Back to Journals » Journal of Pain Research » Volume 16
Efficacy of Combining Traditional Chinese Manual Therapy (Tuina) and Specific Therapeutic Neck Exercise in Young Adults with Non-Specific Chronic Neck Pain: Study Protocol for a Randomized Controlled Trial
Authors Chen L, Zhang Q, Huang Z, Da W, Liu S, Xue C, Ding C, Chen D, Fan T, Shi Q, Li X
Received 8 June 2023
Accepted for publication 7 September 2023
Published 13 September 2023 Volume 2023:16 Pages 3119—3131
DOI https://doi.org/10.2147/JPR.S424812
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Lin Chen,1,* Qi Zhang,2,* Zheng Huang,1,* Weiwei Da,1 Shuang Liu,1 Chunchun Xue,1 Chao Ding,1 Deta Chen,1 Tianyou Fan,1 Qi Shi,1 Xiaofeng Li1
1Department of Orthopedics, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Department of Traditional Chinese Medicine, Xuhui District Tianping Street Community Health Center, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaofeng Li, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, 274 Middle Zhijiang Road, Shanghai, 200071, People’s Republic of China, Tel +86-13816504131, Email [email protected]
Purpose: Non-specific chronic neck pain (NSCNP) is an increasingly common musculoskeletal disease and an important issue in the global healthcare system. Some studies have shown that the combination of manual therapy and exercise is effective in treating NSCNP but still with several limitations. Traditional Chinese manual therapy (tuina) is a Chinese manual therapy that consists of soft tissue manipulation and spinal manipulation. This study aims to design a randomized controlled trial to assess the effect of a tuina combined with specific therapeutic neck exercise modified protocol for NSCNP patients.
Patients and Methods: This is a study protocol for a randomized, participant-, assessor- and analyst-blinded controlled trial. Eighty-eight eligible NSCNP patients will be randomly allocated into tuina combined with specific therapeutic neck exercise group (TSTE group) and tuina combined with sham therapeutic neck exercise group (TS group) in a ratio of 1:1. All participants will receive 8 treatment sessions applied in 4 weeks and then be followed up for another 12 weeks. Clinical data will be collected at baseline, during treatment phase (at the 2- and 4-week) and at the 8-, 12-, 16-week follow-ups. The primary outcome is the changes in neck pain intensity (visual analogue scale). The secondary outcomes include neck disability (Neck Disability Index), cervical range of motion (ROM), neck muscle endurance, cervical muscle cross-sectional area, cervical curvature and analgesic consumption. Adverse events will be collected and recorded throughout the study.
Conclusion: We will discuss whether our tuina combined with specific therapeutic neck exercise modified protocol is more effective at improving pericervical muscle endurance, ROM, cervical muscle cross-sectional area and cervical curvature than tuina alone, thereby decreases neck pain and disability in individuals with NSCNP more effectively.
Trial Registration: Chinese Clinical Trials Registry, ChiCTR2300067903. Registered on 31 January 2023.
Keywords: manual therapy, non-specific neck pain, specific therapeutic exercise, randomized controlled trial
Introduction
Neck pain is one of the highly prevalent musculoskeletal disorders which causes considerable economic impact in the world.1 As measured by disability-adjusted life years for aged 25 to 49, neck pain ranks 19th according to the data from Global Burden of Disease Study.2 Non-specific neck pain is neck pain without any identifiable pathoanatomical cause.3 When non-specific neck pain persists for more than 12 weeks, it is called non-specific chronic neck pain (NSCNP), with a prevalence rate ranging from 30% to 50%, and 11.5% of the cases limit daily activities.4
Many studies have demonstrated that conservative treatments such as manual therapy, exercise, acupuncture and usual medical care (ie, face-to-face interview, education, reassurance, medication) are effective for patients with NSCNP.5–8 Systematic review has also found that combining manual therapy with exercise is better than manual therapy or exercise alone.9 Moreover, clinical practice guideline for the management of NSCNP also advocates clinicians should primarily apply cervical manual therapy combining with exercise therapy in NSCNP patients.10 However, during the process of implementation, some limitations are still existed in a combination of manipulation and exercise.
First, a home exercise regime is usually used in the studies. Although participants are periodically asked and monitored for the performance in home exercises, the execution of the home exercises is still not fully controlled. Second, a standardized program with the same dosage of exercise is given to all participants, while the importance of precise exercise tailoring is highlighted by non-specific neck pain clinical guidelines.11,12 Furthermore, the combination of manipulation and exercise is in a simple and independent form, which cannot reflect complementary advantages of each other.
Therefore, in this study, we will conduct a modified protocol in which we combine traditional Chinese manual therapy (tuina) and specific therapeutic neck exercise. Besides, we adopt the form of doctor-patient cooperation. Thus, participants can directly engage in exercise during the tuina treatment process. Our hypothesis is that the modified protocol would have more advantages in efficacy and satisfaction to treat NSCNP than tuina alone.
