunofluorescence to clearly understand the cross talking between endothelial cells and podocytes. Results (Figures 14–18) showed that in glomeruli, ET-1, Ang-2, Tie-2 were mainly expressed in endothelial cells, ETAR, Ang-1 were mainly expressed in podocytes.
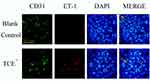 |
Figure 14 The location of ET-1 (×400). ET-1 was mainly expressed on endothelial cells. Yellow arrow: endothelial cells. |
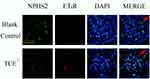 |
Figure 15 The location of ETAR (×400). ETAR was mainly expressed on podocytes. Red arrow: podocytes. |
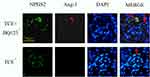 |
Figure 16 The location of Ang-1 (×400). Ang-1 was mainly expressed on podocytes. Red arrow: podocytes. |
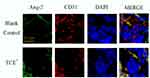 |
Figure 17 The location of Ang-2 (×400). Ang-2 was mainly expressed on endothelial cells. Yellow arrow: endothelial cells. |
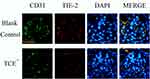 |
Figure 18 The location of Tie-2 (×400). Tie-2 was mainly expressed on endothelial cells. Yellow arrow: endothelial cells. |
The Gene Levels of Ang-1/Ang-2
Ang-1 and Ang-2 gene levels were detected by qRT-PCR. Results (Figure 19) showed that compared to solvent control group mice, the gene level of Ang-2 was significantly increased in TCE sensitized positive mice (P<0.05). Compared to TCE sensitized positive mice, the gene level of Ang-1 was significantly increased and Ang-2 decreased in TCE+BQ123 sensitized positive group (P<0.05). Compared to solvent control group mice, the ratio of Ang-1/Ang-2 was significantly decreased in TCE sensitized positive group, but significantly increased in TCE+BQ123 sensitized positive group mice (P<0.05).
Discussion
TCE is a kind of chlorinated solvent and currently 80–90% of TCE is used for metals degreasing.27 TCE is also a main industrial toxic byproduct that widely exists in soil, air and water.28 Exposure to TCE may cause nervous system damage, reproductive and developmental disorders, cancer, and OMLDT. Till now, there is still no specific drug for OMLDT treatment. In OMLDT patients, part of them may display severe renal injury. Our previous studies have investigated the mechanisms of renal endothelial cells and podocyte damage, respectively. In this study, we combined the TCE sensitized mouse model with OMLDT patients to investigate the cross talk between glomerular endothelial cells and podocytes. This study focused on the glomerular injury of OMLDT patients and will provide a new direction for the treatment of renal injury.
ET-1 and its two G protein-coupled receptors (ETAR and ETBR) play a significant role in regulating blood vessel stability.29 The overexpression of ET-1 is also considered to be associated with inflammation. Studies found that ET-1 triggers the release of NO through ETBR and subsequently causing vascular relaxation,30 while causes sustained powerful vasoconstriction by binding to ETAR.31 Angiopoietins as the vascular growth factors are important in regulating vascular remodeling, stabilization and maturation.21 Previous studies also found that Ang-1 and Ang-2 are involved in podocyte injury.32 In this study, we found that ET-1 and Ang-2 were highly expressed in OMLDT patients and TCE sensitized positive mice but Ang-1 was inhibited. TCE sensitized positive mice renal injury got alleviated after injecting with BQ123. The ET-1 and Angiopoietins were also associated with renal injury. Was renal Ang-1 inhibited by the overexpressed ET-1?
Because the two different endothelin receptors put two different biological effects, it is better to investigate their functions by using the two specific inhibitors for ETAR (BQ123) and ETBR (BQ788) respectively. We found that compared to TCE sensitized positive mice, ETAR and Ang-2 were inhibited by injecting with BQ123, Ang-1 and its phosphorylated receptor pTie-2 were upregulated by injecting with BQ123. Besides, ETBR was inhibited by injecting with BQ788, but Ang-1 and its phosphorylated receptor pTie-2 were not upregulated, Ang-2 was not inhibited by injecting with BQ788. We conclude that the glomerulus Ang-1/Ang-2 expression was regulated by ET-1/ETAR in TCE sensitized mice.
