Back to Journals » Cancer Management and Research » Volume 12
EGLIF-CAR-T Cells Secreting PD-1 Blocking Antibodies Significantly Mediate the Elimination of Gastric Cancer
Authors Zhou JT, Liu JH, Song TT, Ma B, Amidula N, Bai C
Received 4 May 2020
Accepted for publication 22 August 2020
Published 23 September 2020 Volume 2020:12 Pages 8893—8902
DOI https://doi.org/10.2147/CMAR.S260915
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Harikrishna Nakshatri
Jing-Tao Zhou,1,* Jiang-Hao Liu,2,* Ting-Ting Song,2 Bo Ma,3 Nuermaimait Amidula,1 Chao Bai4
1General Surgery, The Seventh Affiliated Hospital of Xinjiang Medical University, Urumqi, People’s Republic of China; 2General Surgery, Xinjiang Uygur Autonomous Region Eighth People’s Hospital, Urumqi, People’s Republic of China; 3General Surgery, The Second Affiliated Hospital of Xinjiang Medical University, Urumqi, People’s Republic of China; 4Department of Vascular and Thyroid Surgery, The First Affiliated Hospital of Xinjiang Medical University, Urumqi 830000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Bai
Department of Vascular and Thyroid Surgery, The First Affiliated Hospital of Xinjiang Medical University, No. 137 South Liyushan Road, New District, Urumqi 830000, People’s Republic of China
Tel +86 991-4364657
Email [email protected]
Objective: To investigate the anti-tumor effects of programmed cell death protein 1 (PD-1) scFv-secreting EGFR-chimeric antigen receptor-modified (CAR)-T cells against gastric cancer.
Methods: Second-generation EGFR-CAR-T cells and fourth-generation PD-1 scFv-secreting EGFR-CAR-T cells were engineered. The anti-tumor activities of chimeric antigen receptor-modified (CAR)-T cells were analyzed in vitro by long-term co-culture with gastric cancer cells. The tumor scavenging capacity in vivo was evaluated in xenograft and PDX mouse models.
Results: EGFR-CAR-T cells secreting PD-1 scFv showed enhanced long-term tumor cell killing capacity in vitro. These cells also showed significant anti-tumor effect in the subcutaneous xenograft model of gastric cancer as well as in the PDX model, and autocrine PD-1 antibody secretion significantly increased tumor infiltration of the CAR-T cells.
Conclusion: EGFR-CAR-T cells secreting PD-1 scFv are highly effective against gastric cancer and offer new insights into anti-cancer immunotherapy.
Keywords: EGLIF-CAR-T cells, PD-1 blocking antibodies, elimination, gastric cancer
Introduction
Gastric cancer is the third most common cause of cancer-related deaths worldwide, with approximately one million new cases diagnosed each year.1,2 Despite significant advances in surgical and chemotherapeutical approaches, the five-year survival rate for patients with advanced gastric cancer is a dismal 5%–20%, while the median overall survival (OS) is only 10 months. Therefore, there is an urgent need for new strategies to extend patient survival and improve prognosis.3 Chimeric antigen receptor-modified (CAR)-T cells offer an alternative immunotherapeutic strategy. The CAR consists of an extracellular single-chain variable fragment (scFv domain) that targets tumor cells, a short transmembrane domain, and an intracellular co-stimulatory domain comprising of tandemly assembled T cell signaling moieties.4,5 Preclinical studies have shown that Her-targeted CAR-T cells can retard gastric cancer progression.6,7
EGFR is a transmembrane glycoprotein of the ERBB receptor tyrosine kinase family that is overexpressed in many human cancers due to amplification and/or mutation of the EGFR gene, and is closely related to tumor recurrence, angiogenesis and metastasis.8,9 The extracellular domain of EGFR, which is overexpressed on the surface of tumor cells, is an ideal tumor-specific and immunogenic antigen. In fact, the monoclonal antibody against EGFR had shown encouraging results against lung cancer, and head and neck cancer.8,10,11 Therefore, EGFR is a potential target for adoptive cellular immunotherapy as well.
