Back to Journals » Journal of Pain Research » Volume 16
Efficacy of Radiofrequency Thermocoagulation of the Thoracic Sympathetic Nerve versus Chemical Excision in Pain Caused by Raynaud’s Disease
Authors Xin B , Xie K , Huang B, Yao M
Received 30 November 2022
Accepted for publication 28 January 2023
Published 5 March 2023 Volume 2023:16 Pages 649—658
DOI https://doi.org/10.2147/JPR.S398298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Bingyue Xin, Keyue Xie, Bing Huang, Ming Yao
Department of Anesthesiology and Pain Research Center, The First Hospital of Jiaxing or The Affiliated Hospital of Jiaxing University, Jiaxing, People’s Republic of China
Correspondence: Ming Yao, Department of Anesthesiology and Pain Research Center, The First Hospital of Jiaxing or The Affiliated Hospital of Jiaxing University, No. 1882 South Zhonghuan Road, Nanhu District, Jiaxing, Zhejiang Province, People’s Republic of China, Email [email protected]
Objective: To investigate the effectiveness and safety of computed tomography (CT)-guided radiofrequency thermocoagulation (RFTC) of the thoracic sympathetic nerve versus chemical resection (CTS) for the treatment of pain caused by Raynaud’s disease.
Methods: Patients who underwent CTS or thoracic sympathetic nerve RFTC between March 2012 and March 2021 were enrolled in this retrospective study. There were 28 cases in the alcohol group (Group A) and 44 in the radiofrequency group (Group R). Visual analog scores (VAS) were collected from patients at different time points, as well as preoperative and postoperative finger end perfusion index (PI) and hand temperature (T). The efficiency, postoperative recurrence rate, complications, and improvement in postoperative quality of life were observed in both groups.
Results: Pain scores at different follow-up times after surgery decreased in both groups compared to the preoperative period (P < 0.05). Postoperative T and PI were higher in both groups than preoperatively all (P < 0.05). The recurrence rate was higher in the R group than in the A group. Postoperative complications were observed in 13.6% and 25% of patients in groups R and A, respectively. Meanwhile, the postoperative quality of life improved in both groups, but the radiofrequency (RF) group was better than the alcohol group in terms of improvement in quality of life (P < 0.05).
Conclusion: Both CT-guided CTS and RFTC of the thoracic sympathetic nerve provided good treatment outcomes. However, the RF group was superior to the alcohol group in terms of complication rate and quality of life improvement.
Keywords: Raynaud’s disease, sympathetic nerve, radiofrequency thermocoagulation, chemical thoracic sympathectomy, CT guidance
Introduction
Raynaud’s disease is a dysfunctional vascular disorder characterized by pallor and bruising of the extremities in response to cold stimuli or emotional stress. Its clinical types are classified as primary and secondary. The former is idiopathic and usually does not leave serious complications. In contrast, secondary Raynaud’s disease is mostly complicated by connective tissue diseases, such as systemic sclerosis, systemic lupus erythematosus, rheumatoid arthritis, and Sjögren’s syndrome.1 Patients with secondary Raynaud’s disease may develop serious complications, such as ulceration of the fingers or toes and ischemic necrosis of the tissues. Currently, vasoactive drugs, mainly calcium channel blockers, remain an important clinical treatment for Raynaud’s disease; however, previous studies have shown that relying on drugs alone does not seem to have the desired efficacy and commonly leads to some drug side effects. Calcium channel blockers seem to be associated with many adverse effects, such as headache, flushing, and ankle swelling.2,3 Recurrent episodes of experiencing cold and pain in extremities make patients suffer, thus seriously affecting their quality of life. Therefore, it is necessary to explore more effective treatment modalities to improve the prognosis of patients with Raynaud’s disease.
