Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Efficacy and Safety of Microwave Ablation Assisted by Ultrasound Fusion Imaging for Primary and Secondary Liver Cancers with a Diameter of 3–7 Cm
Authors Yang J , Liang S, Liu H, Hu C, Guan S, Kang H, Xu E, Yan R
Received 20 June 2023
Accepted for publication 12 October 2023
Published 18 October 2023 Volume 2023:10 Pages 1839—1848
DOI https://doi.org/10.2147/JHC.S424009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Mohamed Shaker
Jing Yang,1,* Shuang Liang,1,* Huahui Liu,1 Cai Hu,1 Sainan Guan,1 Haiyu Kang,1 Erjiao Xu,1 Ronghua Yan1,2
1Department of Medical Ultrasonics, The Eighth Affiliated Hospital of Sun Yat-sen University, Shenzhen, Guangdong Province, People’s Republic of China; 2Department of Radiology, Peking University Shenzhen Hospital, Shenzhen, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ronghua Yan; Erjiao Xu, Department of Medical Ultrasonics, The Eighth Affiliated Hospital of Sun Yat-sen University, No. 3025 Shennan Middle Road, Futian Street, Futian District, Shenzhen City, Guangdong Province, People’s Republic of China, Tel +86 755 83982222, Fax +86 755 83980805, Email [email protected]; [email protected]
Purpose: To investigate the efficacy and safety of microwave ablation (MWA) assisted by ultrasound fusion imaging (FI) for primary and secondary liver cancers with a diameter of 3– 7 cm.
Patients and Methods: A retrospective analysis was conducted on patients with primary and secondary liver cancers (3– 7 cm) who underwent MWA with ultrasound FI assistance in our hospital from April 2020 to May 2022. Technical success, technique efficacy, local tumor progression (LTP), major complication, intrahepatic distant recurrence (IDR), and overall survival (OS) were assessed during the follow-up period. In addition, the ablation results of tumors between the medium-sized group (3.1– 5.0 cm) and large-sized group (5.1– 7.0 cm) were compared.
Results: 31 patients with 35 primary and secondary liver cancers were treated with MWA assisted by ultrasound FI. Complete ablation was achieved in 34 lesions with a technical success rate of 97.1%. Major complications occurred in 6.5% of patients (2/31), while no ablation-related deaths were reported. The median follow-up time of this study was 24 months (range:10 to 35 months). The technique efficacy rate was 97.1% (34/35), with LTP occurring in three lesions at a rate of 8.8% (3/34). The incidence of IDR was 38.7% (12/31) and the 2-year cumulative OS rate reached 96.7%. Moreover, there were no statistical differences in technique efficacy rate (p=0.286), LTP rate (p=0.328), major complication rate (p=0.503), IDR (p=0.857), and OS (p=0.118) between medium-sized group and large-sized group.
Conclusion: Ultrasound FI-assisted MWA has the potential to be an effective and safe therapeutic strategy for primary and secondary liver cancers ranging from 3– 7 cm in size.
