Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Efficacy and Tolerability of Hyperdiluted Calcium Hydroxylapatite (Radiesse) for Neck Rejuvenation: Clinical and Ultrasonographic Assessment
Authors Trindade de Almeida AR, Marques ERMC, Contin LA, Trindade de Almeida C, Muniz M
Received 4 March 2023
Accepted for publication 13 May 2023
Published 25 May 2023 Volume 2023:16 Pages 1341—1349
DOI https://doi.org/10.2147/CCID.S407561
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Ada Regina Trindade de Almeida,1,2 Elisa Raquel Martins C Marques,1,2 Letícia Arsie Contin,1,2 Camila Trindade de Almeida,2 Mariana Muniz3
1Hospital do Servidor Público Municipal, São Paulo, SP, Brazil; 2For Trials Pesquisa Clínica, São Paulo, SP, Brazil; 3Dermatology Private Office, São Paulo, SP, Brazil
Correspondence: Ada Regina Trindade de Almeida, R. Turiassu, 390 - cjs 113-114 - Perdizes, São Paulo, SP, 05005-000, Brazil, Email [email protected]
Background: The subdermal injection of calcium hydroxylapatite (CaHA) can improve the mechanical properties of the skin, providing immediate correction and stimulating the endogenous production of collagen, elastin, angiogenesis, and dermal cell proliferation; however, few studies have examined the neck region.
Purpose: This study assessed the clinical and ultrasonographic improvement induced by two sessions of subdermal injection of hyperdiluted (1:4) CaHA for neck rejuvenation in 22 women.
Patients and Methods: A quasi-experimental longitudinal trial (before and after) was performed by enrolling 22 adult women with mild and moderate neck aging (grades 1 and 2) on Merz Neck Volume Scale at rest (laxity) and Neck Horizontal Lines. They were submitted to two sessions of subdermal hyperdiluted CaHA (D0 and D45) treatments and assessed clinically and ultrasonographically at D0, D45, and D120. The main outcomes were the blinded photographic assessment (Merz scales of neck laxity and horizontal lines), dermal thickness (at three points), and the GAIS (Global Aesthetic Improvement Scale) score.
Results: The mean (SD) age was 43.5 (5.6) years. At the inclusion, the Merz scores for neck laxity were mild for 27% and moderate for 73% of participants, and the Merz scores for necklines were mild for 32% and moderate for 68%. At D120, scores decreased in 86% (95% CI 68– 99%) of the participants by at least one degree on the necklines scale and in 82% (95% CI 73– 90%) for neck laxity. According to the GAIS, 91% (95% CI 77– 99%) of the participants evidenced improvement at D120. The mean dermal thickness increased by 15% (95% CI 8– 21%) at D120. No severe adverse effects were recorded, and high satisfaction was reported by 82% of the participants.
Conclusion: Two sessions of subdermal hyperdiluted CaHA were well-tolerated and improved necklines, neck laxity, and dermal thickness in adult women with mild and moderate cervical aging.
Keywords: rejuvenation, biostimulatory treatment, neocollagenesis, calcium hydroxylapatite, neck, collagen, dermis
Introduction
Cervical aging, similar to facial aging, is a multifactorial condition influenced by sun exposure, gravity, hormonal changes, genetic background, and postural characteristics, which modulate the decrease in dermal collagen and elastin.1,2 It is clinically manifested as skin thinning, flaccidity, and dyschromia. Although rhytids are a frequent aesthetic complaint in medical offices, there is a paucity of systematic studies on the assessment of rejuvenation procedures for the neck.