Previous studies found that the overexpression of Ang-1 down-regulated Fas and Fas/L expression via the PI3K/Akt pathway.33 Sun et al found that Ang-2 up-regulated the expression levels of Bax and cleaved caspase-3 but down-regulated the expression level of Bcl-2.34 In this study, we found that the Ang-1/Ang-2 gene level was decreased in TCE sensitized positive group compared to solvent control group. We further detected the glomerular endothelial cell apoptosis rate, found that the cell apoptosis rate was (16.96±7.17)% in TCE sensitized positive group, but (2.95±0.84)% in solvent control group. The cell apoptosis rate was decreased by injecting with BQ123. The promotor of apoptosis proteins Fas, Fas/L, Bax, Bad, pro-caspase-3 and cleaved- caspase-3 also downregulated by injecting with BQ123, the inhibitor of apoptosis proteins PI3K, pAKT, eNOS, pBad and Bcl-2 upregulated by injecting with BQ123. The endothelial cell injury was also alleviated. So we conclude that both increased Ang-2 and decreased Ang-1 were associated with TCE-induced glomerular apoptosis.
We also found that the TCE sensitized positive mouse glomeruli podocytes got obvious injury, but the loss of nephrin and podocin was prevented by injecting with the ETAR antagonist. Indicating that the overexpression of ET-1 induced nephrin shedding was also mediated by ET-1/ETAR. Collino et al. got the same results.16
In summary, ET-1 was a key factor which associates with OMLDT patients' renal injury. Serum level of Ang-1 was negatively associated with ET-1, ET-1 was positively associated with renal injury in OMLDT patients. The endothelial cells secreted ET-1 promoted podocyte injury in TCE sensitized mice. The elevated ET-1 inhibited podocytes to secret Ang-1 and promoted Ang-2 expression in endothelial cells by combining to ETAR which located at podocytes, the disruption of Ang-1/Ang-2 induced the glomerular endothelial cell apoptosis and podocytes injury. This study found the new pathway in renal endothelial cells and podocytes cross talking. Suggesting that the ETAR inhibitor may have a significant effect in reducing renal endothelial cell injury. There are several limitations of this study. The first limitation was that the glomerular endothelial cells and podocytes could not be separated due to technical limitations. The second limitation was that the TCE sensitized kidney injury was a kind of immune injury in this study; the model could not be well mimicked using in vitro cell experiments.
Conclusion
ET-1/ETAR-Angs/Tie-2 pathway mediated the injury between glomerular endothelial cells and podocytes. BQ123 alleviated the endothelial cells and podocyte injury and renal inflammation by promoting Ang-1 expression and inhibiting Ang-2 expression. This study added a piece of important information for a better understanding of the mechanisms underlying TCE-induced renal injury. The expression of Endothelin-1 could affect renal podocyte injury, suggest that the disorder of vascular endothelial system may exist in OMLDT patients. This conclusion provided a new treatment direction for TCE-induced kidney immune injury.
Ethical Statement
This work has received approval for research ethics from Anhui Medical University and a proof/certificate of approval is available upon request (Ethical approval number: 20,131,419). The experimental animal protocol was approved by Anhui Medical University (animal ethical committee number: SCXK 2017-001).
