Back to Journals » Journal of Pain Research » Volume 17
Efficacy and Safety of High-Voltage Pulsed Radiofrequency versus Standard-Voltage Pulsed Radiofrequency for Patients with Neuropathic Pain: A Literature Review and Meta-Analysis
Authors Wang Y, Jia Y , Wang Z, Feng G, Ma Y, Fan Z, Liu M, Feng K, Wang T
Received 13 September 2023
Accepted for publication 23 February 2024
Published 4 March 2024 Volume 2024:17 Pages 851—863
DOI https://doi.org/10.2147/JPR.S439909
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Yu Wang,1,2 Yitong Jia,3 Zheng Wang,4 Guang Feng,3 Yanhui Ma,3 Zhen Fan,3 Miao Liu,3 Kunpeng Feng,3 Tianlong Wang3
1Department of Neurosurgery, Beijing Boai Hospital, China Rehabilitation Research Center, Beijing, People’s Republic of China; 2College of Rehabilitation, Capital Medical University, Beijing, People’s Republic of China; 3Department of Anesthesiology, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of General Surgery, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Tianlong Wang, Department of Anesthesiology, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China, Email [email protected]
Background: Neuropathic pain (NP) is recognized as one of the most difficult pain syndromes which lacks a safe, well-tolerated and effective treatment. Pulsed radiofrequency (PRF), a novel and minimally invasive interventions, has been introduced to alleviate various types of NP. Previous studies reported PRF with higher voltage could further improve the treatment efficacy. Therefore, we conducted this systematic review and meta-analysis to determine whether high-voltage PRF is superior to standard-voltage PRF for the treatment of NP patients.
Methods: Databases published from the date of inception until 15 March 2022 on PubMed/MEDLINE, EMBASE, Web of Science and the Cochrane Library were searched for RCTs comparing high-voltage PRF and standard-voltage PRF in NP patients. The primary outcome measures were the efficiency rates of NP patients with high-voltage PRF or standard-voltage PRF treatment. Data analysis was conducted using the Review Manager software (RevMan V.5.3).
Results: Six RCTs involving 423 patients were included in our meta-analysis. Compared with standard-voltage PRF group, the high-voltage PRF group attained a higher efficiency rate at 1 month (P = 0.04; I2 = 0%), 3 months (P = 0.04; I2 = 0%), 6 months (P = 0.002; I2 = 0%) post-procedure respectively. There was no significant difference in the complications between the two groups.
Conclusion: Our study supported that high-voltage PRF attained more satisfactory efficacy than standard-voltage PRF without increased side effects. High-voltage PRF could be a promising, effective, minimally invasive technology for NP patients.
Keywords: trigeminal neuralgia, pulsed radiofrequency, high-voltage, standard-voltage, efficacy
Background
Neuropathic pain (NP) is recognized as one of the most difficult pain syndromes due to involvement of somatosensory system.1,2 It is reported that the incidence rate of NP ranged from 0.9% to 17.9% worldwide.3 The characteristic of NP is usually electric shock-like, burning, pricking and squeezing sensations which leads to sleep disturbances, anxiety and depression and seriously impairs patients’ quality of life (QoL).4,5 Pharmacological treatments, such as antiepileptic drugs, γ-aminobutyric acid (GABA) inhibitors, serotonin-noradrenaline reuptake inhibitor or tricyclic antidepressants remain first-line therapy for peripheral and central NP.6,7 However, unsatisfactory efficacy and several adverse effects associated with oral medication limit its clinical utility.1 Patients who respond poorly to pharmacological treatments often receive invasive therapy including microvascular decompression (MVD), gamma knife radiosurgery, percutaneous micro-balloon compression or partial sensory rhizotomy. Nevertheless, surgical interventions are of high risk for occurrence of complications.8–10 Hence, there is an overwhelming requirement for developing a safe, well tolerated, and effective treatment option for NP.
Pulsed radiofrequency (PRF), a novel and minimally invasive interventions, has been introduced to alleviate various types of NP.11–14 Different from conventional radiofrequency (CRF), PRF exerts its analgesic effect through electric fields by interfering with action potential generation and ectopic firing of neuronal membrane without affecting the structural integrity of the nerve.11,15 The standard PRF parameters are an output voltage of 45 V, a pulse frequency of 2 Hz, output frequency of 500kHz with a continuous current action and an intermission period of 480 ms. Within the interval period, the heat of the electrode tip can be dissipated, and the temperature will not exceed 42°C.16,17 Thus, PRF has been regarded as a safe and promising technique for the treatment of NP.