Calcium hydroxylapatite (CaHA) is a biocompatible, biodegradable, and resorbable biostimulator product. The commercial formulation known as Radiesse (Merz North America, Raleigh, NC) is composed of 30% synthetic CaHA microspheres (25–45μm diameter) suspended in a 70% aqueous carboxymethyl cellulose gel carrier. This formulation has been used to stimulate facial and body collagen and elastin production, angiogenesis, and dermal cell proliferation, improving the skin’s mechanical characteristics. When CaHA is hyperdiluted, biostimulation occurs without the relevant dermal volumization.3,4
Studies have demonstrated that subdermal injections of CaHA can induce extracellular matrix remodeling, improving quality, promoting local skin tightening, and reducing wrinkles. Data on the use of diluted (ratio of 1:1, CaHA: diluent) or hyperdiluted (ratio of ≥1:2, CaHA: diluent) CaHA for the treatment of the neck and décolletage areas derive from three small studies.5
This work aimed to evaluate the clinical and ultrasonographic improvement induced by two sessions of subdermal injection of hyperdiluted (1:4) CaHA for neck rejuvenation in 22 women.
Patients and Methods
A prospective, quasi-experimental (before and after), evaluator-blinded, single-center study was performed to evaluate the clinical and ultrasonographic improvement induced by two sessions of subdermal injection of hyperdiluted (1:4) CaHA for neck rejuvenation.
The protocol was approved by the institution’s review board (Comitê de Ética e Pesquisa da Universidade São Francisco (protocol #5.159.039)), and all the participants provided written informed consent to publish their data in this paper. The study complies with the Declaration of Helsinki.
Twenty-two healthy adult women from São Paulo-SP (Brazil) aged between 35 and 55 years, with mild or moderate horizontal neck lines and mild or moderate neck laxity (Neck Volume (laxity) Scale at rest) and Neck Horizontal Lines were enrolled in the study between October 2021 and April 2022.6,7 The exclusion criteria were pregnancy, lactation, hypersensitivity to CaHA or lidocaine, use of anticoagulants or immunosuppressive drugs, local infection, blood cell dyscrasias, diabetes mellitus, allergy to local anesthetics, and previous procedures on the neck (eg, lifting, lipoaspiration, microfocused ultrasound, polydioxanone implants, hyaluronic acid, polylactic acid, and CaHA).
The study comprised three visits: D0 (inclusion + assessment + procedure), D45 (assessment + procedure), and D120 (final assessment).
To dilute the CaHA, a LuerLock 5-mL syringe containing 2 mL of 2% lidocaine was connected to the original 1.5 mL syringe through a transfer adapter in a sterile mixing environment. Then, the LuerLock 5-mL syringe containing the mixture (1.5 mL CaHA plus 2 mL of 2% lidocaine) was connected to another LuerLock 5-mL syringe containing 4 mL of 0.9% saline. At least 20 passes between the two 5-mL syringes were performed to adequately mix the final 7.5 mL total volume. The injection was performed using the original 1.5mL CaHA syringe. The neck was then divided into five columns. Each column received 1.5 mL of hyperdiluted CaHA, injected by retrograde parallel linear threads of 0.1 mL aliquots per pass, using a 27G ¾ long needle, adapted to the original syringe (Figure 1). All the patients were submitted to the same therapeutic regimen by the same injector.
