Back to Journals » Journal of Pain Research » Volume 13
Electroacupuncture Treatment Attenuates Paclitaxel-Induced Neuropathic Pain in Rats via Inhibiting Spinal Glia and the TLR4/NF-κB Pathway
Authors Zhao YX , Yao MJ, Liu Q , Xin JJ, Gao JH, Yu XC
Received 4 December 2019
Accepted for publication 14 January 2020
Published 29 January 2020 Volume 2020:13 Pages 239—250
DOI https://doi.org/10.2147/JPR.S241101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Yu-Xue Zhao, 1 Ming-Jiang Yao, 2, 3 Qun Liu, 1 Juan-Juan Xin, 1 Jun-Hong Gao, 1 Xiao-Chun Yu 1
1Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing 100700, People’s Republic of China; 2Institute of Basic Medical Sciences, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing 100091, People’s Republic of China; 3Key Laboratory of Pharmacology of Chinese Materia Medica, Beijing 100091, People’s Republic of China
Correspondence: Yu-Xue Zhao; Xiao-Chun Yu
Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Dongcheng District, Beijing 100700, People’s Republic of China
Email [email protected]; [email protected]
Background and Purpose: Neuropathic pain is a major side-effect of paclitaxel (PTX) chemotherapy. Although the precise mechanisms responsible for this pain are unclear, the activation of neuroglia and upregulation of the TLR4/NF-κB pathway are known to be involved. In this study, we determined whether electroacupuncture (EA) could limit mechanical hypersensitivity resulting from the chemotherapeutic drug PTX in rats, and investigated the potential mechanisms involved.
Methods: Rats intraperitoneally received a cumulative dose of 8 mg/kg PTX (2 mg/kg per day) or vehicle control on alternate days (day 0, 2, 4 and 6). EA treatment (10 Hz, 1 mA) was applied at bilateral ST36 acupoints in rats once every other day on days 0– 14. For sham EA, needles were inserted at ST36 acupoints without electrical stimulation. Mechanical allodynia was measured by mechanical withdrawal latency (MWL) of paws to a mechanical stimulus every 2 days. Protein expression of TLR4 and NF-κB p65, as well as TMEM119 and GFAP (indicators of microglia and astrocytes, respectively) in spinal cord was quantified by Western blot analysis. Levels of inflammatory cytokines IL-1β and TNF-α in spinal cord and serum were detected by ELISA.
Results: Mechanical allodynia induced by PTX in both paws (right and left) of rats was significantly attenuated by EA but not sham EA treatment. In addition, EA, but not sham EA, inhibited the activation of both microglia (TMEM119) and astrocytes (GFAP) in lumbar spinal cord. Moreover, Western blot analysis revealed that protein expression of TLR4 and NF-κB in spinal cord was suppressed by EA but not sham EA treatment. PTX significantly increased inflammatory cytokines in spinal cord and serum, which were ameliorated by EA treatment but not by sham EA.
Conclusion: These results indicate that EA treatment attenuates PTX-induced mechanical allodynia. The putative mechanism corroborating this finding could be related to the suppression of activated microglia and astrocytes in spinal cord, as well as the inhibition of the activated TLR4/NF-κB signaling pathway by EA treatment.
Keywords: electroacupuncture, neuropathic pain, paclitaxel, TLR4/NF-κB pathway, neuroglia
Introduction
Paclitaxel (PTX) is a highly effective and commonly used chemotherapeutic agent derived from the bark of Taxus brevifolia, and has been widely used for the treatment of a broad range of tumors.1 However, PTX therapy frequently induces peripheral neurotoxicity, which typically presents as painful neuropathy that can even lead to discontinuation of treatment.2 It has been reported that PTX-induced neuropathic symptoms occurred in up to 92.8% of all treated patients according to dose intensity,3,4 with the most common complaints being numbness, tingling, burning pain and mechanical allodynia. No optimal therapies have been proven effective to prevent or minimize the nerve injury and neuropathic pain induced by PTX. Thus, the development of a novel analgesic approach during chemotherapy with PTX to alleviate peripheral neuropathy, especially pain symptoms, is highly required.