Acknowledgments
We would like to thank the Platform of Environmental Exposure and Life Health Research in Anhui Medical University.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was partially funded by the National Natural Science Foundation of China grant 81874259, 81673141; National Natural Science Foundation of Anhui Province grant 2008085QH385.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhao N, Song X, Naito H, et al. Trichloroethylene and trichloroethanol induce skin sensitization with focal hepatic necrosis in guinea pigs. J Occup Health. 2020;62(1):e12142. doi:10.1002/1348-9585.12142
2. Urban JD, Wikoff DS, Chappell GA, Harris C, Haws LC. Systematic evaluation of mechanistic data in assessing in utero exposures to trichloroethylene and development of congenital heart defects. Toxicology. 2020;436:152427. doi:10.1016/j.tox.2020.152427
3. Archer NP, Bradford CM, Villanacci JF, et al. Relationship between vapor intrusion and human exposure to trichloroethylene. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2015;50(13):1360–1368. doi:10.1080/10934529.2015.1064275
4. Huang Y, Jiang B, Xia Y, et al. Downregulation of miR-133a contributes to the cardiac developmental toxicity of trichloroethylene in zebrafish. Chemosphere. 2020;251:126610. doi:10.1016/j.chemosphere.2020.126610
5. Ravi S, Lonappan L, Touahar I, Fonteneau É, Vaidyanathan VK, Cabana H. Evaluation of bio-fenton oxidation approach for the remediation of trichloroethylene from aqueous solutions. J Environ Manage. 2020;270:110899. doi:10.1016/j.jenvman.2020.110899
6. Guha N, Loomis D, Grosse Y, et al. Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol. 2012;13(12):1192–1193. doi:10.1016/s1470-2045(12)70485-0
7. Wang H, Nakajima T, Ito Y, et al. Increased risk of occupational trichloroethylene hypersensitivity syndrome at exposure levels higher than 15 mg/L of urinary trichloroacetic acid, regardless of whether the patients had the HLA-B*13:01 allele. Environ Res. 2020;191:109972. doi:10.1016/j.envres.2020.109972
8. Zhang J, Li N, Yang L, et al. Bradykinin contributes to immune liver injury via B2R receptor-mediated pathways in trichloroethylene sensitized mice: a role in Kupffer cell activation. Toxicology. 2019;415:37–48. doi:10.1016/j.tox.2019.01.015
9. Li W, Liu X, Yang X, et al. Effect of trichloroacetaldehyde on the activation of CD4+T cells in occupational medicamentosa-like dermatitis: an in vivo and in vitro study. Toxicology. 2019;423:95–104. doi:10.1016/j.tox.2019.05.014
10. Li B, Xie H, Wang X, et al. Oxidative stress mediates renal endothelial cell damage in trichloroethylene-sensitized mice. J Toxicol Sci. 2019;44(5):317–326. doi:10.2131/jts.44.317
11. Wang G, Zhang J, Dai Y, Xu Q, Zhu Q. Local renal complement activation mediates immune kidney injury by inducing endothelin-1 signalling and inflammation in trichloroethylene-sensitised mice. Toxicol Lett. 2020;333:130–139. doi:10.1016/j.toxlet.2020.07.036
12. Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol. 2008;86(8):485–498. doi:10.1139/Y08-059
13. Shihoya W, Nishizawa T, Okuta A, et al. Activation mechanism of endothelin ETB receptor by endothelin-1. Nature. 2016;537(7620):363–368. doi:10.1038/nature19319
14. De Miguel C, Pollock JS. Does endoplasmic reticulum stress mediate endothelin-1-induced renal inflammation? Am J Physiol Regul Integr Comp Physiol. 2013;305(2):R107–R109. doi:10.1152/ajpregu.00184.2013
15. Sasser JM, Sullivan JC, Hobbs JL, et al. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol. 2007;18(1):143–154. doi:10.1681/ASN.2006030208
16. Collino F, Bussolati B, Gerbaudo E, et al. Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol. 2008;294(5):F1185–F1194. doi:10.1152/ajprenal.00442.2007
17. Isaji T, Osuka K, Ohmichi Y, et al. Expression of angiopoietins and angiogenic signaling pathway molecules in chronic subdural hematomas. J Neurotrauma. 2020;37(23):2493–2498. doi:10.1089/neu.2020.7042
18. Whitehead M, Osborne A, Widdowson PS, Yu-Wai-Man P, Martin KR. Angiopoietins in diabetic retinopathy: current understanding and therapeutic potential. J Diabetes Res. 2019;2019:5140521. doi:10.1155/2019/5140521
19. Saharinen P, Leppänen VM, Alitalo K. SnapShot: angiopoietins and their functions. Cell. 2017;171(3):724–724.e1. doi:10.1016/j.cell.2017.10.009
20. Satchell SC, Harper SJ, Tooke JE, Kerjaschki D, Saleem MA, Mathieson PW. Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J Am Soc Nephrol. 2002;13(2):544–550.