Thoracoscopic thoracic sympathectomy has been shown to improve hand microcirculation in patients with Raynaud’s disease.4 Endoscopic thoracic sympathectomy (ETS) has been widely used to treat hyperhidrosis and other upper extremity and head and neck disorders; nonetheless, it has been associated with serious complications and a high incidence.5,6 In addition, thoracoscopic surgery requires general anesthesia with tracheal intubation, which leads to increased surgical risk. It is also very invasive and costly, making it less acceptable to most patients. In recent years, chemical disruption and radiofrequency techniques have been carried out for an application in sympathectomy,7–10 a minimally invasive surgery, which can ensure both surgical safety and effective treatment. The study by Tao J. Chun et al included 72 patients who underwent chemical lumbar sympathectomy for cold allergy of the hands and feet, with a postoperative treatment efficiency of 87.5% and a recurrence rate of 31.9% after two years, which confirmed that chemical lumbar sympathectomy is a safe, cost-effective and efficient treatment for cold allergy of the hands and feet.7 A study by Huang H et al included 17 patients who underwent radiofrequency thermocoagulation of the thoracic sympathetic nerve for Raynaud’s disease and found that postoperative finger perfusion index (PI) and palm temperature (T) were significantly higher than preoperatively, with a mean 4.6-fold increase in PI and a mean 3.6°C increase in T at the end of treatment, a negative postoperative cold water stimulation test, and a recurrence rate of 11.8% at 15 months of follow-up.10 Both chemical sympathectomy and sympathectomy with radiofrequency thermocoagulation have the advantages of high success rates, minimal trauma, and reproducibility. This study focused on comparing these two different surgical approaches in terms of efficiency, recurrence rate, and complications in the treatment of Raynaud’s disease, aiming to provide a new direction and clinical basis for the treatment of Raynaud’s disease.
Materials and Methods
Patients
This is a retrospective cohort study of patients diagnosed with Raynaud’s disease at our institution from March 2012 to March 2021. The Ethics Committee of Jiaxing First Hospital reviewed and approved this study. The study was conducted in accordance with the 1964 Declaration of Helsinki and its subsequent amendments, as well as the International Association for the Study of Pain (IASP) guidelines for pain research in animals and humans. The choice of surgical procedure is based on the patient’s informed consent, and the patient is fully informed of the advantages and disadvantages of both procedures as well as the possible risks and complications. We finally divided the patients into two groups according to the procedure: the CTS group (n=28) and the RFTC group (n=44).
Methods
A total of 72 patients who were diagnosed with Raynaud’s disease and underwent CT-guided radiofrequency thermocoagulation or chemical disruption of the thoracic sympathetic chain under local anesthesia between March 2012 and March 2021 were retrospectively included. Among these patients, 44 were in the radiofrequency group (Group R) and 28 were in the alcohol group (Group A). All patients were informed of the risks and possible complications of the procedure and signed an informed consent form before treatment.
Inclusion criteria were the following: (1) those who met the diagnostic criteria related to Raynaud’s disease11; (2) did not obtain significant improvement after conservative measures, drugs, and acupuncture treatment, or could not tolerate adverse drug reactions; (3) those who were able to actively cooperate to complete treatment, review and follow up.
Exclusion criteria were: (1) diagnosis of combined hypothyroidism, anemia, diabetic peripheral neuropathy, peripheral arteriosclerosis, or hypothalamic dysfunction; (2) lack of effective contact information to achieve follow-up; (3) refusal to participate in follow-up or withdrawal from the study.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Jiaxing University, located in Jiaxing City, Zhejiang Province, China.
Surgical Process
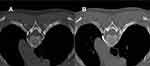
Both CTS and RFTC were performed under CT guidance with intraoperative monitoring using ECG, SpO2, NIBP, PI, and T. In the alcohol group, the patient was placed in a prone position on the CT treatment bed with a thin pillow in front of the chest to expose the puncture site. After a single CT scan, the layer on which the cephalic margin of the fourth rib is located was clarified. In certain cases, it was necessary to locate the absence of bone on the cephalic margin of the rib. Also, the target point of the puncture was close to the anterior edge of the fourth rib (Figure 1A). After determining the target site, the initial puncture path was drawn, the body puncture site was defined, and the body surface was localized with infrared light. All surgeries were performed by a pain physician with at least three years of experience in CT-guided CTS surgery. Local infiltration anesthesia was done with 1% lidocaine, and a 10-cm 22G puncture needle was used to puncture according to the preset path, and the tip of the needle was adjusted to reach the vicinity of the small head of the fourth rib and the outer edge of the rib pleura. Special attention was paid to whether there was chest tightness and dyspnea during the puncture. After the injection of 1% lidocaine 2.7mL and 30% iohexol 0.3mL, the distribution of the drug was determined by CT and 3D reconstruction, and the blocking effect was judged. After the lidocaine subsided (left for about 20 min), 2.5 mL of anhydrous alcohol was injected (Figure 1B).