 |
Figure 1 Schematic representation of the retrograde parallel linear subdermal injections in a participant of the study (above the platysma muscle). Notes: The injection lines should be aligned vertically with the topography of the sternocleidomastoid muscle, taking into account its origin, middle, and insertion, dividing the anterior neck into five equivalent columns (A). Injection points should be placed at intervals of 1 cm along these vertical lines (B). Schematic representation of the parallel retroinjections (C). Data from these studies.4,8 |
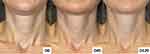
Standardized photographs were taken before the treatments at D0 and D45 as well as at D120 for randomized blinded consensual analysis (by two experienced blinded dermatologists) of the grades of neck laxity and horizontal necklines. They were measured byMerz scales of neck laxity and horizontal lines, Neck Volume Scale at rest and Neck Horizontal Lines [© 2020 Merz Pharmaceuticals GmbH], respectively, at each visit.6 The categories of Merz Scale of Laxity at rest are: 0 – “No sagging”, 1 – “Mid sagging”, 2 – “Moderate sagging”, 3 – “Severe sagging”, 4 – “Very severe sagging”. The Merz Scale Neck Horizontal Lines, in dynamic are: 0 – “No lines”, 1 – “Mild lines”, 2 – “Moderate lines”, 3 – “Severe lines”, 4 – “Very severe lines”. Additionally, the assessment of the 5-point GAIS (Global Aesthetic Improvement Scale) was consensually graded at D45 and D120, in comparison with D0. The categories of improvement were: 1 – “Exceptional improvement”, 2 – “Very improved”, 3 – “Improved”, 4 – “Unaltered”, and 5 – “Worsened”.9
The participants were also assessed at D0, D45, and D120 for dermal thickness through high-frequency ultrasound (DermaScan-C, Cortex Technology, Denmark) at three different points at the same level: at the thyroid prominence (A), medially to the sternocleidomastoid muscle (B), and laterally to the sternocleidomastoid muscle (C). These measurements were performed by the same experienced dermatologist in a controlled environment.10
Adverse effects were queried and recorded at each visit. At D120, the subjective satisfaction of the participants was assessed using a 5-point visual analog scale, which ranges from “Very unsatisfied” to “Very satisfied”.
Categorical variables (dichotomous and ordinal) are represented by a percentage, and their 95% confidence interval (95% CI) was assessed by bootstrap analysis with 5000 resamples.11,12 Quantitative data are reported as the mean (standard deviation) or median (p25-p75) if the normality was not confirmed by the Shapiro–Wilk test.13 Correlations between quantitative variables were assessed by the Spearman rank coefficient (rho).14
The clinical and ultrasonographic outcomes of the visits were compared using a generalized mixed model, followed by a post hoc sequential Šidák correction.15 Significance was set as a two-tailed p-value <0.05.16 Data were analyzed using IBM SPSS v25 software.
The sample size (n = 22) was estimated by the expectation of improved scores in at least 60% of the participants at D120, considering alpha = 0.01 and power of 90%.17
Results
All the participants completed the protocol. The main demographic and clinical data at the baseline (D0) are presented in Table 1. The baseline scores of laxity and horizontal necklines were correlated (rho = 0.65, p < 0.01); however, they were not correlated with age (p ≥ 0.05).
 |
Table 1 Main Demographic and Clinical Data of the Sample (n = 22) |
Figures 2 and 3 display the Merz scales grades from the consensual blind assessment by two dermatologists of horizontal necklines and neck laxity at D0, D45, and D120. The participants presented progressive improvement in horizontal necklines (p < 0.01); at D45, in 72.7%, the Merz score had decreased by at least one degree, and in 27.3%, scores continued to improve (at least one point) to D120. For 86.4% (95% CI 68.3–99.9%) of the participants, the scores had decreased at D120 (at least one point) compared to D0 (Figure 4).
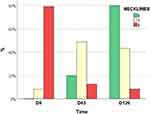 |
Figure 2 Grades of Merz horizontal necklines at each visit (n = 22). |
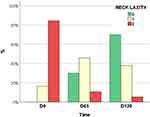 |
Figure 3 Grades of Merz neck laxity at each visit (n = 22). |
In neck laxity, there was a time-dependent improvement (p < 0.01); at D45, the Merz score had decreased by at least one degree in 72.7% of participants, and in 36.4%, scores continued to improve to D120. For 81.8% (95% CI 72.7–90.1%) of the participants, scores had decreased at D120 compared to D0 (Figure 4).
The dermal thicknesses at neck points A, B, and C at each visit are presented in Figure 5 and illustrated in Figure 6. There was an overall time-dependent improvement in dermal thickness (p < 0.01), which was more prominent laterally (at point C) than medially. The pooled mean dermal thickness of the points in the neck had increased by 14.9% (95% CI 8.5–21.3%) at D120. However, the final increase of the thickness at the central point (A) was 9.6%, at the median point (B) was 11.1%, and at the external point (C) was 23.0%.