Electroacupuncture (EA) has been proven to be clinically efficacious in alleviating various types of pain. Emerging experimental and clinical studies have reported that EA can be potentially used as an effective therapy in the treatment of PTX-induced peripheral neuropathy. Basic research demonstrated that EA significantly inhibited mechanical allodynia/hyperalgesia in rodents with PTX-evoked peripheral neuropathy.5,6 In addition, combined treatment with EA and gabapentin (an analgesic drug) showed robust and enduring analgesic actions against PTX-induced neuropathy.7 Furthermore, a phase IIA trial showed that acupuncture attenuated the severity of peripheral neuropathy induced by PTX chemotherapy in breast cancer patients.8 According to previous basic studies, the anti-nociceptive effect of EA in relieving PTX-induced neuropathic pain was mediated via spinal opioid receptors and the adrenergic pathway, which is similar to the mechanism underlying EA’s analgesic actions in other pain models.9–11 Nevertheless, the molecular mechanisms behind the EA intervention against PTX-caused neuropathy have not yet been fully elucidated. Since chemotherapy-induced pain is usually complicated by more complex components, the analgesic actions of EA may involve other potentially delicate mechanisms.
A growing body of evidence suggests that activated glial cells in the spinal cord play a prominent role in pathological pain modulation.12,13 It has been reported that PTX treatment induces activation of glial cells (microglia and astrocytes) in the spinal cord.14–18 Thus, glial cells appear to be potential targets for the treatment of PTX-induced neuropathic pain. Both microglia and astrocytes express toll-like receptor 4 (TLR4) in the central nervous system.19,20 TLR4 plays an essential role in the transition and induction of pain. It was reported that PTX treatment could result in increased expression of TLR4 and the activation of its downstream nuclear factor-κB (NF-κB) signaling pathway in the spinal cord, leading to the production of pro-inflammatory cytokines,21,22 which, in turn, contributed to the occurrence and development of chemotherapy-induced neuropathic pain.23 In addition, previous studies have proved the inhibitory action of EA in abnormal activation of spinal glia, TLR4 and NF-κB in various animal models of pain and inflammation.24–28
In the present study, we hypothesized that EA treatment could attenuate PTX-induced mechanical allodynia through the inhibition of spinal glia and their downstream TLR4/NF-κB pathway. In brief, we identified the analgesic effect of EA on a PTX-induced neuropathic model in rats, and the changes in protein expression of TMEM119 (microglia), GFAP (astrocytes), TLR4 and NF-κB in the lumbar spinal cord (L3–L6) of experimental rats, as well as the concentrations of inflammatory cytokines in spinal cord and serum.
Materials and Methods
Animals
Male Sprague–Dawley rats (from China Academy of Military Science, Beijing, People’s Republic of China), weighing 120–150 g, were used in this study. Experimental rats were housed four to five per cage under conditions of controlled temperature (23–25°C) and light (07:00 h on, 19:00 h off). Standard food and water were supplied ad libitum. Rats received care consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee of China Academy of Chinese Medical Sciences (reference no. 2015010801). All efforts were made to minimize discomfort and the number of animals used. A total of 29 rats were randomly divided into the following four groups: Control group (n=7), PTX group (n=8), PTX + EA group (n=7) and PTX + sham EA group (n=7). PTX injection (6 mg/mL, Beijing Union Pharmaceutical Factory, Beijing, People’s Republic of China) was diluted with PBS solution to make PTX solution with a concentration of 2 mg/mL, and then administered (2 mg/kg, i.p.) to rats on four alternate days (cumulative dose 8 mg/kg, i.p.) to induce peripheral neuropathic pain. Control rats were given i.p. injections of vehicle (PBS solution) (Figure 1).
 |
Figure 1 Schematic representation of the experimental procedure. Abbreviations: PTX, paclitaxel; VEH, vehicle; EA, electroacupuncture. |
EA Treatment
Acupoints of ST36 (Zusanli) on bilateral hind limbs were used for EA treatment.29 The acupoint of ST36 in rats is located 10 mm below the knee joint and 5 mm lateral to the anterior tubercle of the tibia. Acupuncture needles at each hind limb were inserted as follows: one into the ST36 acupoint and the other one 5 mm below the ST36 acupoint (5 mm in depth). The tips of the electrode cables from an electroacupuncture apparatus (HANS-200A) were connected to a pair of steel acupuncture needles, and constant current pulses (10 Hz, 1 mA) were applied for 30 min. For sham EA, acupuncture needles were inserted into points on the bilateral hind limbs of rats in the same way as in the EA group, but without electrical current. Both EA and sham EA procedures were performed under isoflurane anesthesia. After the first administration of PTX, EA or sham EA treatment was applied every other day for 14 days following the initiation of PTX (Figure 1).