Keywords: fusion imaging, microwave ablation, liver cancers, ultrasound
Introduction
Thermal ablation, mainly radiofrequency ablation (RFA) and microwave ablation (MWA) has become one of the main curative methods for small-sized liver cancers.1,2 However, the incidence of residual and local tumor progression (LTP) rises with the augmentation of tumor diameter.3 The literature reported that for tumors less than 3 cm, the complete ablation rate was over 90%, and the LTP was about 10%.4,5 When the tumor diameter exceeded 3 cm, the complete ablation rate was about 76.5%-93%, and the LTP rate could be as high as 25%-70%.6–8 In addition, major complications occurred in approximately 9.2% of ablation procedures, with a mortality rate of 0.2% for large-sized lesions.9,10 The main reasons are as follows: Firstly, as tumor size increases, its spatial structure becomes more complex, making it difficult to obtain sufficient ablation coverage with thermal ablation and increasing the risk of thermal damage to surrounding vital structures.6 Secondly, larger tumors usually require more complex ablation planning with multiple ablation needles and multiple ablation insertions to cover the entire tumor.11 Thirdly, microvascular infiltration and satellite lesions are more common in larger tumors, lending to a higher incidence of residual and LTP.12,13 Therefore, surgical resection is often considered the main treatment for liver cancers above 3 cm.13,14 However, some patients with recurrent liver cancers or insufficient liver functional reserve cannot tolerate this procedure. Additionally, liver transplantation is only available to limited patients due to disease-related and social factors.15 In recent years, thermal ablation therapy is becoming more feasible for tumors larger than 3 cm due to improvements in ablation techniques and strategies, particularly with MWA, which has a higher thermal efficiency and larger ablation range than RFA.16 Related studies had shown that thermal ablation was still feasible for liver cancers within 7 cm.6,14,15
The ablation effect was mainly enhanced by double or multiple antennas/electrodes placed simultaneously, thermal ablation combined with transarterial chemoembolization (TACE), or utilizing 3D visualization preoperative planning system to assist ablation.6,17–19 The literature reported that thermal ablation combined with TACE was superior to thermal ablation alone for liver cancer with a diameter of 3–7 cm that could not be surgically resected.20–23 Nevertheless, these strategies increased medical expenses inevitably, and the trauma or side effects of TACE could not be ignored.24 Moreover, ensuring an adequate ablation zone to minimize residual and LTP rates remained a challenge.
Ultrasound fusion imaging (FI) is the fusion of real-time ultrasound (US) images with pre-ablation computed tomography (CT), magnetic resonance (MR), or three-dimensional US (3DUS) images in a magnetic positioning system. This technique can play a vital role in preoperative localization and planning, intraoperative guidance and monitoring, and immediate postoperative evaluation during the entire thermal ablation procedure of liver cancers.25,26 Relevant studies had demonstrated that ultrasound FI could improve the technique efficacy and reduce LTP.27 Our previous literature had also reported that ultrasound FI-assisted thermal ablation could be performed for medium-sized liver cancers (3–5 cm) with satisfactory treatment effects.28 However, there were few reports on the effectiveness of ultrasound FI-assisted MWA for liver cancers over 5 cm. Therefore, this study aimed to retrospectively evaluate the efficacy and safety of MWA for primary and secondary liver cancers larger than 3 cm with the assistance of ultrasound FI and to compare the ablation results between medium-sized (3.1–5.0 cm) and large-sized (5.1–7.0 cm) tumors.
Materials and Methods
Study Population
This study involved patients with primary and secondary liver cancers who received ultrasound-guided MWA under the ultrasound FI assistance in our hospital from April 2020 to May 2022. After evaluation by a multidisciplinary team (MDT), all patients deemed unsuitable for surgical resection or who declined it were preliminarily included in the study population. The inclusion criteria were: (1) primary and secondary liver cancers confirmed by pathological result or hepatocellular carcinomas (HCC) meet the clinical diagnostic criteria;11,25–27 (2) lesion diameter between 3.1 cm-7.0 cm; (3) no radiologic evidence of major vascular invasion. The exclusion criteria were: (1) failure of ultrasound FI alignment; (2) no evaluation by contrast-enhanced CT/MR within 3 months after MWA procedures and subsequent regular follow-up; (3) combination therapy with TACE.
This retrospective study was approved by the Ethics Committee of the Eighth Affiliated Hospital of Sun Yat-Sen University and complied with the Helsinki human trial regulations (The approval number was ZB-KYIRB-2023-012-01).
Patient consent for the review of their medical data was waived, but we guaranteed anonymization of participant data to ensure privacy and confidentiality. In addition, written informed consent was obtained from all patients for the MWA procedure and contrast-enhanced ultrasound (CEUS) examination.
Instruments and Equipment
Ultrasound machine: GE Logiq E9 ultrasound scanner (General Electric, United States of America) was equipped with a C1-6VN convex array probe (frequency 1–6MHZ), built-in Volume Navigation (General Electric, United States of America) ultrasound FI system, as well as specific ultrasound contrast imaging technology.
Ultrasound contrast agents: SonoVue (Bracco, Milan, Italy) was prepared by suspending it with 5 mL of normal saline to form a suspension. Each injection involved a rapid bolus of 1.0 to 1.5 mL through the antecubital vein, followed by a 5 mL normal saline flush.