For CT-guided RFTC, the steps for positioning and puncture were the same as before (Figure 2). However, the RF needle tip was required to puncture the anterior margin of the 4th rib cranium, followed by a motor electrophysiology test using 0.4–1.0 V, 2 HZ, which was considered positive if muscle fiber tremors were present; the sensory electrophysiology test was performed using a current of 0.1–0.5 V, 50 HZ, which was considered positive if numbness, tingling, and other discomforts in the local area were present. After testing for safety, radiofrequency thermocoagulation was then performed at 95°C, 300S, for 2 cycles. The PI and T were recorded at two time points, ie, 5 min before the start of the operation, which was recorded as T0, and 5 min after the end of the operation, which was recorded as T1.
Observations and Follow-Up
The primary outcomes included VAS scores and postoperative recurrence rates. The VAS score was used to assess the severity of patients with Raynaud’s disease.12 VAS scores were collected from patients before surgery, 1 day after surgery, 1 month after surgery, 3 months after surgery, 6 months after surgery, 12 months after surgery, and 24 months after surgery to assess the change in severity for all patients on a scale of 0–10, where 0 indicated no pain and 10 indicated the most severe pain. The VAS scores were weighted, and the VAS weighting values were calculated as follows: (VAS-WV) = (A-B)/A, where A was the preoperative VAS score and B was the follow-up time point VAS score. The efficacy was judged according to the weighted value results, which were specifically classified as follows: VAS-WV < 50% as ineffective, 50% ≤ VAS-WV ≤ 75% as remission, and VAS-WV > 75% as improvement, and the total effective rate = (remission + improvement)/total number of cases × 100%. Patients were observed for treatment efficiency at 1 day after surgery, 1 month after surgery, 3 months after surgery, 6 months after surgery, 12 months after surgery, and 24 months after surgery. Recurrence was defined as VAS-WV <50% during follow-up. The relapse rate was the number of relapses/total number of patients followed up. Secondary outcomes included complications and adverse effects. In addition, general patient information, including gender, age, height, weight, duration of disease, history of underlying disease, PI, and T before and after treatment, were collected from the electronic medical system or the surgical record book.
Statistical Analysis
SPSS 26.0 software was used for statistical analysis. The Kolmogorov–Smirnov test was used to determine whether the variable data obeyed normality. Statistical descriptions of non-normally distributed data were expressed as median (interquartile spacing), and the Wilcoxon rank sum test was used to analyze the differences; normally distributed data were expressed as mean ± standard deviation (±s), and independent samples t-test was used to analyze the differences. Count data were expressed as N (%), and Pearson’s chi-square test or Fisher’s exact probability method was used to analyze the differences. P< 0.05 was considered a statistically significant difference.
Results
Clinical Features
In this study, a total of 106 patients with Raynaud’s disease admitted to our pain department and treated with surgery from March 2012 to March 2021 were screened. Among the 31 patients excluded from the study, 27 were excluded because of unreachable problems and not because of other diseases. A total of 75 patients were finally enrolled, and 3 cases were dislodged during the follow-up process, of which 1 patient passed away and 2 patients were lost to follow-up. Finally, a total of 72 patients completed all the postoperative follow-up contents, with a total of 28 cases in the CTS group (Group A) and 44 cases in the RFTC group (Group R), as shown in Figure 3.
 |
Figure 3 Flow Diagram. |
The baseline data of the two groups are shown in Table 1. There were no significant differences in gender, age, height, weight, BMI, disease duration, and comorbidities (P > 0.05).
 |
Table 1 Comparison of Patient Clinical Features in Two Groups |
VAS Score of Patients
VAS scores were performed in both groups before surgery, 1 day after surgery, 1 month after surgery, 3 months after surgery, 6 months after surgery, 12 months after surgery, and 24 months after surgery, respectively, as shown in Figure 4.
 |
Figure 4 Line plots of VAS scores in both groups. Abbreviations: Group R, radiofrequency thermocoagulation; Group A, chemical thoracic sympathectomy; VAS, visual analog scale. |
The VAS scores of patients in both groups at different time points after surgery were significantly lower than those before surgery (P < 0.05). However, the VAS scores of patients in Group R and Group A were not statistically different (P > 0.05), as shown in Table 2.
 |
Table 2 Comparison of VAS Scores at Different Time Points Before and After Surgery and Comparison of VAS Scores Between the Two Groups at Each Time Point |
T and PI Before and After Surgery
When making between-group comparisons, there were no statistically significant differences in indicators at either T0 or T1 (p>0.05). Intra-group comparisons revealed that T and PI, in both groups were significantly higher postoperatively compared to preoperatively (P < 0.05), as shown in Table 3.