In the GAIS assessment (Figure 7), there was an overall time-dependent improvement (p < 0.01); at D45, 86.4% of the participants exhibited improvement, and 50.0% continued to improve to D120. A total of 90.9% (95% CI 77.3–99.9%) of the participants had improved at D120 compared to D0.
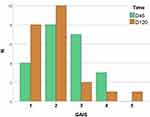 |
Figure 7 Grades of GAIS (Global Aesthetic Improvement Score) compared to D0 (n = 22): 1 – “Exceptionally improved” 2 – “Very improved”, 3 – “Improved”, 4 – “Unaltered”, 5 – “Worsened”. |
No serious adverse events were reported after the procedures. At D45, one participant (5%) reported mild pain and ecchymoses, and another participant (5%) reported pain with neck movement. At D120, one participant (5%) reported a nodule at one injection site, which dissolved with massage; one participant reported ecchymoses; another participant (5%) reported mild pain and a sensation of “heat”; and another participant (5%) reported a sensation of rough skin. All these effects were transitory, and none required specific treatment.
Regarding subjective satisfaction with the results, at D120, 82% (95% CI 64–96%) of the participants reported being “Very satisfied”, 9% were “Satisfied”, 5% were “Neutral”, and 5% were “Unsatisfied”.
Discussion
Two sessions of hyperdiluted (1:4) subdermal CaHA injection provided consistent improvement in clinical and ultrasonographic parameters among adult women with mild and moderate cervical aging. Moreover, it presented a safe profile, well-tolerated adverse effects, and high patient satisfaction.
As aging is a multidimensional process, aesthetic treatments require the rigorous assessment of each patient to offer the best indication for individual demands and the most satisfactory results.1 When well-indicated, minimally invasive aesthetic treatments engender highly tolerable adverse effects, natural results, and prompt resumption of daily activities.18,19
Neck rejuvenation should be promoted by a combination of measures aimed to prevent the aging process, such as environmental exposure (eg, ultraviolet radiation, air pollution), poor posture, pigmentary alterations, surface changes, skin thinning, and the activity of platysma.20–22 Assessing aging severity and targeting the correct layer for treatment are vital to determining the optimal treatment approach.
Consensus recommendations regarding the use of diluted and hyperdiluted CaHA have been developed by panels of global expert aesthetic physicians from Asia, Brazil, and the United States.4,23–25 For the treatment of the neck, the Brazilian panel agrees that one syringe per session is usually indicated and that product application can be performed by retrograde injections. According to skin thickness, a dilution of 1:2 to 1:4 is indicated, and a short linear threading technique using a needle is an alternative option for product application, following a gentle massage.4
Greater CaHA dilutions (≥1:2) provide more biostimulation than volumization, allowing the diffusion of the product after superficial injections without nodule formation and the visualization of the product throughout the skin, which is suitable for neck rejuvenation for highly mobile and usually thin skin.4,23 The use of a cannula or other protocols of injection (eg, volume, dilution, the interval between the sessions, and non-parallel retroinjections) should be explored through controlled studies.
The effects of hyperdiluted CaHA on neocollagenesis and dermal remodeling of the neck and décolletage areas were assessed in 20 women.5 Patients received multiple linear subdermal injections of diluted CaHA at baseline and 4 months. Each syringe of CaHA was diluted with saline: 1:2 for normal skin, 1:4 for thin skin, and 1:6 for atrophic skin. Through ultrasound scanning, increased dermal thickness, skin elasticity, histological elastin, angiogenesis, collagen I, and collagen III expressions were progressively evidenced at 4 and 7 months. Clinical efficacy was confirmed by patients and blinded investigators at 4 and 7 months by improved GAIS scores. Furthermore, in agreement with our results, injection-related adverse events, such as bruising and swelling, were resolved spontaneously within 1 week of the procedure. Notably, the clinical and histologic improvement was progressive for up to 7 months of follow-up.