The aim of our study is to determine whether our tuina combined with specific therapeutic neck exercise modified protocol is more effective at improving pericervical muscle endurance, ROM, cervical muscle cross-sectional area and cervical curvature than tuina alone, thereby decreasing neck pain and disability in individuals with NSCNP more effectively.
Patients and Methods
Study Design and Setting
This single-center, participant-, assessor- and analyst-blinded randomized controlled trial will be conducted at Shanghai Municipal Hospital of Traditional Chinese Medicine. A total of 88 eligible NSCNP participants will be randomly assigned to tuina combined with specific therapeutic neck exercise group (TSTE group) and tuina combined with sham therapeutic neck exercise group (TS group) in a 1:1 allocation ratio to receive 8-session of tuina combined with specific therapeutic neck exercise or tuina combined with sham therapeutic neck exercise over a 4-week treatment period and a 3-month follow-up period. The trial will follow the guidelines of Consolidated Standards of Reporting Trials (CONSORT)13 and fulfil the requirements of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist (Supplementary Material).14 The flowchart of the trial process is shown in Figure 1.
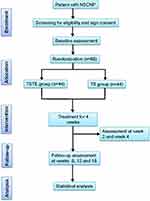 |
Figure 1 Flow chart of study procedures. |
Recruitment and Ethic
All participants will be recruited from the Shanghai Municipal Hospital of Traditional Chinese Medicine and through advertisements on hospital social Internet media (WeChat). The trial protocol has been approved by the ethics committee of Shanghai Municipal Hospital of Traditional Chinese Medicine on 30 May 2022 (No. 2022SHL-KY-29-02) and registered in the Chinese Clinical Trial Registry (ChiCTR2300067903). All interested participants will be required to sign the written informed consent form with a clear understanding of the study before participating in. The study schedule is as follows in Table 1.
 |
Table 1 Schedule of Enrollment, Intervention and Assessments |
Inclusion Criteria
Participants will be recruited if they satisfy the following criteria:15,16
- Aged 18–35 years, either gender.
- Current neck pain (sense of pain anywhere in cervical region or bilateral scapular region).
- Experienced non-specific chronic neck pain for at least 12 weeks.
- Mean neck pain intensity ≥30 mm on a 100 mm visual analog scale during the preceding week.
- Willing to participate and prohibit any other treatment which may affect trial results.
Exclusion Criteria
Participants will be excluded if they have any of the following conditions:17,18
- Neck pain with radicular syndrome, disc protrusion or prolapse, whiplash, fractures of cervical, congenital spine deformity, spinal stenosis or neoplasm.
- A reported red flag medical history, including inflammatory rheumatic diseases, oncological diseases, metabolic diseases, neurological disease, fibromyalgia syndrome.
- A history of previous cervical spine surgery or invasive treatment of the spine.
- Severe psychiatric disorders and somatic comorbidity.
- Pregnancy or preparing for pregnancy during the study period.
- Undergoing any treatment for neck pain or had received related treatment in the previous three months.
Physician will diagnose the exclusion criteria based on symptoms, mandatory physical examination, X-ray and MRI.
Randomization and Allocation Concealment
The randomization sequence will be computer-generated with the software SPSS 21.0 (SPSS Statistics, IBM Corp., Armonk, NY, USA) by an independent statistician, who will not participate in the implementation or later statistical work of the trial. Random numbers will be placed in sealed and opaque envelopes, and an independent researcher will ensure the concealment of the allocation sequence. The therapists will sequentially open the envelopes and allocate the patients accordingly in the trial.
Blinding
The assessor, analyst and participants will be blinded to the allocated group, except for the therapists. The participants will not be informed of group allocation throughout the trial, and they will be explained to receive either of the two different types of manual therapy. To prevent exchange of study information, each participant will be treated separately, and a blinding test will be conducted at week 4 to assess the success of blinding. In addition, researchers will be trained the specifications of the trial and required to strictly abide by the principle of task separation.
Qualification of Practitioners
Only traditional Chinese manual therapy therapists registered Chinese Medicine Practitioners with at least 3 years of tuina experience will perform the treatment in the study. All therapists will be trained in administering tuina protocol.
Interventions
The interventions will be applied to eligible NSCNP patients in two groups for 8 sessions over 4 weeks (2 sessions per week with a minimum interval of 2 days). Each participant will be placed in a constant room temperature of 23–25 ℃ and performed in a sitting position during the treatment. Each treatment will last for 35 min. In order to improve participants’ adherence, participants will accept the treatments and imaging examinations for free, as well as two additional free tuina treatments after the last follow-up assessment.