 |
Table 3 T and PI Before and After Treatment |
Treatment Effects
The efficiency rates of patients in group R at 1 day, 1 month, 3 months, 6 months, 12 months, and 24 months postoperatively were 100%, 95%, 84%, 34%, 43%, and 32%, respectively, while in group A the rates were 100%, 97%, 71%, 50%, 39%, and 43%, respectively. There were no significant differences (P > 0.05) in the comparison of the efficiency rates in the two groups of patients at each time point, as shown in Table 4.
 |
Table 4 The Effective Rates at Different Time Points After Surgery |
The long-term observation results revealed that the recurrence rate at ≥ 6 months was 61.4% in the RF group and 32.1% in the alcohol group; the observed difference was not statistically significant [Group R vs Group A: 27 (61.4%) vs 9 (32.1%), P>0.05].
Kaplan-Meir analysis of recurrence-free survival in both groups is shown in Figure 5.
Postoperative Complications
Compensatory hyperhidrosis was the most common postoperative complication after thoracic sympathectomy. The results of this study showed a statistically significant difference between the incidence of compensatory hyperhidrosis in group R and group A [group R vs group A: 2 (4.5%) vs 6 (21.4%), P < 0.05]. The incidence of postoperative chronic pain was mainly concentrated in the chest and back, and there was no difference in incidence between the two groups. There were no serious complications or deaths in either group (Table 5).
 |
Table 5 Postoperative Complications |
Quality of Life After Surgery
In the assessment of patients’ postoperative quality of life, one patient in group R and one in group A complained of decreased postoperative quality of life, while 65.9% of the subjects reported a significant improvement in quality of life after radiofrequency thermocoagulation, and 35.7% of the subjects in group A reported an improvement in quality of life. The results showed a statistically significant difference between group R and group A in terms of postoperative improvement in quality of life [group R vs group A: 29 (65.9%) vs 10 (35.7%), p<0.05] (Table 6).
 |
Table 6 Quality of Life After Surgery |
Discussion
Previous studies have shown that sympathetic nerves and their secretion of norepinephrine have an important role in inflammatory pain.13–15 Yoo et al found that early administration of thoracic sympathetic block (TSB) significantly reduces chronic pain, edema, and warmth in the upper extremity.16 In addition, Oh et al found that thoracic nerve root radiofrequency thermocoagulation could effectively control cancer pain and refractory chest wall pain in a short period.17 The above study explains why thoracic sympathectomy can effectively treat end extremity pain in patients with Raynaud’s disease. Studies have also shown that cutaneous microcirculation causes vasoconstriction through excitation of sympathetic nerves and release of norepinephrine,18 therefore blocking sympathetic nerves to diastole peripheral blood vessels can lead to the improvement of cold extremity symptoms in patients with Raynaud’s disease. At present, CT-guided chemical thoracic sympathectomy (CTS) is considered a relatively mature technique that can effectively improve patient’s symptoms of limb ischemia and can significantly reduce pain after the operation. However, the technique still has certain defects. Eg, abnormal diffusion of anhydrous alcohol may cause Horner’s syndrome;7 if the drug is inadvertently introduced into the blood, it may lead to patient’s death in more serious cases. In recent years, radiofrequency thermocoagulation has been gradually applied to sympathectomy, and a number of CT-guided thoracic sympathetic chain radiofrequency thermocoagulation (RFTC) procedures for Raynaud’s disease were carried out at our pain department.
This study explored the safety and efficacy of CTS and RFTC in the treatment of Raynaud’s disease and provided new ideas for the treatment of Raynaud’s disease. The follow-up results showed a significant decrease in pain levels in patients with Raynaud’s disease after surgery compared to the preoperative period. Statistical analysis of hand temperature (T) and perfusion index (PI) before and after surgery in both groups revealed that T and PI in both groups showed a significant increase at T1. In contrast, no significant differences were seen when T and PI after treatment were compared between groups A and R. Evidently, both chemical modulation and radiofrequency thermocoagulation could achieve good thoracic sympathetic disruption. Two patients in group A complained of chest tightness and shortness of breath during the procedure, and the CT scan did not show that the needle tip was positioned too deeply or that it pierced the ribbed pleura, which was considered as a transient autonomic response enhancement of unknown origin. None of the patients in the R group experienced any significant abnormal sensations or complications intraoperatively. In terms of efficiency, both surgical treatments achieved satisfactory results in the short term, but the recurrence rate was higher at long-term follow-up, which we speculate may be due to the fact that the alcohol injected during chemical thoracic sympathectomy is gradually metabolized over time, resulting in poorer long-term results. Whereas radiofrequency thermocoagulation technique may be due to the small extent of puncture needle destruction, which cannot spread like alcohol, we believe that it can reduce the recurrence rate more effectively if using using double or multiple needle radiofrequency.