Although CAR-T cell therapy has been effective against hematological tumors, it has not achieved the same success in solid tumors.12 This is partially due to the immunosuppressive tumor microenvironment (TME) which blocks CAR-T cell activity by triggering inhibitory signals through the PD-1/PD-L1 pathway.13,14 PD-1 is an inhibitory receptor that is not expressed on the resting T cells in cancer patients but increases significantly in the functionally depleted T cells.15 Its ligand PD-L1 is expressed on the surface of antigen presenting cells (APCs) and tumor cells, and is significantly up-regulated upon stimulation by local inflammatory cytokines such as type I and type II interferons. The interaction of PD-L1 and PD-1 leads to the apoptosis and functional failure of T cells.16 Therefore, blocking this immune checkpoint can partially restore T cell function and improve tumor cell clearance.17 The anti-PD-1 monoclonal antibodies pembrolizumab and nivolumab were approved by the Food and Drug Administration (FDA) in 2014, and have achieved satisfactory results in melanoma, non-small cell lung cancer (NSCLC), renal cell cancer (RCC) and other malignant tumors.18–20 However, most patients do not respond optimally to the immune checkpoint blockade (ICB), and some are completely unresponsive. Therefore, a better understanding of the pathways and molecular mechanisms that block the PD-1/PD-L1 pathway is needed. Studies on animal models and several clinical trials have shown that ICB therapy affects the tumor infiltrating lymphocytes (TILs) and other immune cells in the tumor microenvironment.21,22 Based on these findings, we hypothesized that CAR-T cells producing anti-PD-1 antibodies will be resistant to immunosuppression and show enhanced toxicity against solid tumors. To this end, we engineered fourth generation EGFR-CAR-T cells secreting PD-1 scFv for ICB therapy against EGFR-overexpressing gastric cancer cells, and analyzed its anti-tumor efficacy.
Materials and Methods
Cell Lines and Culture Conditions
Fresh blood samples were collected from six healthy volunteers, and peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation using LymphoprepTM (Axis-Shield, Norseland). The CD3+ T cells were enriched by magnetic separation (Miltenyi Biotec Inc, Auburn, CA, USA), and cultured in X–VIVO15 medium (Lonza, Switzerland) supplemented with 5% human AB serum (Valley Biomedical Inc, Winchester, VA, USA.), 10 mM N-acetyl L-cysteine (Sigma Aldrich, St. Louis, MO, USA) and 300 U/mL human IL-2 (PeproTech, Rocky Hill, CT, USA). The gastric cancer cell lines MKN28, MGC-803, MKN7 and MGC-27 were obtained from the American Type Culture Collection (ATCC). MKN28 and MKN7 cells were cultured in RPMI-1640 medium (Hyclone, Logan, UT, USA), and the MGC-803 and MGC-27 cells in Dulbecco’s modified Eagle medium (DMEM) (Hyclone), both supplemented with 10% fetal calf serum, 2 mM L-glutamine (Gibco, Gaithersburg, MD, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Sangong Biotech, Shanghai, China).
Patient Tissue Samples
32 tumor tissues and three normal gastric tissue specimens were obtained from 32 gastric cancer patients, of which 3 normal tissues originated from 3 of 32 tumor patients, processed as per standard protocols and subjected to hematoxylin and eosin staining. The collection of human tissue samples was approved by the Seventh Affiliated Hospital of Xinjiang Medical University and all patients and healthy volunteers provided informed consent.
Lentiviral Transduction of T Cells
Primary CD3+ T cells were activated with human CD3/CD28 beads (Invitrogen, Carlsbad, CA, USA) at the ratio of 2:1 (magnetic beads: cells) in T cell culture medium. Forty-eight hours later, the activated cells were transduced with the lentivirus at a MOI of 8 with 7 μg/mL polybrene (Yeasen Biotech, China), centrifuged at 1200 g for 60 minutes, and incubated overnight at 37°C with 5% CO2. After 5 days, the T cells were harvested and the percentage of transduced CAR-T cells was detected by flow cytometry.
Flow Cytometry
The harvested cells were centrifuged, washed thrice with FACS wash buffer (1×PBS containing 0.5% BSA and 0.03% sodium azide), and stained with anti-EGFR-PE (BD, San Jose, CA, USA), anti-Fab-FITC (eBioscience, CA, USA), anti-CD3-FITC (eBioscience, CA, USA), anti-CD4-PE (BD, CA, USA), anti-CD8-APC (eBioscience, CA, USA) and anti-PD-1-PB450 (eBioscience, CA, USA) antibodies as appropriate. The intracellular IFN-γ in T cells was also stained using anti-IFN-γ-FITC antibody (BD, CA, USA). In addition, the peripheral blood of the tumor-bearing mice was stained with the CD3-PerCP/CD4-FITC/CD8-PE TruCOUNT kit (BD Bioscience), and the CD4+ and CD8+ T cells were quantified according to the manufacturer’s instructions.