Traditional Chinese Manual Therapy Combined with Specific Therapeutic Neck Exercise Group
In TSTE group, the therapist will administer a five-step protocol intended to alleviate neck pain and improve neck function, which includes four-step tuina protocol (local manipulation, cervical spine manipulation, cervicothoracic thrust manipulation, ear acupressure) and one-step specific therapeutic neck exercise protocol. The specific protocol used is as follows.
Step 1: Local Manipulation
Participants are instructed to sit and relax their neck naturally. The therapist first will apply kneading manipulation to relax the muscles and soft tissues of the neck, shoulders and upper back for 3 minutes. Then, the therapist will press and knead the acupoints in traditional Chinese medicine such as DU16 (Du meridian 16), SI14 (Small intestine meridian 14), LI11 (Large intestine meridian 11), GB20 and GB21 (Gallbladder Meridian 20 and 21) for 1 min each. The strength of pressing and kneading acupoints depends on participant’s feeling, which is usually described as sour swelling, heaviness, numbness or dull pain.
Step 2: Specific Therapeutic Neck Exercise
The specific therapeutic neck exercise protocol was modified based on the technique standardized by Akodu et al.19 For the specific therapeutic neck exercise, the cervical flexion, extension and rotation exercises will be performed with the aid of therapist. All the cervical exercises will be performed in a sitting position with the head in a neutral position. To perform the cervical flexion exercise, the therapist places his right palm on the forehead of the participant and his left hand on the seventh cervical vertebra behind the neck to maintain the stability of the neck movement. Then, the participant will press the therapist’s right hand in order to indicate that the therapist should apply similar forces in the opposite direction. Using these balanced forces, the therapist instructed the participant to perform isometric contraction. The participant will perform the isometric contraction for 6 seconds, counting from 1 to 6, and gradually increase the intensity of the contraction to maximal strength. When the participant’s contraction intensity reaches the maximum, the therapist’s right hand will appropriately reduce the resistance and guide the participant to conduct slow and uniform flexion resistance movement until the flexion reaches the maximum angle. This movement continues for about 10 seconds and the cervical flexion exercise will be applied three repetitions with an interval of 10 seconds each time. Cervical extension and rotation exercises are similar to the flexion exercise, except that the therapist’s right hand is placed on the back of the participant’s head or the corresponding cheek.
The step 1 (local manipulation) and step 2 (specific therapeutic neck exercise) will be carried out alternately and repeated three times to ensure that the neck muscles are fully exercised and relaxed.
Step 3: Cervical Spine Manipulation
The cervical spine manipulation is a non-fixed-point rotational manipulation,20 which is not performed on a specific cervical segment, but by twisting the head to transmit the force to the cervical spine to achieve a therapeutic effect. The participant is manipulated in an upright seated position, with the therapist standing behind him to perform the oblique pulling and the rotation-traction manipulation on the left and right sides, respectively. The oblique pulling manipulation is done with one hand to hold the participant’s jaw bone and the other to hold the neck, with the two hands exerting cooperative force in the opposite directions, allowing the neck to be slightly twisted to the obvious resistance point. Take the oblique pulling manipulation on the left as an example. First, the therapist guides the participant’s head into flexion and extreme rotation in the left direction. Second, the therapist puts his right hand against the back of the participant’s neck and the left hand against the participant’s jaw to help the participant bend to the left limit again. Finally, the therapist momentarily increases the rotational force and then releases it. During the manipulation, the therapist should listen for cavitation. If no cavitation occurs on the first attempt, the participant will be repositioned, and the therapist will perform a second cervical spine manipulation. A maximum of two attempts can be performed on each participant.
Step 4: Cervicothoracic Thrust Manipulation
The cervicothoracic thrust manipulation is performed with the participant seated with his/her fingers interlocked behind lower cervical spine.21 The therapist stands behind the participant and threads arms through him/her so that hands are on top and inferior to the participant’s hands at the cervicothoracic junction. Then, the participant is told to relax his/her arms and gently recline. When the participant is in a state of natural breathing relaxation after exhalation, the therapist uses legs and waist extension to provide a high velocity, short amplitude distractive thrust directed at the cervicothoracic junction. Similarly, a maximum of two attempts can be performed on each participant depending on whether cavitation occurs.
Step 5: Ear Acupressure
The therapist will massage the auricular points on both ears of the sitting participant with his hands. Based on the classical theory of traditional Chinese medicine and expert experience, participants will receive ear acupressure on the following auricular points: AH13 (Jingzhui) and AH11 (Xiongzhui). The auricular points’ names and locations follow the “Chinese Standard Ear Acupoints Chart Nomenclature and Location of Auricular Points” (GB/T-13734–2008) (Figure 2). Each auricular point will be moderately stimulated and compressed for 1 min. During the process of ear acupressure, the participant should feel sourness and heat of the ear.
 |
Figure 2 Locations of auricular acupoints (red circles). |
Traditional Chinese Manual Therapy Combined with Sham Therapeutic Neck Exercise Group
In this group, the sequence of treatment protocol and steps of Tuina treatment are the same as those of the TSTE group. In addition to the specific therapeutic neck exercise step, the therapist will lay his hands without therapeutic intention on the corresponding part of the participant, and the participant will be contacted without exerting pressure during the cervical exercises.