MWA system: The MWA system comprises a microwave generator (Great Wall, Nanjing, China) operating at 2450 MHz and an internal cooling microwave antenna. The ablation power was typically selected from 50W to 60W. The duration of each ablation session typically ranged from 3 to 8 minutes, depending on the lesion’s condition.
MWA Procedure
The MWA procedure was carried out by a senior ultrasound interventionalist with over 10 years of clinical experience in MWA, after general anesthesia via endotracheal intubation. Specific ablation strategies were formulated based on lesion location, size, and proximity of important structures. If lesions were near the top of the diaphragm or crucial structures, techniques such as artificial pleural effusion, artificial ascites, or one-lung ventilation could be utilized to enhance the safety and effectiveness of MWA.29,30 Typically, multiple overlapping insertions were performed to cover the target tumor and its 5-mm ablation margin (AM) as comprehensively as possible.
FI
Both 3D US-US FI and CT/MR-US FI were utilized for image-guided MWA. The 3D US-US FI was preferred for lesions with well-defined ultrasound boundaries, while the CT/MR-US FI was often selected for lesions with unclear boundaries on ultrasound or when CT/MR assistance was required to visualize the anatomical structures surrounding the target tumor. The ultrasound FI procedure involved acquiring 3D ultrasound volume images or importing the CT/MR images in DICOM format and aligning them with real-time ultrasound images until an accurate alignment based on hepatic landmarks (mainly intrahepatic vessels) was achieved. The lesion contour was delineated with an ellipsoid on the 3D ultrasound image or CT/MR image, establishing a 5mm-AM, and critical structures around the lesion were also marked if necessary. Further details on the procedures and steps involved in ultrasound FI can be referred to in our previous publications.31
Ultrasound FI was utilized to localize the tumor and vital structures before MWA. During the ablation process, when the target tumor became invisible due to gasification and affected the subsequent needle placement, ultrasound FI could assist in locating and guiding the subsequent puncture needle placement according to the preoperative pre-defined ablation plan. Once the ablation plan was finalized, ultrasound-FI was used in conjunction with CEUS to immediately assess the effectiveness of the ablation. If the non-perfusion zone completely covered the tumor and its AM, the procedure was concluded; otherwise, supplementary ablation was performed immediately under the guidance of ultrasound FI until satisfactory ablation coverage was achieved.
Systemic Treatment
Based on the pathological type and staging of liver cancers, chemotherapy, targeted therapy, and/or immunotherapy methods before or after the MWA procedure were determined through MDT discussion according to relevant guidelines.32,33
Follow Up Information
All patients underwent imaging examinations and laboratory tests within one week to evaluate technical success and exclude early complications. Contrast-enhanced CT/MR was performed within 1–3 months postoperatively to assess technique efficacy, followed by regular follow-up every three months. During follow-up, the occurrence of LTP, intrahepatic distant metastasis (IDR), extrahepatic metastases (EM), overall survival (OS), and major complication were recorded. The definitions of these follow-up indicators were determined according to the criteria defined in the literature.27 The follow-up endpoint was March 31st, 2023.
Statistical Analysis
Categorical variables were presented as frequencies, rates, or percentages. Normally distributed continuous variables were expressed as mean ± standard deviation, while skewed distributions were reported as median (range). Fisher’s exact test was applied to compare categorical variables between groups, while independent samples t-test was utilized for continuous variables. Kaplan-Meier method was employed to analyze LTP, OS, and IDR, and a comparison between the two groups was performed by Log rank test. Statistical significance was defined as a p-value < 0.05 for comparison between groups. SPSS version 21.0.0 (SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
Patients
This study included 31 patients with 35 primary and secondary liver cancers, consisting of 19 HCCs, one intrahepatic cholangiocarcinoma (ICC), and 11 metastatic liver cancers (MLC). Two patients had two lesions larger than 3 cm, and one patient had three such lesions. The baseline characteristics of patients and lesions were presented in Table 1. Among them, 12 patients with liver cancers received systemic treatment.