However, some studies reported that the effective rate of standard PRF for the treatment of NP was not satisfactory.16,18 In recent years, it is demonstrated elevating the output voltage of PRF could improve the treatment efficacy.19–22 Since then, we conducted this systematic review and meta-analysis to determine whether high-voltage PRF is superior to standard-voltage PRF for the treatment of NP patients.
Methods
We performed this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines23 (see Supplemental Table 1). The protocol of the systematic review was registered in the PROSPERO database (registration number: CRD 42022297804) and published on BMJ open.24
Search Strategy and Information Sources
We primarily searched relevant studies published from the date of inception until 15 March 2022 on PubMed/MEDLINE, EMBASE, Web of Science and the Cochrane Library. The conference proceedings for relevant abstracts, clinical trials registers (ClinicalTrials.gov) and the WHO’s International Clinical Trial Registry Platform were also retrieved to identify ongoing studies. All studies were published in English. The search included a broad range of terms and keywords relevant to “pulsed radiofrequency”, “high-voltage”, “neuropathic pain” and “RCT”. Detailed search strategy was shown in Supplemental Table 2.
Eligibility Criteria
We included randomized controlled trials (RCTs) involving PRF for the treatment of NP. The diagnostic criteria for NP was defined by the International Association for the Study of Pain (IASP).2 Experimental animal studies and studies reported no data/results were excluded. The intervention was high-voltage PRF treatment, and the comparator was the standard PRF treatment.
Study Selection and Data Extraction
We determined the specific criteria for selecting studies according to the Population, Intervention, Comparison, Outcome model (PICO) model. Two reviewers independently screened titles and abstracts to identify potential articles. Subsequently, reviewers identified studies meeting the eligibility criteria via screening the full texts. Any discrepancies between the two reviewers were resolved by the involvement of a third reviewer.
A standardized template for data extraction was developed by a researcher. Two researchers independently extracted the data into the electronic form. Original information included name of the first author, sample size, year of publication; baseline characteristics, including age, gender, disease duration, preoperative pain intensity; follow-up period; as well as the efficacy and safety of the treatment.
Outcome Measures
The primary outcome measures were the efficiency rates for NP patients undergoing PRF treatment. The effective rate was defined as the proportion of patients with >50% reduction in pain score after the procedure compared with baseline. Secondary outcome measures included include numeric rating scale (NRS) or visual analogue scale (VAS) score, QoL using a health questionnaire (SF-36) including physical component summary (PCS) and mental component summary (MCS) as well as related complications.
Risk-of-Bias and Quality-of-Evidence Assessment
The Cochrane Risk of Bias (RoB) tool25 was used to assess the potential bias for included studies. Two independent reviewers analyzed RoB and discrepancies was resolved by a third reviewer.
Two reviewers independently evaluated the overall quality of the body of evidence according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.26 According to the GRADE, quality of evidence was rated as high, moderate, low or very low. In cases of disagreements, a third researcher was involved in the discussion.
Statistical Analysis and Data Synthesis
The meta-analysis was conducted utilizing the RevMan 5.3 software (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). With regard to continuous outcomes, standardized mean differences (MD) and 95% CIs were calculated. For dichotomous data, the pooled risk ratio (RR) and 95% CIs were estimated. Continuous data expressed as median (interquartile ranges) were transformed into mean and standard deviation (SD) using mathematical operations. A P value < 0.05 was considered statistically significant.
Heterogeneity was assessed using forest plots and measured by the I2 statistic. The I2 values >50% was considered with substantial heterogeneity, and the random effects model was applied to analyze the outcomes. Otherwise, a fixed-effect model was used for analysis of the data. A funnel plot or Egger test were utilized to evaluate publication bias.