Body Weight Assessment
The body weight of rats was measured on days 0, 1, 3, 5, 7, 9, 11, 13 and 15 and expressed as fold change in body weight with respect to baseline weight (day 0). In addition, we calculated the growth rate of body weight (%) using the following formula:
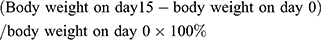
Behavioral Tests
Animals were habituated to the environment for 3 days before behavioral testing. Baseline measurement of withdrawal latencies for both hind paws was performed prior to PTX or vehicle administration. For each testing session, rats were placed on an elevated metal wire mesh floor and confined in individual Perspex boxes and allowed to acclimatize for 30 min. Mechanical withdrawal latencies (MWLs) of both right and left hind paws (response time, s) were measured using a Dynamic Plantar Aesthesiometer (Ugo Basile, Varese, Italy) with a metal filament (0.5 mm in diameter). The Aesthesiometer filament was raised to touch the plantar surface of the hind paws with a constant force at 2 g/s (cut-off force: 50 g) until the paw was withdrawn. At 10 min intervals, the rod was applied to each hind paw three times.28 Behavioral testing was conducted on the day of the first i.p. injection of PTX or vehicle and every other day for 14 days following the initiation of PTX or vehicle administration. The area under the curve (AUC) of withdrawal latencies for both hind paws in each group was calculated to investigate the overall effect.6
Western Blot Assay
After 15 days following the initiation of PTX or vehicle administration, rats were killed and the dissected spinal cords were snap-frozen and stored at −80°C. Whole-cell lysates were prepared from the lumbar region of the spinal cord and protein concentrations were determined using a BCA protein assay kit (Thermo Scientific, USA). Equal amounts of protein extract were resolved by 8–10% SDS-PAGE gels and transferred to a polyvinylidene difluoride (PVDF) membrane (Merck Millipore, USA). Membranes were then incubated with mouse anti-TMEM119 (1:1,000; Proteintech, USA), mouse anti-GFAP (GA5) (1:1,000; Cell Signaling Technology [CST], USA), rabbit anti-TLR4 (1:500; Abcam, UK), rabbit anti-NF-κB p65 (1:1,000; CST, USA) and rabbit anti-GAPDH (1:1,000; CST, USA). All membranes with antibodies were incubated overnight at 4°C and then incubated with goat anti-rabbit IgG (1:3,000; CST, USA) or horse anti-mouse IgG (1:3,000; CST, USA) for 1 h at room temperature. Protein bands were detected with the use of a chemiluminescent substrate (ECL Prime Western Blotting Detection Reagents; Amersham, UK) and visualized using a luminescent image analyzer (ChemiDoc Imaging System, Bio-Rad, USA).
ELISA
Concentrations of IL-1β and TNF-α in spinal cord and serum were measured using commercially available rat ELISA kits (R&D Systems, Minneapolis, USA) according to the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6.0 (La Jolla, CA, USA). All data were presented as mean ± SEM and analyzed with repeated-measures one-way ANOVA followed by Dunnett’s post-hoc test, and two-way ANOVA followed by Bonferroni’s multiple comparison test. In all cases, a value of P<0.05 was considered statistically significant.
Results
Animals’ General Health
Consistent with the results presented in previous studies,30,31 the administration of PTX did not cause apparent mortality and morbidity in experimental rats. None of the rats showed any signs of ill-health, such as alopecia, self-mutilating behavior, or abnormal grooming and locomotor behavior. Rats in all groups gained body weight normally, and there was no significant difference in the average amount of weight gain (ratio of baseline body weight, fold) between groups during 15 days of testing. In addition, no significant difference in final body weight gain (growth rate of body weight, %) was observed among the different groups (Figure 2).