 |
Table 1 Characteristics and Ablation Parameter Data of 31 Patients with 35 Primary and Secondary Liver Cancers |
Immediate Assessment and Supplemental Ablation During Ablation
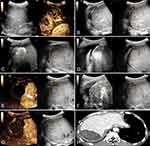
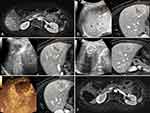
In this study, CT/MR-US FI was applied in 27 lesions, and 3D US-US FI was used in 8 lesions. The success rate of ultrasound FI alignment was 100% (35/35). Based on immediate ultrasound FI combined with CEUS assessment during the MWA procedure, 19 lesions underwent supplementary ablation after immediate assessment, with a supplemental ablation rate of 54.3% (19/35) (Figures 1 and 2). Details were shown in Table 1.
Technical Success
According to the postoperative evaluation, technical success was achieved in 34 of 35 lesions, resulting in a technical success rate of 97.1%. One ICC lesion with a maximum diameter of 68 mm was considered a technical failure based on CEUS and contrast-enhanced MRI results within one week after the MWA procedure. This patient underwent a second MWA eight days after the first procedure, resulting in complete necrosis.
Technique Efficacy
The duration of follow-up for all 31 patients ranged from 10 to 35 months, with a median follow-up duration of 24 months.
After the MWA procedure, contrast-enhanced CT/MR imaging taken within three months showed complete ablation in 34 of 35 lesions, with a technique efficacy rate of 97.1%. One HCC lesion located near the left hepatic vein exhibited residual, probably due to the heat sink effect, ultimately leading to incomplete ablation. This lesion underwent a repeat session of ultrasound-guided MWA treatment and achieved complete ablation.
Complications
There were no deaths related to MWA treatment among these 31 patients. 6.5% (2/31) of patients experienced major complications. One patient had a medium-volume pleural effusion on the right side that was resolved with chest tube drainage. The other patient developed a liver abscess following MWA, which was successfully treated with drainage and antibiotic therapy within 12 days.
Additionally, pain was documented by 15 patients (48.4%), post-ablation syndrome in five patients (16.1%), asymptomatic ascites or pleural effusion in 18 patients (58.1%), asymptomatic biloma in one patient (3.2%) and gross hematuria in one patient (3.2%).
LTP
During follow-up, LTP was observed in three lesions (8.8%, 3/34) between the sixth and 12th month after MWA. These lesions included two MLC from rectal cancer, one located near the diaphragm and the other near the hepatic hilar. The third lesion was an HCC located close to the gallbladder and intestine. All of them underwent repeat MWA treatment, and no further LTP was detected.
OS, IDR, and EM
One patient died from a pulmonary infection during the follow-up period, with a 2-year cumulative OS rate of 96.7%. IDR was observed in 38.7% (12/31) of patients, with 11 receiving repeat MWA and one undergoing surgical treatment. Another patient refused further ablation or surgical treatment and only received conservative treatment. Six patients (19.4%, 6/31) developed EM, including bone, lung, and lymph node metastases.
Comparison Between Groups of Medium-Sized and Large-Sized Liver Cancers
The lesions were divided into two groups based on maximum diameter: a medium-sized group (3.1–5.0 cm, 25 tumors in 22 patients) and a large-sized group (5.1–7.0 cm, 10 tumors in 9 patients). There were no statistical differences in patient characteristics between the groups, except for the lesion size (Table 2). When comparing efficacy and safety, no significant differences were found in technique efficacy rate (p=0.286), LTP (p=0.328), major complication rate (p=0.503), IDR (p=0.857), and OS (p=0.118) (Table 2). However, the large-sized group required more MWA parameters, including double antenna use (p=0.001), more punctures (p=0.002), and longer ablation time (p=0.026) (Table 2).
 |
Table 2 Comparison of General Data, Ablation Parameters and Therapeutic Outcomes Between Medium-Sized and Large-Sized Groups |
Discussion
The present study demonstrated a high technical efficacy rate of 97.1% for MWA in the treatment of primary and secondary liver cancers, with a low LTP rate of 8.8% and a major complication rate of 6.5%. Notably, these favorable results were obtained with the assistance of ultrasound FI and without the combination of TACE, indicating that ultrasound FI might play an important role in the MWA of medium to large-sized liver cancers.