Permitted and Prohibited Concomitant Treatments
Additional treatment for neck pain will be banned during the trial, which includes but is not limited to acupuncture, physical therapy, drug injections, oral muscle relaxants and surgery. However, participants will be allowed to have general light exercises. The researchers will re-emphasize the prohibited concomitant treatments at every visit, and any change in concurrent treatment will be recorded.
Evaluation
All participants will complete a questionnaire during the initial screening visit regarding demographic data (age, gender, weight, height, education level, occupation), as well as information on past neck pain such as duration, treatment method and curative effect. X-ray and magnetic resonance imaging will also be examined for analysis before randomization.
Outcomes
The primary outcome will be neck pain intensity. The secondary outcomes include neck disability, cervical range of motion, neck muscle endurance, cervical muscle cross-sectional area, cervical curvature and analgesic consumption. All the following outcomes will be assessed at baseline, during treatment phase (at the 2- and 4-week) and at the 8-, 12-, 16-week follow-ups, except for the cervical range of motion, neck muscle endurance, cervical muscle cross-sectional area and cervical curvature, which will be only conducted at baseline and 4-week.
Primary Outcome Measurement
Pain Intensity
The visual analogue scale (VAS) created by Scott and Huskisson22 will be used to evaluate the intensity of neck pain during the past week perceived by the subjects. The subjects will place a vertical mark at the point on a horizontal line of 10 cm that represented their level of pain, where 0 indicated “no pain” and 10 indicated “the worst pain imaginable”.23 In patients with chronic neck pain, a change of 0.8 cm is a minimal clinically important difference (MCID) score.24
Secondary Outcome Measurements
Neck Disability
The Chinese version of the Neck Disability Index (NDI) will be used to measure the neck-related disability in two groups. This index contains ten sections including daily living activity (seven sections), pain (two sections), and concentration (one section). Each section is scored from 0 (no disability) to 5 (total disability) and total scores range from 0 to 50, with higher scores corresponding to higher degree of disability.25
Cervical Range of Motion
Cervical range of motion (CROM) in six conventional movements (flexion, extension, right and left lateral flexion, right and left rotation) will be assessed using a cervical goniometer (CROM Deluxe, Performance Attainment Associates), which has been shown to have high levels of repeatability with an intra-tester reliability (ICC) ranging from 0.89 to 0.98.26 The patients will be measured while sitting in a relaxed straight-back seated position, with both feet flat on the ground, hips and knees at a right angle.
Neck Muscle Endurance
Neck flexor and extensor muscle endurance tests will be measured in seconds and performed in lines with the previous studies.27,28 For the flexor muscles, participants are in supine position with legs straight and arms by side. They are asked to flex the upper cervical spine and lift their head approximately 2.5 cm off the bed while maintaining retraction until exhaustion. During the text, the investigator keeps one hand on the bed beneath the participant’s head while has a chronometer in the other hand. Test will be terminated when participants lost their craniocervical flexion upon witnessing the loss of skinfolds posterior to the mandible or dropped their heads on the investigator’s hand. For the extensor muscles, participants are in prone position with legs straight, arms by side and head outside the plinth supported by a pillow. An inclinometer will be placed around participant’s head, and a 2-kg weight applied is suspended from the head above the ears. Participants are asked to support the weight and maintain the head in neutral position as long as possible. Test will be terminated when the head position reached beyond 5° or a maximum endurance test of 5 min. Both of the neck flexor and extensor muscle endurance tests will be repeated twice with an interval of 5 minutes, and the mean of two results will be regarded as the final result.
Cervical Muscle Cross-Sectional Area
The cross-sectional area (CSA) measurement method of cervical muscle was based on the technique standardized by Li et al.29 Digital images from routine neutral position cervical spine MRI will be retrieved from the hospital radiographic system (Winning Health, WiNEX). MR images are acquired on a 1.5-T magnet (SIEMENS) using a standard neurovascular coil. The measurement parameters are consisted of 14 slices with a slice thickness and space of 3.5 mm, FOV 180 × 180 mm, TR 3300 msec, TE 110 msec, matrix size 160 × 256, 1.5 excitations, flip angle 90.