EA Alleviated PTX-Induced Neuropathic Pain
Compared with vehicle (Control), PTX administration caused and maintained neuropathic pain, characterized by a marked reduction in MWLs to mechanical stimulation (2 g/s). As shown in Figure 3A and B, MWL values were decreased by PTX treatment and developed progressively (P<0.05, P<0.01 vs Control) throughout the whole experiment period. Evident tactile allodynia developed following the initial PTX administration and persisted to the end. Compared to PTX, EA significantly attenuated the reduction in MWL induced by PTX administration (P<0.01 vs PTX + EA). In contrast, sham EA exerted no attenuated effect on decreased MWL induced by PTX in response to mechanical stimulation. As shown in Figure 3C, there was no significant difference in MWL (evaluated by AUC) between left and right hind paws, suggesting a symmetrical neuropathy produced by PTX administration. In addition, AUC analysis of continuous MWL values confirmed the prolonged mechanical allodynia produced by PTX in both hind paws and revealed the remarkable analgesic effect of EA, but not sham EA, on PTX-evoked neuropathic pain, by showing a significant increase in AUC percentage in the EA group in comparison with that in the PTX group (P<0.01 vs PTX + EA) (Figure 3C).
EA Attenuated PTX-Induced Glial Activation in Spinal Cord
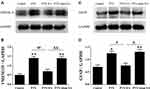
By means of Western blot, the specific markers of microglia (TMEM119) and astrocytes (GFAP) in spinal cord were detected. As shown in Figure 4, the protein levels of spinal TMEM119 and GFAP were significantly increased in the PTX group in comparison with those in the Control (P<0.05, P<0.01), suggesting a remarkable activation of both microglia and astrocytes in spinal cord following PTX administration. EA treatment markedly decreased protein expression of both TMEM119 (P<0.01) (Figure 4A and B) and GFAP (P<0.05) (Figure 4C and D) in spinal cord compared with the PTX group, indicating an evident inhibitory action of EA on PTX-induced spinal glia activation. In contrast, sham EA treatment did not show any effect on the elevated protein expression of either glial marker caused by PTX.
EA Attenuated PTX-Induced TLR4/NF-κB Activation in Spinal Cord
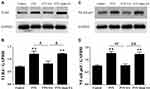
By means of Western blot, protein expression of TLR4 and NF-κB p65 in lumbar spinal cord was detected . As shown in Figure 5, relative protein levels of TLR4 and NF-κB p65 in spinal cord increased significantly in the PTX group compared with those in the Control group (P<0.01). In comparison with the PTX group, EA treatment resulted in a pronounced reduction in both TLR4 (Figure 5A and B) and NF-κB p65 (Figure 5C and D) signals (P<0.05 and P<0.01, respectively), whereas sham EA treatment did not affect the upregulated protein expression of either TLR4 or NF-κB p65 in spinal cord caused by PTX administration.
EA Attenuated the Increase in Inflammatory Cytokines Induced by PTX in Spinal Cord and Serum
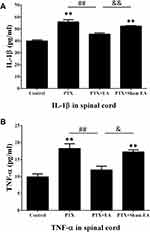
As shown in Figures 6 and 7, increased concentrations of inflammatory cytokines in spinal cord and serum were observed 14 days after the first administration of PTX. Compared to the Control group, concentrations of IL-1β in the PTX group were significantly increased from 39.88±0.89 to 55.91±1.77 pg/mL in spinal cord (P<0.01) and from 25.30±0.91 to 34.51±0.61 pg/mL in serum (P<0.01). Similarly, the levels of TNF-α in both spinal cord (from 9.88±0.87 to 18.23±1.40 pg/mL, P<0.01) and serum (from 6.98±0.33 to 10.96±0.53 pg/mL, P<0.01) were significantly elevated in the PTX group compared to the Control group. Two weeks of EA treatment remarkably reduced spinal and serum concentrations of IL-1β (from 55.91±1.77 to 45.63±1.11 pg/mL, P<0.01, in spinal cord; from 34.51±0.61 to 27.12±0.70 pg/mL, P<0.01, in serum) and TNF-α (from 18.23±1.40 to 11.93±1.11 pg/mL, P<0.01, in spinal cord; from 10.96±0.53 to 8.40±0.12 pg/mL, P<0.01, in serum), compared to the Controls. In contrast, sham EA did not show any obvious effect on attenuating the increase in inflammatory cytokines provoked by PTX administration.
Discussion
The present study confirmed that repeated administrations of PTX in rats resulted in peripheral sensory neuropathy characterized by mechanical allodynia, which was accompanied by increased glial activity and TLR4/NF-κB signals in spinal cord, as well as elevated levels of inflammatory cytokines in spinal cord and serum. The data reported here showed that EA treatment significantly prevented the development of PTX-induced hypersensitive behaviors to mechanical stimulation in both left and right hind paws of rodents. The underlying mechanism that potentiated the anti-nociceptive effect of EA treatment against PTX-induced painful neuropathy may be associated with its regulatory actions on protein expression of neuroglia and TLR4/NF-κB signals in spinal cord, as well as on central and peripheral levels of downstream inflammatory cytokines.