Thermal ablation combined with TACE is currently the most widely used treatment for liver cancers > 3 cm. Patidar et al34 performed RFA combined with TACE in 22 patients with 24 liver cancers of 3 to 7 cm in diameter. The results showed a complete response rate of 87.5% (21/24) and an LTP rate of 7.6% which was similar to our study. However, Liu et al35 reported a lower overall technique efficacy rate of 74.2% (92/124) in the treatment of HCC of 3–10 cm treated with thermal ablation combined with TACE. Lin et al6 performed multi-needle RFA for 3–7cm liver cancers, with an LTP rate as high as 17.4% (12/69). Therefore, fully exploiting ultrasound FI during the MWA procedure could achieve a comparable local control efficacy to TACE combined treatment for liver cancers > 3 cm.
Previous studies36,37 had suggested that even when TACE was combined with thermal ablation, the treatment outcomes were related to the size of the lesions. Liu et al35 reported a technique efficacy rate of 88.6% (62/70) for tumors with a diameter of 3–5 cm, while only 55.6% (30/54) of tumors with a diameter of 5–10 cm could be achieved. However, our subgroup analysis results based on lesion size revealed no statistically significant differences in technical efficiency, LTP, OS, and major complications between medium and large-sized liver cancers. This suggests that ultrasound FI might have great potential as an adjuvant effective therapy for the treatment of liver cancers larger than 3cm, especially those larger than 5 cm. Nevertheless, further research is needed for exploration.
Ultrasound FI has multiple benefits for MWA of medium to large-sized liver cancers, including stereoscopically marking the contour of the target lesions and surrounding structures to determine the ablation range and avoid unnecessary thermal damage. It also helps doctors formulate the ablation planning and guide the insertion of antennas, ensuring accurate placement of the antenna and complete ablation of the lesion, especially in the presence of gasification. In the present study, dual microwave antennas were employed in 34.3% (12/35) of lesions, with a higher requirement observed in 80% (8/10) of large-sized liver cancers compared to only 16% (4/25) of medium-sized liver cancers. Hence, with the aid of ultrasound FI technology, an increasing number of our cases utilize single-needle ablation, thereby reducing the need for multiple microwave antennas and resulting in cost savings.
Moreover, ultrasound FI could evaluate the therapeutic effect of MWA immediately. When combined with CEUS, it could ensure the ablation range covered the target lesion and its 5mm-AM. For lesions with an insufficient ablation range, immediate supplementary ablation would be performed to improve the one-time complete ablation rate and minimize residual and LTP. In this study, 54.3% (19/35) of the lesions underwent immediate supplementary ablation. Thus, with ultrasound FI, the one-time complete ablation rate could be improved and the LTP could be controlled, leading to an improved OS rate and decreased incidence of major complications. This can ultimately save medical costs and resources, particularly for large-sized liver cancers.
However, there were still several limitations. First, it was a single-center retrospective study with a small sample size, especially for large-sized liver cancers that were typically treated with surgical resection. A larger sample size with long-term follow-up should be carried out for further confirmation of these findings. Second, this study included a mixed population of liver cancers (HCC, ICC, and MLC) with different long-term prognoses. However, the satisfactory local control rates (low residual and LTP rate) achieved with ultrasound FI-guided MWA indicated its potential to improve the local efficacy of MWA for medium to large-sized liver cancers. Finally, a comparison between ultrasound FI-assisted MWA and MWA combined with TACE in terms of efficacy and safety could be explored in the future.
Conclusion
In conclusion, ultrasound FI may be of benefit during MWA of primary and secondary liver cancers ranging from 3 to 7 cm in size.
Acknowledgments
Jing Yang and Shuang Liang are co-first authors for this study. This work was supported by the National Natural Science Foundation of China (82272011), the Natural Science Foundation of Guangdong Province, China (2022A1515012155), the Shenzhen Science and Technology Program (JCYJ20220530160208018), and the Futian Healthcare Research Project (FTWS2020022, FTWS2020073, FTWS2020074, FTWS2020075, FTWS2021071).