Results
Literature Search
A total of 854 studies were identified through the initial search. After removing duplicate records, 735 studies remained and 707 were excluded by screening abstracts and title. A total of 28 studies remained for full-text screening. Finally, 6 studies20–22,27–29 with 423 patients were included for data extraction and final analysis (Figure 1).
 |
Figure 1 Flowchart of study selection. |
Study Characteristics
Table 1 showed the characteristics of six included RCTs. The number of patients in included studies ranged from 52 to 115. There were 211 participants in the high-voltage PRF group, and 212 participants in the standard-PRF group. All studies were single center conducted in China. The proportion of male was between 36.8% and 47.2% and the average age was ranging from 60.53 to 72.81 years.
 |
Table 1 Included Trials Characteristics |
The Efficiency Rates
Compared with standard-voltage PRF group, the high-voltage PRF group attained a higher efficiency rate at 1 month (RR: 1.44, 95% CI: 1.13–1.85; P = 0.004; I2 = 0%), 3 months (RR: 1.44, 95% CI: 1.13–1.85; P = 0.004; I2 = 0%), 6 months (RR: 1.51, 95% CI: 1.22–1.87; P = 0.0002; I2 = 0%) post-procedure respectively. There was no significant difference in the effective rate at 1 year postoperatively (RR: 1.88, 95% CI: 0.92–3.84; P = 0.08; I2 = 77%) (Figure 2).
Postoperative Pain Score
The NRS or VAS scores in the high-voltage PRF group were significantly lower than that in the standard-voltage PRF group at 1 week (MD: −0.83, 95% CI: −1.21–-0.44; P < 0.0001; I2=58%), 2 weeks (MD: −0.94, 95% CI: −1.17–-0.70; P < 0.00001; I2=20%), 1 month (MD: −0.86, 95% CI: −1.33–-0.40; P = 0.0003; I2=80%), 3 months (MD: −1.08, 95% CI: −1.28–-0.88; P < 0.00001; I2=26%) and 6 months (MD: −1.34, 95% CI: −1.57–-1.10; P < 0.00001; I2=42%) postoperatively. There was no significant difference in the NRS or VAS score at 3 days postoperatively (MD: −0.24, 95% CI: −0.53–0.04; P = 0.09; I2 = 13%) (Figure 3).
SF-36 Score
PCS Score
The PCS score in the high-voltage PRF group were significantly higher than that in the standard-voltage PRF group at 1 week (MD: 5.54, 95% CI: 2.6–8.48; P = 0.0002; I2=0%), 3 months (MD: 7.5, 95% CI: 5.39–9.62; P < 0.00001; I2=0%) and 6 months (MD: 10.31, 95% CI:8.07–12.55; P < 0.00001; I2=0%) after the procedure. There was no significant difference in the PCS score at 1 month postoperatively (MD: 4.32, 95% CI: −5.31–13.95; P = 0.38; I2=95%) (Figure 4).
 |
Figure 4 (A) The PCS score at 1 week post-procedure; (B) The PCS score at 1 month post-procedure; (C) The PCS score at 3 months post-procedure; (D) The PCS score at 6 months post-procedure. |
MCS Score
Patients in the high-voltage PRF group attained higher MCS Score than that in the standard-voltage PRF group at 1 month (MD: 6.73, 95% CI: 0.28–13.18; P = 0.04; I2=90%) and 3 months (MD: 10.15, 95% CI: 6.74–13.57; P < 0.00001; I2=56%) post-operation. There was no significant difference in the MCS score at 1 week (MD: 7.59, 95% CI: −1.32–16.5; P = 0.09; I2=88%) and 6 months (MD: −1.65, 95% CI: −17.52–14.22; P = 0.84; I2=98%) postoperatively (Figure 5).
 |
Figure 5 (A) The MCS score at 1 week post-procedure; (B) The MCS score at 1 month post-procedure; (C) The MCS score at 3 months post-procedure; (D) The MCS score at 6 months post-procedure. |
Safety Evaluation
There was no significant difference in the complications associated with the PRF operation including intraoperative bradycardia, intraoperative tachycardia, numbness, swelling, worst pain, ocular anesthesia and corneal abrasions (Figure 6).
Risk of Bias
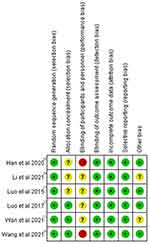
Quality assessment of the 6 RCTs was based on the Cochrane Collaboration’s RoB tool and most of them had a low risk of bias across most domains (Figures 7 and 8). Moreover, GRADEpro Guideline Development Tool was used to create summary of findings for the comparison of high-voltage PRF versus the standard-voltage PRF group for NP patients (Table 2). Funnel plot results for the outcomes reported were illustrated in Supplementary Figures 1–18.