Consistent with previous studies,31–33 our results showed that repeated administrations of PTX did not cause mortality or disability in any experimental rats. In addition, PTX treatment did not show any obvious effect on either body weight or growth rate of body weight in experimental rats. Animals in all groups gained weight normally, indicating that cumulative doses of PTX (8.0 mg/kg) cause no significant impairment in animals’ general health.
In the present study, repeated administrations of PTX produced significant peripheral neuropathy characterized by mechanical allodynia in hind paws of experimental rats, but without any obvious motor deficit or motor impairment (motor function was assessed using the Tarlov motor scale; data not shown). Mechanical hypersensitivity following cumulative administration of PTX was highlighted by a reduction in the time to response to the force applied for hind paw retraction. Moreover, our results showed that there were no significant differences in MWL between left and right hind paws following PTX administration, suggesting that a model of symmetrical neuropathy was established which could mimic the neuropathic pain occurring symmetrically in the extremities of patients treated with PTX.34 In accordance with previous reports,35 we confirmed the anti-nociceptive action of EA treatment against PTX-induced painful neuropathy. Specifically, EA treatment remarkably suppressed the development of PTX-induced mechanical allodynia, with the analgesic effect expressed especially by increasing the time of MWL to response in the assessment of mechanical allodynia. We chose 10 Hz as the EA frequency in the present study since EA at this frequency had a more potent analgesic action against PTX-induced neuropathic pain. This may be attributed to EA being able to induce more types of analgesic opioids in the central nervous system at this frequency than at alternative frequencies.5,9,35
It is well recognized that activation of microglia and astrocytes (i.e. upregulated expression of specific markers) in the spinal cord is crucial to spinal central sensitization in a wide array of pathological pain conditions.18,36 Our results showed a significant enhancement in protein expression of both TMEM119 and GFAP in spinal cord of experimental rats following PTX treatment, indicating the activation of spinal microglia and astrocytes, respectively, which is in agreement with previous studies.17,37 Thus, the increased spinal glial activity may contribute to PTX-induced neuropathic pain in the present study. However, although the activation of astrocytes in the spinal cord is repeatedly observed in PTX-induced peripheral neuropathy, the involvement of microglia remains controversial.18 By detecting protein expression of the microglial markers with Western blot and immunohistological assays, activation of spinal microglia in PTX-induced neuropathic pain was reported in some studies,14,17,37 but not in others.38,39 This discrepancy was reported to be attributed to different animal species, various treatment regimens, and diverse observation time-points.37 In the present study, all experiments were performed on relatively young rats (within 2 months). It is generally recognized that the central nervous system in juvenile rats is more susceptible to abnormal stimulations, which is termed the “juvenile window of susceptibility”.40 Importantly, a previous study reported a remarkable activation of microglia evoked by PTX treatment in the spinal cord of young rats instead of adult animals, which was ascribed to the juvenile window of susceptibility,14 providing a reasonable explanation for the activation of microglial activity following PTX administration in present study.