Two independent reviewers blinded to all clinical information of the patients will use axial T2-weighted images at the upper vertebral endplates from C4 to C6 intervertebral levels for muscle CSA measurement. Muscle areas such as superficial flexors (SFs), deep flexors (DFs), superficial extensors (SEs) and deep extensors (DEs) will be measured in accordance with the MRI atlas of the cervical spine musculature. All CSAs will be performed in square millimeters (mm2) by creating a region of interest (ROI) for each muscle bilaterally. Perpendicular lines from lateral border of the ipsilateral facets will be used as lateral limits for the ROI of SEs.
Given that close relationship between muscle mass and bone strength, ratios of muscle CSA/bony CSA rather than muscle CSA are usually used to eliminate biases caused by physical variations.30 Thus, C4, C5 and C6 vertebral body areas (VBAs) at the levels of upper endplate will be also measured to calculate ratios of muscle CSAs/ VBAs.
Cervical Curvature
The lateral radiographs of cervical vertebrae from the hospital radiographic system (Winning Health, WiNEX) will be individually measured by two reviewers. Borden’s method31 will be used to identify cervical vertebral curve apex, and the schematic diagram is shown in Figure 3.
Analgesic Consumption
Patients will be allowed to take celecoxib (Pfizer Pharmaceutical Co. Ltd., New York State, USA) when they cannot endure neck pain during the trial. The dose and the time of taking celecoxib should be recorded on their case report forms (CRFs).
Adverse Events
Adverse events (AEs) will be monitored at each visit, and any AEs associated with intervention or not will be evaluated. If AEs occurred, researchers will treat the adverse reactions (including observation, medical management, even an early closure of participation) immediately. In addition, the details of AEs will be reported in the CRFs and ethics committee in time. Common AEs in tuina clinical trials include pain, vertigo, syncope, disability, etc. Once any AE occurs, the ethics committee of Shanghai Municipal Hospital of Traditional Chinese Medicine will be responsible for deciding whether to suspend the trial. After investigating and accessing the cause of AEs, participants will be provided with relevant-free medical treatment and appropriate economic compensation.
Blinding Test
Participants will be asked to guess the type of treatment after their final treatment, and the success of the blind strategy will be appraised at the end of the study.
Withdrawal
Participants have the right to discontinue the treatment or withdraw from the study at any time for any reason. Furthermore, if serious AEs occur during the trial, the trial will be forcibly suspended immediately.
Data Collection and Management
The original data will be collected using the printed CRFs and double entered into the electronic case report form (eCRF) by independent researchers timely. Paper CRFs will be stored securely in a locked cabinet in the researcher’s work office, and electronic datasets will be de-identified and blocked. Only authorized data administrators and statistician will be accessed to these paper files and electronic documents. All research documents will be kept strictly and preserved for at least five years after publication.
Monitoring and Quality Control
The Clinical Evaluation and Analysis Centre of Shanghai Municipal Hospital of Traditional Chinese Medicine which is not taking part in and with no conflict of interests in the trial will be responsible for monitoring the data, controlling the bias and suspending or terminating the study. With the management of the Clinical Evaluation and Analysis Centre, quality control will be conducted throughout the trial. Before participating in the trial, all researchers should receive professional trial method training and regular monitoring technique to ensure the consistency of methods. Any corrections or modifications to the study protocol should be submitted to the ethics committee and informed Clinical Evaluation and Analysis Centre.
Sample Size
The sample size calculation will be conducted based on the primary outcome (VAS) and evaluated with the PASS 15 statistical program. According to our small pilot study, the mean and standard deviation of VAS in TS group (n = 11) after intervention was 3.32 and 0.95. While in TSTE group (n = 11), the mean and standard deviation of VAS after intervention was 2.62 and 1.14. The estimated sample size was 74 individuals (37 in each group) to meet the two-tailed test with a significance level of 0.05 and power of 0.80. Considering a 20% dropout rate, a total of 88 participants (44 per group) are required in this trial.
Statistical Analysis
The statistical analysis will be followed the intention-to-treat (ITT) principle, including incomplete data of any participants who withdrew from the RCT during the trial. All the statistical analysis of data will be performed with SPSS 21.0 software by an independent statistician who is blinded to the group allocation. Continuous variables with normal distribution will be reported as means ± standard deviations, whereas continuous variables with non-normal distribution will be presented as median and interquartile range. The categorical variables will be described as counts and percentages. Data normality will be tested through a Kolmogorov–Smirnov test with Lilliefors correction. Parametric statistics or non-parametric statistics will be used for the within- and between-group analyses in accordance with the results of homogeneity and normality analysis. Repeated measures analysis of variance (ANOVA) will be used to analyze the effect of treatment, time and interaction terms between treatment groups versus time. Two-sided paired t tests will be used for within-group comparisons. A chi-squared test or a Fisher’s exact test will be used to analyze categorical variables and adverse effects between groups. The statistical significance will be set at P< 0.05 (two-sided), and the confidence interval will be established at 95%.