Enzyme-Linked Immunosorbent Assay (ELISA)
The effector and target cells were mixed at the ratio of 3:1 in a U-bottomed 96-well plate, and co-cultured for 24 h. The cells were then centrifuged, and the amount of IL-2, IFN-γ and TNF-α in the supernatants were analyzed using specific ELISA kits according to the manufacturer’s instructions (MultiSciences, China).
Co-Culture of T Cells and Tumor Cells
MGC-803 cells were seeded in 6-well tissue culture plates at the density of 1×106 cells/well and cultured for 4 days in complete medium. The CAR-T cells were then added at the effector/target ratio of 1:40, and the co-culture was maintained for 28 days. The T cells were harvested every 7 days and quantified by flow cytometry (CD3 positive) and manual counting. Prior to the third stimulation, the CAR-T cells were stained with Cell Trace Dilution (Invitrogen) and then co-cultured for 96 hours at the effector/target ratio of 3:1. The intensity of the dye was measured by flow cytometry.
Establishment of Xenograft Model and Living Imaging
Five to seven-weeks-old female NOD-SCID IL-2 receptor gamma null (NSG) mice were purchased from Shanghai Runnuo Biotechnology Co. Ltd. (China), and housed at the Experimental Animal Research Center of the Seventh Affiliated Hospital of Xinjiang Medical University. The animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animal published by National Academic Research Council of America. All animal experiments were approved by the Animal Care and Use Committee of the Seventh Affiliated Hospital of Xinjiang Medical University. A xenograft model was established by subcutaneously injecting the mice with MGC-803-Luc cells mixed with Matrigel. Seven days after inoculation, the mice were intravenously injected with PBS, 1×107 EGFR BB-z or 1×107 EGFR BB-z/E30 (n=4). Bioluminescence was measured using the Xenogen IVIS Spectrum System (Life Technologies, USA). The tumors were measured every 7 days, and the mice were euthanized when they appeared morbid. For the PDX model, once the tumors grew to 100 mm3, the mice were randomized into control or treatment groups (n=5) and the latter were injected with 1*107 effector cells via their tail vein. The tumor volume was measured every 3 days. The mice were euthanized if they lost more than 20% of their initial weight or appeared morbid. On the 20th day after tumor cell inoculation, 200 μL blood was collected and the number of CD8+ and CD4+ T cells were quantified as described.
Immunofluorescence Assay
The infiltration of human CAR-T cells in the tumors was assessed by immunostaining cryosections of tumor tissues with anti-CD3, anti-EGFR, and anti-EGFR antibodies (1:150, Thermo Scientific) as per the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6.0. The groups were compared by Student’s t-test and p < 0.05 was considered statistically significant.
Results
Generation of EGFR-Targeting CAR-T Cells
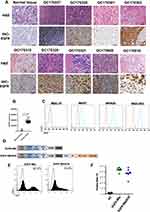
EGFR was highly expressed in 75% of the human gastric cancer tissues (24/32) compared to 3 of the normal tissues (Figure 1A and B). Consistent with this, EGFR was also significantly upregulated in the MKN28, MGC-803 and MKN7 gastric cancer cell lines, while MGC-27 was EGFR-negative (Figure 1C). Subsequently, a second-generation CAR sequence targeting EGFR (EGFR BB-z) was constructed, followed by EGFR BB-z/E30 which incorporated the PD-1 scFv sequence as well (clone 332.8H3).23 The CD3ζ and 4–1BB sequences were included in each construct as the T cell activation and costimulatory signals respectively (Figure 1D). The transduced cells were probed using the anti-Fab antibody in order to detect the EGFR BB-z and EGFR BB-z/E30 sequences (Figure 1E), and around 40% CAR-T cells were stably transduced (Figure 1F).
EGFR-CAR-T Cells Show Antigen-Specific Activation in vitro
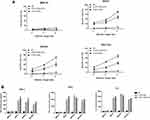
The different gastric cancer cell lines were incubated with the EGFR-CAR-T cells at the effector/target cell ratios of 3:1, 1:1, and 1:3. As indicated by the secreted LDH levels, both EGFR BB-z and EGFR BB-z/E30 cells showed potent dose-dependent cytotoxicity against the EGFR+ MKN28, MGC-803 and MKN7 cells, while the EGFR− MGC-27 cells were unaffected (Figure 2A). In addition, the CAR-T cells secreted significantly higher levels of IL-2, IFN-γ and TNF-α compared to the un-transfected T cells in the presence of the EGFR+ tumor cells, while the EGFR− MGC-27 cells did not increase the basal secretion levels of these cytokines (Figure 2B). Furthermore, there was no significant difference in the cytotoxicity and cytokine secretion levels of the EGFR BB-z and EGFR BB-z/E30 CAR-T cells during the co-culture. Taken together, the EGFR BB-z and EGFR BB-z/E30-CAR T cells were selectively toxic against the EGFR+ tumor cells, and secreted high levels of cytokines upon antigen stimulation.