TLR4 is a critical receptor that is expressed on the surface of glial cells, including microglia and astrocytes, in the central nervous system, mediating glial responses to inflammation and nerve injury that are associated with the pathophysiology of the resulting hyperalgesia, and facilitating inflammatory responses that contribute to the pathology of persistent pain.22,41,42 It has been shown that activated neuroglia in spinal cord produce pro-inflammatory cytokines.43,44 Moreover, recent work has shown that PTX interacts with and activates TLR4 in the spinal cord, leading to the activation of the NF-κB signaling pathway and the release of pro-inflammatory cytokines, including IL-1β and TNF-α.41 The link between spinal TLR4 and the production of central and peripheral inflammatory cytokines could contribute to behavioral hypersensitivity following exposure to chemotherapy drugs.22 The PTX-induced activation of neuroglia and TLR4/NF-κB signals in spinal cord, documented herein, resulted in the spinal and systemic inflammatory responses, as revealed by the significant increase in IL-1β and TNF-α in the spinal cord and serum. Our results suggest that EA treatment exerted significant actions on inhibiting PTX-induced spinal glial activation, suppressing elevated TLR4/NF-κB signals in spinal cord, as well as decreasing the production of spinal and systemic inflammatory cytokines. These results provide a possible mechanism that may potentiate the analgesic effect of EA on PTX-provoked neuropathic pain; that is, EA treatment inhibits spinal microglia and astrocytes activated by PTX administration via downregulating TLR4 expressed on spinal glia, leading to the suppression of NF-κB signals and inhibition of downstream pro-inflammatory cytokines (such as TNF-α and IL-1β), and consequently inhibits the neuropathic pain (Figure 8). It should be noted that the parasympathetic nervous system may also be implicated in mediating the immune regulation of EA. Our previous study reported that EA at ST36 could reduce serum levels of inflammatory cytokines and prevent organ damage via vagal afferent and cholinergic anti-inflammatory pathways in experimental sepsis.27 Moreover, since PTX-induced neuropathy is multifactorial and involves various pathophysiological processes, including nociceptive transmission and neuroinflammation,45,46 the EA treatment should be a multi-target therapy as well. Although the regulatory effect of EA on neuroinflammation has been highlighted in the present study, the anti-nociceptive features of EA should be emphasized as well.47 The production and release of pro-inflammatory cytokines induced by PTX result in the sensitization of nociceptors, thus leading to mechanohypersensitivity.48
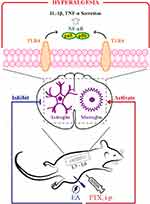 |
Figure 8 Schematic illustration of underlying mechanism in EA-mediated analgesia of PTX-induced neuropathic pain. Abbreviations: TLR4, toll-like receptor 4; PTX, paclitaxel; EA, electroacupuncture. |
Conclusion
In summary, our data suggest that EA treatment attenuates PTX–induced neuropathic pain in experimental rats, which could be associated with the downregulation of glial activity and TLR4/NF-κB signals in the spinal cord, as well as the inhibition of spinal and serous pro-inflammatory cytokines. However, the present study does not provide deep insights into the mechanisms underlying the effects of EA. Further studies will be performed to investigate the role of TLR4/NF-κB in the early stages following PTX administration and the key factors that mediate the actions of EA in the upstream pathways of neuroglia and TLR4.
Acknowledgments
This work was supported by Fundamental Research Funds for the Central Public Welfare Research Institutes (no. 201814009 and no. ZZ13-YQ-067) and National Natural Science Foundation of China Research Grants (no. 81202763 and no. 81873040). We thank Dr. Jun-Ying Wang for providing the antibody of GFAP as a gift.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004–1014. doi:10.1056/NEJM199504133321507
2. Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24(10):1633–1642. doi:10.1200/JCO.2005.04.0543
3. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi:10.1016/j.pain.2014.09.020
4. Pace A, Nisticò C, Cuppone F, et al. Peripheral neurotoxicity of weekly paclitaxel chemotherapy: a schedule or a dose issue? Clin Breast Cancer. 2007;7(7):550–554. doi:10.3816/CBC.2007.n.010