Disclosure
The authors report no conflicts of interest in this work.
References
1. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
2. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
3. Kutlu OC, Chan JA, Aloia TA, et al. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer. 2017;123(10):1817–1827. doi:10.1002/cncr.30531
4. Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802. doi:10.1016/j.jhep.2012.05.007
5. Fang Y, Chen W, Liang X, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(1):193–200. doi:10.1111/jgh.12441
6. Lin CC, Cheng YT, Chen MW, Lin SM. The effectiveness of multiple electrode radiofrequency ablation in patients with hepatocellular carcinoma with lesions more than 3 cm in size and Barcelona clinic liver cancer stage A to B2. Liver Cancer. 2016;5(1):8–20. doi:10.1159/000367755
7. Sun AX, Cheng ZL, Wu PP, et al. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol. 2015;21(10):2997–3004. doi:10.3748/wjg.v21.i10.2997
8. Kim JH, Won HJ, Shin YM, et al. Medium-sized (3.1–5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18(6):1624–1629. doi:10.1245/s10434-011-1673-8
9. Yin XY, Xie XY, Lu MD, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115(9):1914–1923. doi:10.1002/cncr.24196
10. Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology. 2009;251(3):933–940. doi:10.1148/radiol.2513081740
11. Li K, Su ZZ, Xu EJ, Ju JX, Meng XC, Zheng RQ. Improvement of ablative margins by the intraoperative use of CEUS-CT/MR image fusion in hepatocellular carcinoma. BMC Cancer. 2016;16:277. doi:10.1186/s12885-016-2306-1
12. Ye QW, Pang SJ, Yang N, et al. Safety and efficacy of radiofrequency ablation for solitary hepatocellular carcinoma (3–5 cm): a propensity score matching cohort study. J Gastrointest Surg. 2019;23(8):1549–1558. doi:10.1007/s11605-019-04229-6
13. Chong HH, Yang L, Sheng RF, et al. Multi-scale and multi-parametric radiomics of gadoxetate disodium-enhanced MRI predicts microvascular invasion and outcome in patients with solitary hepatocellular carcinoma ≤ 5 cm. Eur Radiol. 2021;31(7):4824–4838. doi:10.1007/s00330-020-07601-2
14. Kariyama K, Nouso K, Wakuta A, et al. Treatment of intermediate-stage hepatocellular carcinoma in japan: position of curative therapies. Liver Cancer. 2020;9(1):41–49. doi:10.1159/000502479
15. N’Kontchou G, Nault JC, Sutter O, et al. Multibipolar radiofrequency ablation for the treatment of mass-forming and infiltrative hepatocellular carcinomas > 5 cm: long-term results. Liver Cancer. 2019;8(3):172–185. doi:10.1159/000489319
16. Chong CCN, Lee KF, Cheung SYS, et al. Prospective double-blinded randomized controlled trial of Microwave versus RadioFrequency Ablation for hepatocellular carcinoma (McRFA trial). HPB. 2020;22(8):1121–1127. doi:10.1016/j.hpb.2020.01.008
17. Francica G, Altiero M, Laccetti E, et al. Long-term follow-up of unresectable medium-large hepatocellular carcinoma nodules treated with radiofrequency ablation using a multiple-electrode switching system. Br J Radiol. 2019;92(1093):20180625. doi:10.1259/bjr.20180625
18. Huang G, Lin M, Xie X, et al. Combined radiofrequency ablation and ethanol injection with a multipronged needle for the treatment of medium and large hepatocellular carcinoma. Eur Radiol. 2014;24(7):1565–1571. doi:10.1007/s00330-014-3151-8
19. An C, Li X, Zhang M, et al. 3D visualization ablation planning system assisted microwave ablation for hepatocellular carcinoma (Diameter >3): a precise clinical application. BMC Cancer. 2020;20(1):44. doi:10.1186/s12885-020-6519-y
20. Yuan P, Zhang Z, Kuai J. Analysis on efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J buon. 2019;24(1):163–170.