21. He FF, Zhang D, Chen Q, et al. Angiopoietin-tie signaling in kidney diseases: an updated review. FEBS Lett. 2019;593(19):2706–2715. doi:10.1002/1873-3468.13568
22. Kerget F, Özkurt Z, Öztürk N, Yılmaz S. The relationship with clinical course and prognosis of serum endothelin-1, angiopoietin-2, and tie-2 levels in Crimean-Congo hemorrhagic fever. Turk J Med Sci. 2019;49(4):1192–1197. doi:10.3906/sag-1812-10
23. Cabral T, Lima LH, Mello LGM, et al. Bevacizumab injection in patients with neovascular age-related macular degeneration increases angiogenic biomarkers. Ophthalmol Retina. 2018;2(1):31–37. doi:10.1016/j.oret.2017.04.004
24. Michinaga S, Tanabe A, Nakaya R, et al. Angiopoietin-1/Tie-2 signal after focal traumatic brain injury is potentiated by BQ788, an ETB receptor antagonist, in the mouse cerebrum: involvement in recovery of blood-brain barrier function. J Neurochem. 2020;154(3):330–348. doi:10.1111/jnc.14957
25. Wang H, Zhang JX, Li SL, et al. An animal model of trichloroethylene-induced skin sensitization in BALB/c mice. Int J Toxicol. 2015;34(5):442–453. doi:10.1177/1091581815591222
26. Correa S, Morrow DA, Braunwald E, et al. Cystatin C for risk stratification in patients after an acute coronary syndrome. J Am Heart Assoc. 2018;7(20):e009077. doi:10.1161/JAHA.118.009077
27. Shih YJ, Hsia KF, Chen CW, Chen CF, Dong CD. Characteristics of trichloroethene (TCE) dechlorination in seawater over a granulated zero-valent iron. Chemosphere. 2019;216:40–47. doi:10.1016/j.chemosphere.2018.10.059
28. Abdraboh ME, Abdeen SH, Salama M, El-Husseiny M, El-Sherbini YM, Eldeen NM. Developmental neurotoxic effects of a low dose of TCE on a 3-D neurosphere system. Biochem Cell Biol. 2018;96(1):50–56. doi:10.1139/bcb-2017-0089
29. Finch J, Riggs DW, O’Toole TE, Pope CA
30. Zhang ZS, Chen W, Li T, Liu LM. Organ-specific changes in vascular reactivity and roles of inducible nitric oxide synthase and endothelin-1 in a rabbit endotoxic shock model. J Trauma Acute Care Surg. 2018;85(4):725–733. doi:10.1097/TA.0000000000002036
31. Taura D, Nakao K, Nakagawa Y, Kinoshita H, Sone M, Nakao K. C-type natriuretic peptide (CNP)/guanylate cyclase B (GC-B) system and endothelin-1(ET-1)/ET receptor A and B system in human vasculature. Can J Physiol Pharmacol. 2020;98(9):611–617. doi:10.1139/cjpp-2019-0686
32. Gao X, Xu H, Liu H, Rao J, Li Y, Zha X. Angiopoietin-like protein 3 regulates the motility and permeability of podocytes by altering nephrin expression in vitro. Biochem Biophys Res Commun. 2010;399(1):31–36. doi:10.1016/j.bbrc.2010.07.027
33. Ren D, Zhu Q, Li J, Ha T, Wang X, Li Y. Overexpression of angiopoietin-1 reduces doxorubicin-induced apoptosis in cardiomyocytes. J Biomed Res. 2012;26(6):432–438. doi:10.7555/JBR.26.20120006
34. Sun NN, Li C, Zhou L, et al. Lentivirus-mediated angiopoietin-2 gene silencing decreases TNF-α induced apoptosis of alveolar epithelium cells. Biochem Cell Biol. 2016;94(5):491–497. doi:10.1139/bcb-2016-0045
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.