5. Meng X, Zhang Y, Li A, et al. The effects of opioid receptor antagonists on electroacupuncture-produced anti-allodynia/hyperalgesia in rats with paclitaxel-evoked peripheral neuropathy. Brain Res. 2011;1414:58–65. doi:10.1016/j.brainres.2011.08.004
6. Choi JW, Kang SY, Choi JG, et al. Analgesic effect of electroacupuncture on paclitaxel-induced neuropathic pain via spinal opioidergic and adrenergic mechanisms in mice. Am J Chin Med. 2015;43(1):57–70. doi:10.1142/S0192415X15500044
7. Kim MJ, Lee JH, Jang JU, Quan FS, Kim SK, Kim W. The efficacy of combination treatment of gabapentin and electro-acupuncture on paclitaxel-induced neuropathic pain. Korean J Physiol Pharmacol. 2017;21(6):657–666. doi:10.4196/kjpp.2017.21.6.657
8. Bao T, Seidman AD, Piulson L, et al. A phase IIA trial of acupuncture to reduce chemotherapy-induced peripheral neuropathy severity during neoadjuvant or adjuvant weekly paclitaxel chemotherapy in breast cancer patients. Eur J Cancer. 2018;101:12–19. doi:10.1016/j.ejca.2018.06.008
9. Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26(1):17–22. doi:10.1016/S0166-2236(02)00006-1
10. Zhang RX, Lao L, Wang L, et al. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020(1–2):12–17. doi:10.1016/j.brainres.2004.05.067
11. Kim HY, Wang J, Lee I, Kim HK, Chung K, Chung JM. Electroacupuncture suppresses capsaicin-induced secondary hyperalgesia through an endogenous spinal opioid mechanism. Pain. 2009;145(3):332–340. doi:10.1016/j.pain.2009.06.035
12. Wu J, Hocevar M, Bie B, Foss JF, Naguib M. Cannabinoid type 2 receptor system modulates paclitaxel-induced microglial dysregulation and central sensitization in rats. J Pain. 2019;20(5):501–514. doi:10.1016/j.jpain.2018.10.007
13. Ochi-ishi R, Nagata K, Inoue T, Tozaki-Saitoh H, Tsuda M, Inoue K. Involvement of the chemokine CCL3 and the purinoceptor p2×7 in the spinal cord in paclitaxel-induced mechanical allodynia. Mol Pain. 2014;10(53):
14. Ruiz-Medina J, Baulies A, Bura SA, Valverde O. Paclitaxel-induced neuropathic pain is age dependent and devolves on glial response. Eur J Pain. 2013;17(1):75–85. doi:10.1002/ejp.2013.17.issue-1
15. Pevida M, Lastra A, Hidalgo A, Baamonde A, Menéndez L. Spinal CCL2 and microglial activation are involved in paclitaxel-evoked cold hyperalgesia. Brain Res Bull. 2013;95:21–27. doi:10.1016/j.brainresbull.2013.03.005
16. Makker PG, Duffy SS, Lees JG, et al. Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLoS One. 2017;12(1):e0170814. doi:10.1371/journal.pone.0170814
17. Burgos E, Gómez-Nicola D, Pascual D, Martín MI, Nieto-Sampedro M, Goicoechea C. Cannabinoid agonist WIN 55,212-2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur J Pharmacol. 2012;682(1–3):62–72. doi:10.1016/j.ejphar.2012.02.008
18. Yan X, Li F, Maixner DW, et al. Interleukin-1beta released by microglia initiates the enhanced glutamatergic activity in the spinal dorsal horn during paclitaxel-associated acute pain syndrome. Glia. 2019;67(3):482–497. doi:10.1002/glia.23557
19. Bruno K, Woller SA, Miller YI, et al. Targeting toll-like receptor-4 (TLR4)-an emerging therapeutic target for persistent pain states. Pain. 2018;159(10):1908–1915. doi:10.1097/j.pain.0000000000001306
20. Iizumi T, Takahashi S, Mashima K, et al. A possible role of microglia-derived nitric oxide by lipopolysaccharide in activation of astroglial pentose-phosphate pathway via the Keap1/Nrf2 system. J Neuroinflammation. 2016;13(1):99. doi:10.1186/s12974-016-0564-0
21. Li Y, Adamek P, Zhang H, Adamek P
22. Li Y, Zhang H, Kosturakis AK, et al. MAPK signaling downstream to TLR4 contributes to paclitaxel-induced peripheral neuropathy. Brain Behav Immun. 2015;49:255–266. doi:10.1016/j.bbi.2015.06.003
23. Liu P, Yuan HB, Zhao S, et al. Activation of GABAB receptor suppresses diabetic neuropathic pain through toll-like receptor 4 signaling pathway in the spinal dorsal horn. Mediators Inflamm. 2018;2018:6016272. doi:10.1155/2018/6016272
24. Xu J, Chen XM, Zheng BJ, Wang XR. Electroacupuncture relieves nerve injury-induced pain hypersensitivity via the inhibition of spinal P2X7 receptor-positive microglia. Anesth Analg. 2016;122(3):882–892. doi:10.1213/ANE.0000000000001097
25. Kang JM, Park HJ, Choi YG, et al. Acupuncture inhibits microglial activation and inflammatory events in the MPTP-induced mouse model. Brain Res. 2007;1131(1):211–219. doi:10.1016/j.brainres.2006.10.089