21. Liu C, Li T, He JT, Shao H. TACE combined with microwave ablation therapy vs. TACE alone for treatment of early- and intermediate-stage hepatocellular carcinomas larger than 5 cm: a meta-analysis. Diagn Interv Radiol. 2020;26(6):575–583. doi:10.5152/dir.2020.19615
22. Young S, Golzarian J. Locoregional therapies in the treatment of 3- to 5-cm hepatocellular carcinoma: critical review of the literature. AJR Am J Roentgenol. 2020;215(1):223–234. doi:10.2214/AJR.19.22098
23. Faiella E, Santucci D, Bernetti C, et al. Combined trans-arterial embolisation and microwave ablation for the treatment of large unresectable hepatic metastases (>3 cm in maximal diameter). Int J Hyperthermia. 2020;37(1):1395–1403. doi:10.1080/02656736.2020.1849823
24. Zhu ZY, Yuan M, Yang PP, et al. Single medium-sized hepatocellular carcinoma treated with sequential conventional transarterial chemoembolization (cTACE) and microwave ablation at 4 weeks versus cTACE alone: a propensity score. World J Surg Oncol. 2022;20(1):192. doi:10.1186/s12957-022-02643-w
25. Ahn SJ, Lee JM, Lee DH, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017;66(2):347–354. doi:10.1016/j.jhep.2016.09.003
26. Xu EJ, Lv SM, Li K, et al. Immediate evaluation and guidance of liver cancer thermal ablation by three-dimensional ultrasound/contrast-enhanced ultrasound fusion imaging. Int J Hyperthermia. 2018;34(6):870–876. doi:10.1080/02656736.2017.1373306
27. Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--A 10-year update. Radiology. 2014;273(1):241–260. doi:10.1148/radiol.14132958
28. Luo L, He X, Li K, et al. Thermal ablation of medium-sized hepatocellular carcinomas using intraoperative ultrasound fusion imaging: a propensity score-matched analysis. Clin Res Hepatol Gastroenterol. 2021;45(5):101581. doi:10.1016/j.clinre.2020.11.011
29. Long Y, Zeng Q, He X, et al. One-lung ventilation for percutaneous thermal ablation of liver tumors in the hepatic dome. Int J Hyperthermia. 2020;37(1):49–54. doi:10.1080/02656736.2019.1708483
30. Huang Q, Li J, Zeng Q, et al. Value of artificial ascites to assist thermal ablation of liver cancer adjacent to the gastrointestinal tract in patients with previous abdominal surgery. BMC Cancer. 2020;20(1):763. doi:10.1186/s12885-020-07261-x
31. Xu E, Long Y, Li K, et al. Comparison of CT/MRI-CEUS and US-CEUS fusion imaging techniques in the assessment of the thermal ablation of liver tumors. Int J Hyperthermia. 2019;35(1):159–167. doi:10.1080/02656736.2018.1487591
32. Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345. doi:10.1200/JCO.20.02672
33. Czauderna C, Luley K, von Bubnoff N, Marquardt JU. Tailored systemic therapy for colorectal cancer liver metastases. Int J Mol Sci. 2021;22(21). doi:10.3390/ijms222111780
34. Patidar Y, Garg L, Mukund A, Sarin SK. Early experience of combination therapy of transarterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma measuring 3–7 cm. Indian J Radiol Imaging. 2019;29(1):47–52. doi:10.4103/ijri.IJRI_352_18
35. Liu W, Xu H, Ying X, et al. Radiofrequency Ablation (RFA) Combined with Transcatheter Arterial Chemoembolization (TACE) for patients with medium-to-large hepatocellular carcinoma: a retrospective analysis of long-term outcome. Med Sci Monit. 2020;26:e923263. doi:10.12659/MSM.923263
36. Wang YH, Liu JF, Li F, et al. Radiofrequency ablation combined with transarterial chemoembolization for unresectable primary liver cancer. Chin Med J. 2009;122(8):889–894.
37. Ni JY, Sun HL, Chen YT, et al. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol. 2014;20(46):17483–17490. doi:10.3748/wjg.v20.i46.17483
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.