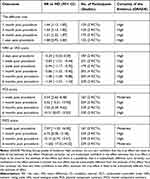 |
Table 2 GRADE Evaluation |
 |
Figure 7 Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies. |
Subgroup Analysis
A subgroup analysis of the MCS score was conducted based on acute or chronic pain. Studies focusing on patients with chronic NP were included in the subgroup analysis. The result revealed that patients in the high-voltage PRF group attained higher MCS score than that in the standard-voltage PRF group at 3 month (MD: 12.23, 95% CI: 8.99–15.46; P < 0.00001; I2=0%). There was no significant difference in the MCS score at 1 month (MD: 6.96, 95% CI: −6.36–20.28; P = 0.31; I2=92%) and 6 months (MD:1.01, 95% CI: −24.82–26.85; P = 0.94; I2=97%) postoperatively (Supplementary Figures 19–21).
Discussion
Our meta-analysis focused on NP patients receiving different output voltage of PRF from previous 6 RCTs, and the results demonstrated high-voltage PRF was associated with higher efficiency rate, lower pain score, higher PCS Score and MCS Score. There was no difference in complications related to PRF between the two groups which revealed that high-voltage PRF would be a promising, minimally invasive therapy with satisfactory efficacy and safety for NP patients.
NP treatment is very tricky due to underlying complex mechanisms.30 Several NP patients respond poorly to traditional analgesics and suffer from intolerable spontaneous pain.31 In recent years, PRF have been increasingly used because of satisfactory analgesic effect with minimally invasive approach. However, some studies reported PRF treatment on NP patients could not achieve satisfactory efficacy in the long duration due to high recurrence rate.32,33 Increasing the output voltage of PRF could increases the output energy of the electric field effect and thus achieve better analgesic effect.34 Consistent with previous reports, our meta-analysis based on 6 published RCTs revealed NP patients undergoing high-voltage PRF attained significantly alleviated pain which could provide more reliable evidence for the choice of PRF modes in clinical practice for pain physicians.
SF-36 is a practical instrument applied to measure patients’ physical and mental health and it was widely used for evaluating the QoL for patients with NP.35,36 The results of our study showed patients who received PRF at a higher voltage attained higher PCS score and MCS score at different time points after the operation. We hypothesize that the significant improvement of QoL is due to the better analgesic effect in high-voltage PRF group. When conducted a subgroup analysis of the MCS score based on chronic pain, the subgroup analysis result revealed that there was no significant difference in the MCS score at 1 month (P = 0.31). However, based on both acute and chronic pain, it was demonstrated that patients in the high-voltage PRF group attained higher MCS score than that in the standard-voltage PRF group at 1 month (P = 0.04). The MCS score have been applied to assess predict depression and mood disorders in various populations including patients with NP.36,37 And there is strong evidence that a lower preoperative mental health status results in poorer functional and pain scores.38 Up to now, there is a lack of study comparing the differences of mental health status between acute and chronic NP after receiving PRF treatment. We hypothesized it might be patients with chronic NP suffered from a longer duration of pain and required longer recovery period to achieve the improvement of mental health status.
PRF therapy was well tolerated because it could release pain via “modulation” rather than thermal destruction of nerve tissue.32 Previous studies demonstrated PRF treatment was a non-neurodestructive and safe technique without serious complications.14,16,39–42 In our study, a few patients developed complications such as intraoperative bradycardia, intraoperative tachycardia, numbness, swelling, etc. Similarly, the degree of these side effects was mild and could improved in a short period. Moreover, there was no statistically significant difference in the complications between the two groups suggesting high-voltage PRF was a fairly safe and effective treatment for NP patients without increasing the risk of intraoperative and post-operative adverse events.
All of the RCTs included in our meta-analysis performed the PRF procedure under the guidance of CT which could prevent neurovascular injury result from inaccurate puncture. However, patients were inevitably exposed to radiation when receiving treatment which is an important problem worthy of attention. In recent years, ultrasonography and electromagnetic navigation was gradually applied to puncture guidance of radiofrequency without the risk of radiation exposure to both patients and hospital staff.43–45 In the future, prospective, randomized controlled clinical trials are needed to furtherly explore the more optimal imaging tool for the guidance of PRF treatment in NP patients.
It is reported that heterogenous device and procedure might affect the analgesic effect of PRF treatment.46,47 In a randomized controlled study, Vigneri et al48 performed high-voltage PRF through multifunctional electrode on dorsal root ganglion and attained satisfactory pain relief for the treatment of chronic lumbosacral radicular pain. In our meta-analysis, PRF procedure in all included studies were needle mediated. Further research is required to determine the specific differences in the treatment of NP via different devices.