Discussion
According to the guidelines of the Orthopaedic Section of the American Physical Therapy Association, manipulative therapy is recommended for NSCNP at different stages.32 Studies have also reported that manipulative therapy such as therapeutic massage, cervical spine manipulation and cervicothoracic thrust manipulation has clinical benefits for treating NSCNP.20,21,33 Traditional Chinese manual therapy (tuina) is a Chinese manual therapy that consists of soft tissue manipulation and spinal manipulation.34 The effect of tuina is attributed to relaxing muscles and tendons, improving local blood circulation, decreasing edema and aseptic inflammation.35 Tuina has been widely used to treat NSCNP worldwide,36 and the efficacy has also been reported.37 However, to our knowledge, there have been few clinical studies on the treatment of combining tuina and specific therapeutic neck exercise in NSCNP.
Evidence shows that specific therapeutic neck exercise is effective for reducing pain and disability in NSCNP with the mechanisms of neurophysiological effects such as reorganization in motor patterns, structural adaptations and increase in strength and endurance.38 Many studies have evaluated the effect of combining manual therapy with specific therapeutic neck exercise on patients with NSCNP. Its purpose is to check the effect in treating this clinical condition.5,39 However, they have not included complete clinical performance protocols, but simply combined manual therapy and therapeutic exercise.
In the current study, we have carried out a modified protocol combining tuina and specific therapeutic neck exercise to treat patients with NSCNP. This protocol has some innovation for several reasons. First, our intervention protocol can be considered as a complete treatment option by itself, without adding other complementary techniques. NSCNP patients can directly engage in specific therapeutic neck exercise during the tuina treatment without the need for additional home exercise which can avoid the uncertainty caused by supervised exercise in terms of treatment effects. Second, the specific therapeutic neck exercise in this study is based on load progression composed of different stages, including activation and recruitment of the deep cervical flexors, co-contraction of the deep and superficial flexors during isometric exercise, and eccentric recruitment of the flexors and extensors. Although exercise can enhance muscle strength, it can also lead to muscle fatigue. However, manual therapy has shown a certain positive effect in improving muscle fatigue, with various effects such as reducing delayed muscle soreness, maintaining exercise performance, and alleviating pain.40 Therefore, alternating tuina and exercise in this study are more beneficial for the therapeutic effects. Lastly, the participant’s specific therapeutic neck exercise is achieved by the resistance exercise with the therapist’s hands, which means the therapist can tailor precise exercise based on the level of each participant.
Given the nature of the study, the limitation of this study is that it is difficult to conduct a double-blinded clinical trial as blinding the therapist is not possible. Another potential limitation of our study is that due to funding constraints, the last follow-up was done after 3 months, so we cannot know the middle- and long-term evolution, which may lead to the need for a future study.
Abbreviations
AEs, Adverse events; CONSORT, Consolidated Standards of Reporting Trials; CSA, cross-sectional area; CRFs, case report forms; CROM, cervical range of motion; DEs, deep extensors; DFs, deep flexors; eCRF, electronic case report form; ICC, intra-tester reliability; ITT, intention-to-treat; MCID, minimal clinically important difference; NSCNP, Non-specific chronic neck pain; NDI, Neck Disability Index; ROI, region of interest; SEs, superficial extensors; SFs, superficial flexors; SPIRIT, Standard Protocol Items, Recommendations for Interventional Trials; Standard protocol items; TSTE, tuina combined with specific therapeutic neck exercise; TS, tuina combined with sham therapeutic neck exercise; VAS, visual analogue scale; VBAs, vertebral body areas.
Trial Status
Protocol: version 1.1, 31 January 2023.
The study launched on 31 January 2023 and participant recruitment is in progress now. Recruitment is expected to be completed by the end of December 2023.
Data Sharing Statement
The data in this trial will be publicly available from the corresponding author upon reasonable request. All data and the protocol will be available after publication in peer-reviewed international journals for 3 years.
Ethics Approval and Informed Consent
The trial protocol has been approved by the ethics committee of Shanghai Municipal Hospital of Traditional Chinese Medicine on 30 May 2022 (No. 2022SHL-KY-29-02). All interested participants will be required to sign the written informed consent form with a clear understanding of the study before participating in. All methods will be conducted in accordance with the ethical standards of the declaration of Helsinki.