Hyperdiluted CaHA induces long-term dermal angiogenesis and collagenesis (types I and III) around its microspheres.26–28 The overall dermis restoration promoted by hyperdiluted CaHA was corroborated by high-frequency ultrasound results that depicted increased dermal thickness.10,29,30 In addition, hyperdiluted CaHA was reported as an effective treatment for dermatoporosis, confirming its effect on dermal recovery in aged skin.31 The systematic investigation of a priori determinants of greater dermal response to CaHA biostimulation is warranted.
The long-term efficacy and safety of two sessions of diluted (1:2) CaHA on the décolletage area were assessed in 20 women.32 Clinical efficacy was evaluated by blinded investigators at 6 weeks, 12 weeks, 180 days, and 360 days using the Merz Décolletage Scales (dynamic and at rest). They observed consistent and progressive improvement in the grades of these clinical scales and patient satisfaction for 1 year after the first procedure.
Skin laxity of the neck was assessed three months after one session of hyperdiluted (1:2) CaHA in 20 patients by a validated 5-point scale of neck laxity.33 Two blinded investigators identified a consistent reduction in grades of neck laxity. In addition, 65% of patients reported being very satisfied, 30% were satisfied, and 5% were neither satisfied nor dissatisfied. Adverse effects were also minor and transient.34
Two participants did not demonstrate clinical improvement after the procedure, in addition, the ultrasonographic assessment showed minimal change in their dermal thickness indeed. These participants did not have any distinguishing factors in terms of age or neck lines severity, although both had hypothyroidism. Further investigations are necessary to determine individual factors that influence the results following CaHA in the neck.
Combined treatments for neck aging, including technologies, botulinum toxin, biostimulators, and topical drugs, are recommended to address its different dimensions.35–37 Other protocols with CaHA associated with microfocused ultrasound resulted in favorable outcomes and high patient satisfaction.1,38 Systematic approaches for the assessment and combination of these treatments for neck aging are warranted.
Our study has limitations, as it was monocentric and lacked head-to-head controls, despite natural improvement is not expected for aging disorders. Nevertheless, the highly satisfactory clinical results proven by the improved ultrasound scan measurements and the near-absence of adverse effects reinforce the safety and effectiveness of hyperdiluted CaHA for neck rejuvenation. Further controlled trials with longer follow-up periods and different treatment regimens (eg, injection technique, dilution, and application volume) should explore how to maximize these results.
Conclusion
Two sessions of subdermal hyperdiluted CaHA injections were well-tolerated and improved necklines, neck laxity, and dermal thickness in adult women with mild and moderate cervical aging.
Ethical Statement
This project was approved by the institution’s review board: Comitê de Ética e Pesquisa da Universidade São Francisco (protocol #5.159.039).
Informed-Consent Statement
Written informed consent was obtained from patients to publish their data in this paper.
Acknowledgments
To the patients who allowed the publication of their data and the anonymized photos. The authors also thank Prof. Hélio A. Miot for editorial and writing assistance and statistical analysis of the data.
Merz Aesthetics provides the syringes of Radiesse®, the financial support for the injectors, and data management, however, it did not interfere with the study design, the participants’ enrollment, the procedures, or data analysis.
Disclosure
Dr Trindade de Almeida and Dr Mariana Muniz are speakers, consultants, and researchers of Merz Aesthetics. Dr Trindade de Almeida is speaker, consultant and researcher of Allergan Aesthetics. The remaining authors had no conflict of interest to declare.
References
1. Casabona G, Nogueira Teixeira D. Microfocused ultrasound in combination with diluted calcium hydroxylapatite for improving skin laxity and the appearance of lines in the neck and decolletage. J Cosmet Dermatol. 2018;17:66–72. doi:10.1111/jocd.12475
2. Shadfar S, Perkins SW. Anatomy and physiology of the aging neck. Facial Plast Surg Clin North Am. 2014;22:161–170. doi:10.1016/j.fsc.2014.01.009
3. Lizzul PF, Narurkar VA. The role of calcium hydroxylapatite (radiesse) in nonsurgical aesthetic rejuvenation. J Drugs Dermatol. 2010;9:446–450.