PD-1 scFv Secretion Maintains the Functional Potency of EGFR-CAR-T Cells After Long-Term Stimulation in vitro
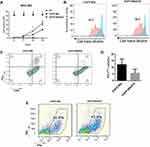
To determine the effect of the secreted PD-1 scFv on EGFR-CAR-T cell activity after long-term antigen stimulation, the effector cells were co-cultured with MGC-803 for 28 days. The total number of EGFR BB-z/E30 CAR-T cells were significantly higher compared to the EGFR BB-z cells after long-term stimulation (Figure 3A), which was also confirmed by the higher proliferation rates in the former (Figure 3B). Furthermore, the PD-1 expression levels were significantly lower on EGFR BB-z/E30 cells compared to EGFR BB-z cells during the long-term stimulation (Figure 3C and D), while the proportion of IFNγ-secreting cells was significantly higher in the former (Figure 3E). In conclusion, secretion of PD-1 scFv maintains the proliferative capacity and anti-tumor function of EGFR-CAR-T cells following long-term antigenic stimulation, which can translate to better therapeutic effects.
PD-1 scFv Enhanced the Anti-Tumor Effect of EGFR-CAR-T Cells in a Heterologous Tumor Model
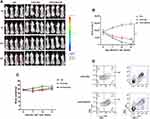
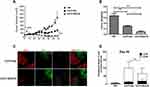
The anti-tumor activity of EGFR BB-z and EGFR BB-z/E30-CAR-T cells was analyzed in NOD/SCID mice bearing subcutaneous MGC-803-luc xenograft tumors, and tumor growth inhibition was tracked through in vivo fluorescence imaging. As shown in Figure 4A, both EGFR BB-z and EGFR BB-z/E30-CAR-T cells inhibited tumor growth, although the latter showed greater efficacy (Figure 4B). In addition, more EGFR BB-z/E30-CAR-T cells infiltrated the tumor tissues compared to the EGFR BB-z cells, and the surface expression of PD-1 was significantly lower on the infiltrating EGFR BB-z/E30 cells (Figure 4D). In terms of biocompatibility, no significant changes were observed in the bodyweight of the mice throughout the experiment (Figure 4C). Taken together, EGFR BB-z/E30-CAR-T cells are highly potent against gastric tumors without any obvious side effects.
PD-1 scFv Enhanced the Anti-Tumor Activity of EGFR-CAR-T Cells in a PDX Model
We next established a gastric cancer PDX model, and injected the tumor-bearing mice with PBS, EGFR BB-z or EGFR BB-z/E30-CAR-T cells. While both the engineered CAR T cells inhibited tumor growth, the EGFR BB-z/E30 cells showed greater tumor clearance and a prolonged inhibitory effect compared to the EGFR BB-z cells (Figure 5A). Consistent with this, the tumors were significantly smaller in the EGFR BB-z/E30 versus the EGFR BB-z-CAR-T cells-treated mice (Figure 5B). The EGFR BB-z/E30-CAR T cells also infiltrated to a greater extent in the tumors, which corresponded to significantly lower fewer EGFR+ tumor cells compared to that in the EGFR BB-z group (Figure 5C). However, 20 days after reintroduction of effector cells, the number of circulating EGFR BB-z/E30 cells was less than that of EGFR BB-z cells (Figure 5D). This could be due to the inherent differences between both cell types in terms of proliferative capacity. In conclusion, EGFR BB-z/E30-CAR T cells effectively inhibited the growth of PDX gastric tumors by targeting the EGFR+ cells.