Acknowledgments
The authors would like to thank all the participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study is partly sponsored by the Future Plan for Traditional Chinese Medicine Inheritance and Development of Shanghai Municipal Hospital of Traditional Chinese Medicine (WLJH2021ZY-ZYY013), the Training Program for High-caliber Talents of Clinical Research at Affiliated Hospitals of SHUTCM (2023LCRC18), the Medical Research Program of Shanghai Xuhui District (SHXH202224), the Domestic Science and Technology Cooperation Project of Shanghai Science and Technology Innovation Action Plan (No. 22015830700). The study funder has no role in the study design, data collection, management, analysis, interpretation, or manuscript writing publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Safiri S, Kolahi AA, Hoy D, et al. Global, regional, and national burden of neck pain in the general population, 1990–2017: systematic analysis of the Global Burden of Disease Study 2017. BMJ. 2020;368:m791. doi:10.1136/bmj.m791
2. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/s0140-6736(20)30925-9
3. Binder A. The diagnosis and treatment of nonspecific neck pain and whiplash. Eura Medicophys. 2007;43(1):79–89.
4. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the bone and joint decade 2000–2010 task force on neck pain and its associated disorders. Spine. 2008;33(4 Suppl):S39–51. doi:10.1097/BRS.0b013e31816454c8
5. Groisman S, Malysz T, de Souza da Silva L, et al. Osteopathic manipulative treatment combined with exercise improves pain and disability in individuals with non-specific chronic neck pain: a pragmatic randomized controlled trial. J Bodyw Mov Ther. 2020;24(2):189–195. doi:10.1016/j.jbmt.2019.11.002
6. Kim JI, Kim YI, Kim E, et al. Effectiveness and safety of polydioxanone thread embedding acupuncture compared to physical therapy in the treatment of patients with non-specific chronic neck pain: study protocol for an assessor-blinded, randomized, controlled, clinical trial. Medicine. 2019;98(32):e16768. doi:10.1097/md.0000000000016768
7. Cheng Z, Chen Z, Xie F, et al. Efficacy of Yijinjing combined with Tuina for patients with non-specific chronic neck pain: study protocol for a randomized controlled trial. Trials. 2021;22(1):586. doi:10.1186/s13063-021-05557-2
8. Bronfort G, Evans R, Anderson AV, Svendsen KH, Bracha Y, Grimm RH. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156(1 Pt 1):1–10. doi:10.7326/0003-4819-156-1-201201030-00002
9. Hidalgo B, Hall T, Bossert J, Dugeny A, Cagnie B, Pitance L. The efficacy of manual therapy and exercise for treating non-specific neck pain: a systematic review. J Back Musculoskelet Rehabil. 2017;30(6):1149–1169. doi:10.3233/bmr-169615
10. Bier JD, Scholten-Peeters WGM, Staal JB, et al. Clinical practice guideline for physical therapy assessment and treatment in patients with nonspecific neck pain. Phys Ther. 2018;98(3):162–171. doi:10.1093/ptj/pzx118
11. Parikh P, Santaguida P, Macdermid J, Gross A, Eshtiaghi A. Comparison of CPG’s for the diagnosis, prognosis and management of non-specific neck pain: a systematic review. BMC Musculoskelet Disord. 2019;20(1):81. doi:10.1186/s12891-019-2441-3
12. Corp N, Mansell G, Stynes S, et al. Evidence-based treatment recommendations for neck and low back pain across Europe: a systematic review of guidelines. Eur J Pain. 2021;25(2):275–295. doi:10.1002/ejp.1679
13. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi:10.1136/bmj.i5239
14. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346(jan08 15):e7586. doi:10.1136/bmj.e7586
15. Guzman J, Haldeman S, Carroll LJ, et al. Clinical practice implications of the bone and joint decade 2000–2010 Task force on neck pain and its associated disorders: from concepts and findings to recommendations. Spine. 2008;33(4 Suppl):S199–S213. doi:10.1097/BRS.0b013e3181644641
16. Cohen SP. Epidemiology, diagnosis, and treatment of neck pain. Mayo Clin Proc. 2015;90(2):284–299. doi:10.1016/j.mayocp.2014.09.008
17. Allende S, Anandan A, Lauche R, Cramer H. Effect of yoga on chronic non-specific neck pain: an unconditional growth model. Complement Ther Med. 2018;40:237–242. doi:10.1016/j.ctim.2017.11.018
18. Bernal-Utrera C, Gonzalez-Gerez JJ, Anarte-Lazo E, Rodriguez-Blanco C. Manual therapy versus therapeutic exercise in non-specific chronic neck pain: a randomized controlled trial. Trials. 2020;21(1):682. doi:10.1186/s13063-020-04610-w
19. Akodu AK, Nwanne CA, Fapojuwo OA. Efficacy of neck stabilization and Pilates exercises on pain, sleep disturbance and kinesiophobia in patients with non-specific chronic neck pain: a randomized controlled trial. J Bodyw Mov Ther. 2021;26:411–419. doi:10.1016/j.jbmt.2020.09.008
20. Huang X, Lin D, Liang Z, et al. Mechanical parameters and trajectory of two Chinese cervical manipulations compared by a motion capture system. Front Bioeng Biotechnol. 2021;9:714292. doi:10.3389/fbioe.2021.714292
21. Grimes JK, Puentedura EJ, Cheng MS, Seitz AL. The comparative effects of upper thoracic spine thrust manipulation techniques in individuals with subacromial pain syndrome: a randomized clinical trial. J Orthop Sports Phys Ther. 2019;49(10):716–724. doi:10.2519/jospt.2019.8484
22. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175–184. doi:10.1016/0304-3959(76)90113-5
23. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi:10.1016/0304-3959(83)90126-4
24. Lauche R, Langhorst J, Dobos GJ, Cramer H. Clinically meaningful differences in pain, disability and quality of life for chronic nonspecific neck pain - A reanalysis of 4 randomized controlled trials of cupping therapy. Complement Ther Med. 2013;21(4):342–347. doi:10.1016/j.ctim.2013.04.005