4. de Almeida AT, Figueredo V, da Cunha ALG, et al. Consensus recommendations for the use of hyperdiluted calcium hydroxyapatite (radiesse) as a face and body biostimulatory agent. Plast Reconstr Surg. 2019;7:e2160. doi:10.1097/GOX.0000000000002160
5. Yutskovskaya YA, Kogan EA. Improved neocollagenesis and skin mechanical properties after injection of diluted calcium hydroxylapatite in the neck and decolletage: a pilot study. J Drugs Dermatol. 2017;16:68–74.
6. Stella E, Di Petrillo A. Standard evaluation of the patient: the merz scale. In: Goisis M, editor. Injections in Aesthetic Medicine. Milano: Springer; 2014:33–50.
7. Sattler G, Carruthers A, Carruthers J, et al. Validated assessment scale for neck volume. Dermatol Surg. 2012;38:343–350. doi:10.1111/j.1524-4725.2011.02253.x
8. Chao YY, Chiu HH, Howell DJ. A novel injection technique for horizontal neck lines correction using calcium hydroxylapatite. Dermatol Surg. 2011;37:1542–1545. doi:10.1111/j.1524-4725.2011.02086.x
9. Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of restylane versus zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–595. doi:10.1046/j.1524-4725.2003.29150.x
10. Caetano LD, Soares JL, Bagatin E, Miot HA. Reliable assessment of forearm photoageing by high-frequency ultrasound: a cross-sectional study. Int J Cosmet Sci. 2016;38:170–177. doi:10.1111/ics.12272
11. Miola AC, Miot HA. Comparing categorical variables in clinical and experimental studies. J Vasc Bras. 2022;21:e20210225. doi:10.1590/1677-5449.20210225
12. Bland JM, Altman DG. Statistics notes: bootstrap resampling methods. BMJ. 2015;350:h2622. doi:10.1136/bmj.h2622
13. Miot HA. Assessing normality of data in clinical and experimental trials. J Vasc Bras. 2017;16:88–91. doi:10.1590/1677-5449.041117
14. Miot HA. Correlation analysis in clinical and experimental studies. J Vasc Bras. 2018;17:275–279. doi:10.1590/1677-5449.174118
15. Duricki DA, Soleman S, Moon LD. Analysis of longitudinal data from animals with missing values using SPSS. Nat Protoc. 2016;11:1112–1129. doi:10.1038/nprot.2016.048
16. Miola AC, Miot HA. P-value and effect-size in clinical and experimental studies. J Vasc Bras. 2021;20:e20210038. doi:10.1590/1677-5449.210038
17. Miot HA. Sample size in clinical and experimental studies. J Vasc Bras. 2011;10:275–278. doi:10.1590/S1677-54492011000400001
18. Mobayed N, Nguyen JK, Jagdeo J. Minimally invasive facial cosmetic procedures for the millennial aesthetic patient. J Drugs Dermatol. 2020;19:100–103. doi:10.36849/JDD.2020.4641
19. Matarasso A, Nikfarjam J, Abramowitz L. Incorporating minimally invasive procedures into an aesthetic surgery practice. Clin Plast Surg. 2016;43:449–457. doi:10.1016/j.cps.2016.03.001
20. Sturm A, Shokri T, Ducic Y. Nonsurgical rejuvenation of the neck. Facial Plast Surg Clin North Am. 2022;30:407–417. doi:10.1016/j.fsc.2022.03.014
21. Noormohammadpour P, Ehsani A, Mahmoudi H, et al. Botulinum toxin injection as a single or combined treatment with non-cross-linked high molecular weight and low molecular weight hyaluronic acid gel for neck rejuvenation: a randomized clinical trial. Dermatol Ther. 2022;35:e15673. doi:10.1111/dth.15673