In the present study, postoperative recurrence was the primary endpoint. A total of 72 patients were followed up by telephone for a period of 2 years after surgery, showing that the recurrence rate was lower in group A than in group R. However, there was no statistical difference between the groups, indicating that both surgical methods provided significant short-term efficacy, which is in line with our expectation related to good diffusivity of anhydrous alcohol, representing both an advantage of alcohol and a potential risk factor. A certain degree of alcohol diffusion can expand the destruction of the thoracic sympathetic chain and achieve a better therapeutic effect. As shown in Figure 1A, in chemical thoracic sympathetic disfigurement, the tip of the puncture needle did not completely reach the anterior margin of the small head of the 4th rib, but after injection of the drug, the CT subcontrast image still met the drug diffusion requirements and achieved a satisfactory treatment effect. In contrast, radiofrequency thermocoagulation requires that the radiofrequency needle be ideally aligned, and the closer the needle tip is to the sympathetic chain, the more perfect the theoretical destruction is. Accordingly, we assumed that the higher recurrence rate in the RF group was likely due to the demanding puncture technique. The range of RF action was much smaller than that of anhydrous alcohol, and the mild deviation of the puncture needle tip also decreased the disfiguring effect, requiring several adjustments of the puncture needle position throughout the procedure.
Compensatory hyperhidrosis is an important complication after thoracic sympathectomy, and several publications have reported on the effectiveness of thoracic sympathectomy in hyperhidrosis.19 After removal of the thoracic sympathetic chain, the symptoms of hand sweating usually resolve along with dry hands and compensatory hyperhidrosis. The sympathetic postganglionic fibers that innervate the small sweat glands in the body release acetylcholine to promote sweat secretion, and sympathetic blockade can significantly reduce sweating in the corresponding areas. Our previous study also demonstrated that hand sweating could be treated after thoracic sympathetic chain resection at the T4 level; however, the treatment could lead to varying degrees of hand dryness and compensatory hyperhidrosis.20 Whereas compensatory hyperhidrosis is often the main cause of reduced postoperative quality of life in patients, all six cases in the alcohol group who complained of reduced quality of life had significant postoperative compensatory hyperhidrosis, which was more common in the chest and back. Also, it was less common in the RF group. We speculate that this may be related to the fact that after the injection of anhydrous alcohol at the T4 level, due to its diffusion among the segments, the multi-segmental disruption results in significant anhidrosis on the operative side and severe compensatory hyperhidrosis in the area dominated by the remaining segments. In contrast, the small ablation area produced by the single needle, which was used in RF treatment in the present study, the mild destruction in case of poor alignment, and the more limited segments being affected may have resulted in a less effective treatment with a low and mild complication rate. Horner’s syndrome is another common complication after thoracic sympathetic modulation, and due to the mobility of alcohol, we were unable to completely stop its diffusion to the cephalad side even with a controlled injection rate.7 Ding et al21 noted that anhydrous alcohol’s diffusion might also damage surrounding vital tissues and organs.
The present study has some limitations, and expanded sample size and a multicenter unfolding study would be more convincing. We expect that the application of multi-needle radiofrequency or precise localization means in thoracic sympathectomy will provide a new outlook for treating Raynaud’s disease.
Limitations
This was a single-center, observational, non-randomized controlled study, which is the main limitation of this study.
Conclusions
CT-guided RFTC of the thoracic sympathetic nerve has similar efficacy to CTS in treating Raynaud’s disease but with a lower overall complication rate and greater safety than chemical disruption.
Funding
Zhejiang Province and city jointly built key disciplines - pain medicine (2019-ss-ttyx); Jiaxing City Key Laboratory of Nerve and Pain.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001;357(9273):2042–2048. doi:10.1016/S0140-6736(00)05118-7
2. Ennis H, Hughes M, Anderson ME, et al. Calcium channel blockers for primary Raynaud’s phenomenon. Cochrane Database Syst Rev. 2016;2(2):CD002069. doi:10.1002/14651858.CD002069.pub5
3. Belch JJF, Ho M. Pharmacotherapy of Raynaudʼs Phenomenon. Drugs. 1996;52(5):682–695. doi:10.2165/00003495-199652050-00006
4. Maga P, Kuzdzal J, Nizankowski R, et al. Long-term effects of thoracic sympathectomy on microcirculation in the hands of patients with primary Raynaud disease. J Thorac Cardiovasc Surg. 2007;133:1428–1433.