Discussion
CAR-T cell therapy has achieved excellent clinical outcomes in hematological malignancies and is currently being developed for the treatment of solid tumors as well.12 Since gastric tumor tissues overexpress multiple tumor-specific antigens, including EGFR, Her2, MSLN etc., CAR-T cells are a highly promising option for treating this malignancy. However, CAR-T cells are often ineffective against solid tumors due to the immunosuppressive microenvironment.24–26 Immune checkpoint blockers, especially antibodies targeting PD-1 and PD-L1, can overcome the inhibition of CAR-T cells and significantly increase their efficacy against solid tumors.27 Although the combination of anti-PD-1 antibody and CAR-T cells has shown encouraging results in some clinical trials, systemic administration of the antibody is associated with adverse effects.28 In addition, the high cost of the combination therapy limits its widespread use. An alternative strategy is to engineer CAR-T cells that release PD-1 antibody in an autocrine manner. To this end, we constructed a second-generation EGFR BB-z-CAR sequence targeting the EGFR-overexpressing gastric cancer cells, and then incorporated the PD-1 scFv sequence resulting in fourth-generation EGFR BB-z/E30-CAR T cells that secrete the variable fragments of anti-PD-1 antibody. While both CAR-T cells displayed cytotoxic effects against the gastric cancer cells in an antigen-dependent manner, autocrine PD-1 antibody secretion significantly augmented the tumor cell killing activity of the EGFR BB-z-CAR-T cells after long-term stimulation. In the xenograft tumor model as well, the EGFR BB-z/E30 cells achieved better clearance of the EGFR+ tumor cells compared to the EGFR BB-z cells. It is worth noting that when evaluating the antitumor activity of EGFR BB-z/E30 in the PDX model, EGFR BB-z and EGFR BB-z/E30 can still be detected in peripheral blood on the 20th day. The number of EGFR BB-z/E30 is lower than that of EGFR BB-z, but the tumor infiltration of EGFR BB-z/E30 is much higher than that of EGFR BB-z. It shows that after CAR-T cell therapy, only T cells that fully infiltrate the tumor tissue can play a good anti-tumor effect.
Conclusion
These PD-1 scFv-secreting CAR-T cells provide a novel immunotherapeutic strategy against EGFR+ gastric cancer, and can be adapted to target other tumor-specific antigens like MSLN and Her2 as well.29 However, since CAR-T cells targeting a single antigen may have off-target effects, we will consider adopting the dual-receptor CAR-T cell strategy.30,31 In addition, we will also improve the efficacy of transfecting large fragment genes encoding both dual-receptor CAR and PD-1 antibody by electroporation, so as to increase its clinical potential.
Disclosure
The authors declare that they have no conflict of interests for this work.
References
1. Cheng J, Cai M, Shuai X, et al. First-line systemic therapy for advanced gastric cancer: a systematic review and network meta-analysis. Ther Adv Med Oncol. 2019;11:1758835919877726. doi:10.1177/1758835919877726
2. Lorenzen S, Stahl M, Hofheinz R, et al. Influence of taxanes on treatment sequence in gastric cancer. Onkologie. 2020;43:42–47. doi:10.1159/000503428
3. Selim JH, Shaheen S, Sheu W, et al. Targeted and novel therapy in advanced gastric cancer. Exp Hematol Oncol. 2019;8:1–23. doi:10.1186/s40164-019-0149-6
4. Holstein SA, Lunning MA. CAR T‐cell therapy in hematologic malignancies: a voyage in progress. Clin Pharmacol Ther. 2020;107:112–122. doi:10.1002/cpt.1674
5. Schepisi G, Cursano MC, Casadei C, et al. CAR-T cell therapy: a potential new strategy against prostate cancer. J Immunother Cancer. 2019;7:1–11. doi:10.1186/s40425-019-0741-7
6. Han Y, Liu C, Li G, et al. Antitumor effects and persistence of a novel HER2 CAR T cells directed to gastric cancer in preclinical models. Am J Cancer Res. 2018;8:106–119.
7. Song Y, Tong C, Wang Y, et al. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell. 2018;9:867–878. doi:10.1007/s13238-017-0384-8
8. Liu SH, Chen XF, Xie ZB, et al. EGFR monoclonal antibody panitumumab inhibits chronic proliferative cholangitis by downregulating EGFR. Int J Mol Med. 2019;44:79–88. doi:10.3892/ijmm.2019.4190
9. Jia Y, Zhao S, Jiang T, et al. Impact of EGFR-TKIs combined with PD-L1 antibody on the lung tissue of EGFR-driven tumor-bearing mice. Lung Cancer. 2019;137:85–93. doi:10.1016/j.lungcan.2019.09.016
10. Alsaleh K, Elsherify M, Safwat R, et al. Phase II/III randomized controlled trial of concomitant hyperfractionated radiotherapy plus cetuximab (Anti-EGFR antibody) or chemotherapy in locally advanced head and neck cancer. Gulf J Oncolog. 2019;1:6–12.