22. Perez P, Hohman MH. Neck Rejuvenation. Treasure Island (FL): StatPearls; 2022.
23. Goldie K, Peeters W, Alghoul M, et al. Global consensus guidelines for the injection of diluted and hyperdiluted calcium hydroxylapatite for skin tightening. Dermatol Surg. 2018;44(Suppl 1):S32–S41. doi:10.1097/DSS.0000000000001685
24. Lorenc ZP, Black JM, Cheung JS, et al. Skin tightening with hyperdilute CaHA: dilution practices and practical guidance for clinical practice. Aesthet Surg J. 2022;42:NP29–NP37. doi:10.1093/asj/sjab269
25. Corduff N, Chen JF, Chen YH, et al. Pan-Asian consensus on calcium hydroxyapatite for skin biostimulation, contouring, and combination treatments. J Clin Aesthet Dermatol. 2021;14:E76–E85.
26. Figueredo VO, Miot HA, Soares Dias J, Nunes GJB, Barros de Souza M, Bagatin E. Efficacy and safety of 2 injection techniques for hand biostimulatory treatment with diluted calcium hydroxylapatite. Dermatol Surg. 2020;46(Suppl 1):S54–S61. doi:10.1097/DSS.0000000000002334
27. Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008;34(Suppl 1):S64–S67. doi:10.1111/j.1524-4725.2008.34245.x
28. Marmur ES, Phelps R, Goldberg DJ. Clinical, histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler. J Cosmet Laser Ther. 2004;6:223–226. doi:10.1080/147641704100003048
29. Caetano LD, de Oliveira Mendes T, Bagatin E, Miot HA, Soares JL, Martin AA. In vivo confocal Raman spectroscopy for intrinsic aging and photoaging assessment. J Dermatol Sci. 2017;88:199–206. doi:10.1016/j.jdermsci.2017.07.011
30. Carvalho PRS, Sumita JM, Soares JLM, Sanudo A, Bagatin E. Forearm skin aging: characterization by instrumental measurements. Int J Cosmet Sci. 2017;39:564–571. doi:10.1111/ics.12407
31. Souza GP, Serra MS. Treatment of actinic purpura with calcium hydroxyapatite. Surg Cosmet Dermatol. 2018;10:355–358.
32. Fabi SG, Alhaddad M, Boen M, Goldman M. Prospective clinical trial evaluating the long-term safety and efficacy of calcium hydroxylapatite for chest rejuvenation. J Drugs Dermatol. 2021;20:534–537. doi:10.36849/JDD.5680
33. Guida S, Longhitano S, Spadafora M, et al. Hyperdiluted calcium hydroxylapatite for the treatment of skin laxity of the neck. Dermatol Ther. 2021;34:e15090. doi:10.1111/dth.15090
34. Ianhez M, Souza MB, Miot HA. Frequency of complications of aesthetic facial fillers in Brazil. Plast Reconstr Surg. 2022;149:599e–601e. doi:10.1097/PRS.0000000000008839
35. Vanaman M, Fabi SG, Cox SE. Neck rejuvenation using a combination approach: our experience and a review of the literature. Dermatol Surg. 2016;42(Suppl 2):S94–S100. doi:10.1097/DSS.0000000000000699
36. Friedman O, Shamban A, Fabi S, Duncan DI, Artzi O. The aging neck-a case base treatment algorithm. J Cosmet Dermatol. 2021;20:569–576. doi:10.1111/jocd.13877
37. Doh EJ, Kim J, Lee DH, Park JY. Neck rejuvenation using a multimodal approach in Asians. J Dermatolog Treat. 2018;29:400–404. doi:10.1080/09546634.2017.1395801
38. Yutskovskaya YA, Sergeeva AD, Kogan EA. Combination of calcium hydroxylapatite diluted with normal saline and microfocused ultrasound with visualization for skin tightening. J Drugs Dermatol. 2020;19:405–411. doi:10.36849/JDD.2020.4625
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.