5. Ojimba TA, Cameron AE. Drawbacks of endoscopic thoracic sympathectomy. Br J Surg. 2004;91(3):264–269. doi:10.1002/bjs.4511
6. Licht PB, Pilegaard HK. Severity of compensatory sweating after thoracoscopic sympathectomy. Ann Thorac Surg. 2004;78(2):427–431.
7. Tao J, Zhu J, Wang T, et al. CT-guided chemical lumbar sympathectomy in the treatment of cold hypersensitivity in the hands and feet. Pain Physician. 2021;24(4):E459–E466.
8. Liu QY, Huang B, Chen YJ, et al. Prevention and treatment of Horner syndrome in treatment of head and face hyperhidrosis by thoracic sympathetic nerve modulation. Zhonghua Yi Xue Za Zhi. 2017;97(46):3624–3627.
9. Tao J, Huang B, Tang J, et al. Comparison of efficacy and safety of lumbar sympathetic radiofrequency thermocoagulation versus chemical lumbar sympathectomy in the treatment of cold hypersensitivity in the hands and feet: a retrospective study. Pain Physician. 2022;25(2):E357–E364.
10. Huang H, Qiu W, Chen Q, et al. Computed tomography (CT)-guided percutaneous thoracic sympathetic chain radiofrequency thermocoagulation for Raynaud disease. Med Sci Monit. 2019;25:7391–7395. doi:10.12659/MSM.917392
11. Maverakis E, Patel F, Kronenberg DG, et al. International consensus criteria for the diagnosis of Raynaud’s phenomenon. J Autoimmun. 2014;48:60–65. doi:10.1016/j.jaut.2014.01.020
12. Andrigueti FV, Ebbing PCC, Arismendi MI, et al. Evaluation of the effect of sildenafil on the microvascular blood flow in patients with systemic sclerosis: a randomised, double-blind, placebo-controlled study. Clin Exp Rheumatol. 2017;106(4):151–158.
13. Xie W, Strong JA, Zhang J-M. Localized sympathectomy reduces peripheral nerve regeneration and pain behaviors in 2 rat neuropathic pain models. Pain. 2020;161(8):1925–1936. doi:10.1097/j.pain.0000000000001887
14. Schlereth T, Drummond PD, Birklein F. Inflammation in CRPS: role of the sympathetic supply. Auton Neurosci. 2014;182:102–107. doi:10.1016/j.autneu.2013.12.011
15. Drummond PD. Noradrenaline increases hyperalgesia to heat in skin sensitized by capsaicin. Pain. 1995;60(3):311–315. doi:10.1016/0304-3959(94)00130-7
16. Yoo HS, Nahm FS, Lee PB, et al. Early thoracic sympathetic block improves the treatment effect for upper extremity neuropathic pain. Anesth Analg. 2011;113(3):605–609. doi:10.1213/ANE.0b013e3182274803
17. Oh TK, Kim NW, Yim J, et al. Effect of radiofrequency thermocoagulation of thoracic nerve roots in patients with cancer and intractable chest wall pain. Pain Physician. 2018;21(4):E323–E329.
18. Cracowski JL, Roustit M. Human Skin Microcirculation. Compr Physiol. 2020;10(3):1105–1154.
19. Dumont P, Denoyer A, Robin P. Long-term results of thoracoscopic sympathectomy for hyperhidrosis. Ann Thorac Surg. 2004;78(5):1801–1807. doi:10.1016/j.athoracsur.2004.03.012
20. Guo J-G, Fei Y, Huang B, et al. CT-guided thoracic sympathetic blockade for palmar hyperhidrosis: immediate results and postoperative quality of life. J Clin Neurosci. 2016;34:89–93. doi:10.1016/j.jocn.2016.05.031
21. Ding Y, Yao P, Li H, et al. Evaluation of combined radiofrequency and chemical blockade of multi-segmental lumbar sympathetic ganglia in painful diabetic peripheral neuropathy. J Pain Res. 2018;11:1375–1382. doi:10.2147/JPR.S175514
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.