11. McDaid WJ, Greene MK, Johnston MC, et al. Repurposing of Cetuximab in antibody-directed chemotherapy-loaded nanoparticles in EGFR therapy-resistant pancreatic tumours. Nanoscale. 2019;11:20261–20273. doi:10.1039/C9NR07257H
12. Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128. doi:10.3389/fimmu.2019.00128
13. Akbari P, Huijbers EJM, Themeli M, et al. The tumor vasculature an attractive CAR T cell target in solid tumors. Angiogenesis. 2019;22:473–475. doi:10.1007/s10456-019-09687-9
14. Bagley SJ, Orourke DM. Clinical investigation of CAR T cells for solid tumors: lessons learned and future directions. Pharmacol Ther. 2020;205:107419. doi:10.1016/j.pharmthera.2019.107419
15. Meleveedu K, Chen D, Nadiminti K, et al. PD-1/PD-L1 expression in extramedullary lesions of acute myeloid leukemia. Leuk Lymphoma. 2019:1–4. doi:10.1080/10428194.2019.1675880
16. Liu Y, Zugazagoitia J, Ahmed FS, et al. Immune cell PD-L1 co-localizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26:970–977. doi:10.1158/1078-0432.CCR-19-1040
17. Qorraj M, Bottcher M, Mougiakakos D. PD-L1/PD-1: new kid on the “immune metabolic” block. Oncotarget. 2017;8:73364–73365. doi:10.18632/oncotarget.20639
18. Tang B, Yan X, Sheng X, et al. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. J Hematol Oncol. 2019;12:1–15. doi:10.1186/s13045-018-0693-2
19. Jia M, Feng W, Kang S, et al. Evaluation of the efficacy and safety of anti-PD-1 and anti-PD-L1 antibody in the treatment of non-small cell lung cancer (NSCLC): a meta-analysis. J Thorac Dis. 2015;7:455–461. doi:10.3978/j.issn.2072-1439.2015.02.06
20. Mizutani K, Horie K, Kato T, et al. Serum PD-1 levels measured by ELISA using Nivolumab increased in advanced RCC patients: novel approach to develop companion diagnostics for antibody therapy. J Cancer Res Clin Oncol. 2019;145:1661–1663. doi:10.1007/s00432-018-2806-2
21. Wang W, Carper K, Malone FR, et al. PD-L1/PD-1 signal deficiency promotes allogeneic immune responses and accelerates heart allograft rejection. Transplantation. 2008;86:836–844. doi:10.1097/TP.0b013e3181861932
22. Mizuno R, Sugiura D, Shimizu K, et al. PD-1 primarily targets TCR signal in the inhibition of functional T cell activation. Front Immunol. 2019;10:630. doi:10.3389/fimmu.2019.00630
23. Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4:124–135. doi:10.1158/2326-6066.CIR-15-0151
24. Benli Yavuz B, Koc M, Kozacioglu S, et al. Prognostic importance of PTEN, EGFR, HER-2, and IGF-1R in gastric cancer patients treated with postoperative chemoradiation. Turk J Med Sci. 2019;49:1025–1032. doi:10.3906/sag-1802-34
25. Hucl T, Brody JR, Gallmeier E, et al. High cancer-specific expression of mesothelin (MSLN) is attributable to an upstream enhancer containing a transcription enhancer factor dependent MCAT motif. Cancer Res. 2007;67:9055–9065. doi:10.1158/0008-5472.CAN-07-0474
26. Giraldo NA, Sanchez-Salas R, Peske JD, et al. The clinical role of the TME in solid cancer. Br J Cancer. 2019;120:45–53. doi:10.1038/s41416-018-0327-z
27. Ready N, Ott PA, Hellmann MD, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol. 2020;15:426–435. doi:10.1016/j.jtho.2019.10.004
28. Lainez S, Tissot C, Cottier M, et al. EBUS-TBNA can distinguish sarcoid-like side effect of nivolumab treatment from tumor progression in non-small cell lung cancer. Respiration. 2017;94:518–521. doi:10.1159/000480155
29. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–1384. doi:10.1016/S1470-2045(18)30481-9
30. Li J, Gu H, Yang J, et al. [Research progress of the side effect and application prospects of CAR-T cells in the treatment of malignant tumors]. Zhonghua Xue Ye Xue Za Zhi. 2016;37:169–173. doi:10.3760/cma.j.issn.0253-2727.2016.02.019
31. Kloss CC, Condomines M, Cartellieri M, et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi:10.1038/nbt.2459
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.