26. Chen T, Xiong Y, Long M, et al. Electro-acupuncture pretreatment at zusanli (ST36) acupoint attenuates lipopolysaccharide-induced inflammation in rats by inhibiting Ca2+ influx associated with cannabinoid CB2 receptors. Inflammation. 2019;42(1):211–220. doi:10.1007/s10753-018-0885-5
27. Zhao YX, He W, Jing XH, et al. Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evid Based Complement Alternat Med. 2012;2012:627023. doi:10.1155/2012/627023
28. Wang JY, Gao YH, Qiao LN, et al. Repeated electroacupuncture treatment attenuated hyperalgesia through suppression of spinal glial activation in chronic neuropathic pain rats. BMC Complement Altern Med. 2018;18(1):74. doi:10.1186/s12906-018-2134-8
29. Zhao Y, Cui C, Yu X, et al. Electroacupuncture ameliorates abnormal defaecation and regulates corticotrophin-releasing factor in a rat model of stress. Acupunct Med. 2017;35(2):114–121. doi:10.1136/acupmed-2016-011080
30. Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122(3):245–257. doi:10.1016/j.pain.2006.01.037
31. Cata JP, Weng HR, Dougherty PM. The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain Res. 2008;1229:100–110. doi:10.1016/j.brainres.2008.07.001
32. Al-Massri KF, Ahmed LA, El-Abhar HS. Pregabalin and lacosamide ameliorate paclitaxel-induced peripheral neuropathy via inhibition of JAK/STAT signaling pathway and Notch-1 receptor. Neurochem Int. 2018;120:164–171. doi:10.1016/j.neuint.2018.08.007
33. Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94(3):293–304. doi:10.1016/S0304-3959(01)00363-3
34. Wang MS, Davis AA, Culver DG, Wang Q, Powers JC, Glass JD. Calpain inhibition protects against Taxol-induced sensory neuropathy. Brain. 2004;127(Pt 3):671–679. doi:10.1093/brain/awh078
35. Zhang Y, Li A, Xin J, et al. Electroacupuncture alleviates chemotherapy-induced pain through inhibiting phosphorylation of spinal CaMKII in rats. Eur J Pain. 2018;22(4):679–690. doi:10.1002/ejp.2018.22.issue-4
36. Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–455. doi:10.1016/S0166-2236(00)01854-3
37. Ha JW, You MJ, Park HS, Kim JW, Kwon MS. Differential effect of LPS and paclitaxel on microglial functional phenotypes and circulating cytokines: the possible role of CX3CR1 and IL-4/10 in blocking persistent inflammation. Arch Pharm Res. 2019;42(4):359–368. doi:10.1007/s12272-019-01137-w
38. Zhang H, Yoon SY, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain. 2012;13(3):293–303. doi:10.1016/j.jpain.2011.12.002
39. Rahn EJ, Deng L, Thakur GA, et al. Prophylactic cannabinoid administration blocks the development of paclitaxel-induced neuropathic nociception during analgesic treatment and following cessation of drug delivery. Mol Pain. 2014;10:27. doi:10.1186/1744-8069-10-27
40. Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120(Pt 3):435–444. doi:10.1093/brain/120.3.435
41. Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain. 2014;15(7):712–725. doi:10.1016/j.jpain.2014.04.001
42. Wu Z, Wang S, Wu I, Mata M, Fink DJ. Activation of TLR-4 to produce tumour necrosis factor-α in neuropathic pain caused by paclitaxel. Eur J Pain. 2015;19(7):889–898. doi:10.1002/ejp.613
43. Gui Y, Zhang J, Chen L, et al. Icariin, a flavonoid with anti-cancer effects, alleviated paclitaxel-induced neuropathic pain in a SIRT1-dependent manner. Mol Pain. 2018;14:1744806918768970. doi:10.1177/1744806918768970
44. Qin B, Li Y, Liu X, Gong D, Zheng W. Notch activation enhances microglial CX3CR1/P38 MAPK pathway in rat’s model of vincristine-induced peripheral neuropathy. Neurosci Lett. 2019;11:134624.
45. Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174. doi:10.3389/fnmol.2017.00174
46. Boyette-Davis JA, Walters ET, Dougherty PM. Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag. 2015;5(4):285–296. doi:10.2217/pmt.15.19
47. Jiang YL, Yin XH, Shen YF, He XF, Fang JQ. Low frequency electroacupuncture alleviated spinal nerve ligation induced mechanical allodynia by inhibiting TRPV1 upregulation in ipsilateral undamaged dorsal root ganglia in rats. Evid Based Complement Alternat Med. 2013;2013:170910. doi:10.1155/2013/170910
48. Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291(1–3):1–9. doi:10.1016/j.tox.2011.10.019
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.