25. Vernon H, Mior S. The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–415.
26. Audette I, Dumas JP, Côté JN, De Serres SJ. Validity and between-day reliability of the cervical range of motion (CROM) device. J Orthop Sports Phys Ther. 2010;40(5):318–323. doi:10.2519/jospt.2010.3180
27. Fazli F, Farahmand B, Azadinia F, Amiri A. The effect of ergonomic latex pillow on head and neck posture and muscle endurance in patients with cervical spondylosis: a randomized controlled trial. J Chiropr Med. 2019;18(3):155–162. doi:10.1016/j.jcm.2019.02.003
28. Carvalho GF, Luedtke K, Szikszay TM, Bevilaqua-Grossi D, May A. Muscle endurance training of the neck triggers migraine attacks. Cephalalgia. 2021;41(3):383–391. doi:10.1177/0333102420970184
29. Li Z, Zhang W, Wu W, Wei C, Chen X, Lin J. Is there cervical spine muscle weakness in patients with Hirayama disease? A morphological study about cross-sectional areas of muscles on MRI. Eur Spine J. 2020;29(5):1022–1028. doi:10.1007/s00586-020-06290-1
30. Thakar S, Kurudi Siddappa A, Aryan S, Mohan D, Sai Kiran NA, Hegde AS. Does the mesodermal derangement in Chiari Type I malformation extend to the cervical spine? Evidence from an analytical morphometric study on cervical paraspinal muscles. J Neurosurg Spine. 2017;27(4):421–427. doi:10.3171/2016.12.Spine16914
31. Borden AG, Rechtman AM, Gershon-Cohen J. The normal cervical lordosis. Radiology. 1960;74(5):806–809. doi:10.1148/74.5.806
32. Blanpied PR, Gross AR, Elliott JM, et al. Neck pain: revision 2017. J Orthop Sports Phys Ther. 2017;47(7):A1–a83. doi:10.2519/jospt.2017.0302
33. Sherman KJ, Cherkin DC, Hawkes RJ, Miglioretti DL, Deyo RA. Randomized trial of therapeutic massage for chronic neck pain. Clin J Pain. 2009;25(3):233–238. doi:10.1097/AJP.0b013e31818b7912
34. Wu Z, Kong L, Zhu Q, et al. Efficacy of tuina in patients with chronic neck pain: study protocol for a randomized controlled trial. Trials. 2019;20(1):59. doi:10.1186/s13063-018-3096-3
35. Goats GC. Massage--the scientific basis of an ancient art: part 2. Physiological and therapeutic effects. Br J Sports Med. 1994;28(3):153–156. doi:10.1136/bjsm.28.3.153
36. Gong Z, Guo Y, Liu X, Ai K, Li W, Li J. Bibliometric analysis of research trends on tuina manipulation for neck pain treatment over the past 10 years. J Pain Res. 2023;16:2063–2077. doi:10.2147/jpr.S410603
37. Cheng ZJ, Zhang SP, Gu YJ, et al. Effectiveness of Tuina therapy combined with Yijinjing exercise in the treatment of nonspecific chronic neck pain: a randomized clinical trial. JAMA network open. 2022;5(12):e2246538. doi:10.1001/jamanetworkopen.2022.46538
38. Villanueva-Ruiz I, Falla D, Lascurain-Aguirrebeña I. Effectiveness of specific neck exercise for nonspecific neck pain; usefulness of strategies for patient selection and tailored exercise-a systematic review with meta-analysis. Phys Ther. 2022;102(2): pzab259.
39. Bernal-Utrera C, Anarte-Lazo E, Gonzalez-Gerez JJ, et al. Effect of combined manual therapy and therapeutic exercise protocols on the postural stability of patients with non-specific chronic neck pain. a secondary analysis of randomized controlled trial. J Clin Med. 2021;11(1):84. doi:10.3390/jcm11010084
40. Fuller JT, Thomson RL, Howe PR, Buckley JD. Vibration therapy is no more effective than the standard practice of massage and stretching for promoting recovery from muscle damage after eccentric exercise. Clin J Sport Med. 2015;25(4):332–337. doi:10.1097/jsm.0000000000000149
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