At present, there is no standard optimal parameters for PRF procedure on the treatment of NP. Several research have been conducted to determine the ideal parameters including output voltage, exposure time, treatment targets and so on. The animal experiment and clinical study all supported that increasing exposure time could improve the analgesic effect of PRF treatment.47,49 Our previous study and Ding et al’s study showed patients with acute zoster-related trigeminal neuralgia who received PRF therapy on gasserian ganglion attained more satisfactory effect than treating peripheral nerves.50,51 Moreover, PRF treatment seems to be more effective when performed in the earlier stages.49,51 In the future, research remains to be conducted to evaluate whether increasing exposure time, choosing the more suitable treatment location or receiving the procedure in earlier stages could furtherly improve the therapeutic effect of high-voltage PRF for NP patients.
Limitation
Our study has several limitations need to be mentioned. First, all of the 6 RCTs were single-center trials with relatively small sample size, power to evaluate cross-center differences was probably not enough. Secondly, we only included studies published on English, which could also have increased the publication bias. Thirdly, all of the clinical trials included in this study were conducted in People’s Republic of China which limited racial/ethnic diversity of our study population. Moreover, the heterogeneous pathophysiology among different studies increased the heterogeneity of our meta-analysis and made it difficult for additional analysis.
Conclusions
Compared with standard-voltage PRF, high-voltage PRF attained more satisfactory efficacy without increased side effects. Our meta-analysis suggested high-voltage PRF was a promising, effective, minimally invasive technology for patients suffering from NP. In the future, prospective, multi-centered, high-quality RCTs with large sample size is required to provide more reliable evidence for the effectiveness and safety of high-voltage PRF for the treatment of NP.
Abbreviations
NP, neuropathic pain; QoL, quality of life; MVD, microvascular decompression; PRF, pulsed radiofrequency; CRF, conventional radiofrequency; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; IASP, International Association for the Study of Pain; PICO, Population, Intervention, Comparison, Outcome model; NRS, numeric rating scale; VAS, visual analogue scale; RoB, Risk of Bias; GRADEL Grading of Recommendations Assessment, Development and Evaluation; MD, mean differences; RR, risk ratio; SD, standard deviation.
Data Sharing Statement
The datasets of the current study are available from the corresponding authors upon reasonable request.
Consent for Publication
All authors consented.
Declarations
Ethical approval was waived as our systematic review was based on published literature.
Acknowledgments
The authors would like to thank the participants of the study for their cooperation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. YW, YJ and ZW contributed equally to this paper and are co-first authors. TW is responsible as corresponding author.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interest in this work.
References
1. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3(1):17002. doi:10.1038/nrdp.2017.2
2. Ochoa JL. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2009;72(14):1282–1283. doi:10.1212/01.wnl.0000346325.50431.5f
3. van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. doi:10.1016/j.pain.2013.11.013
4. Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152(12):2836–2843. doi:10.1016/j.pain.2011.09.014
5. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
6. Cruccu G, Gronseth G, Alksne J, et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15(10):1013–1028. doi:10.1111/j.1468-1331.2008.02185.x
7. Tan T, Barry P, Reken S, Baker M. Pharmacological management of neuropathic pain in non-specialist settings: summary of NICE guidance. BMJ. 2010;340:c1079.
8. Meng J, Zhao HY, Zhuo XJ, Shen QH. Postoperative analgesic effects of serratus anterior plane block for thoracic and breast surgery: a meta-analysis of randomized controlled trials. Pain Physician. 2023;26(2):E51–E62.
9. Guo S, Shen M, Zhang L, et al. The effect of interventional pain management on treating postherpetic neuralgia. Indian J Dermatol. 2019;64(3):251. doi:10.4103/ijd.IJD_130_18
10. Morra ME, Elgebaly A, Elmaraezy A, et al. Therapeutic efficacy and safety of botulinum toxin a therapy in trigeminal neuralgia: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain. 2016;17(1):63. doi:10.1186/s10194-016-0651-8
11. Chalermkitpanit P, Pannangpetch P, Kositworakitkun Y, et al. Ultrasound-guided pulsed radiofrequency of cervical nerve root for cervical radicular pain: a prospective randomized controlled trial. Spine J. 2023;23(5):651–655. doi:10.1016/j.spinee.2023.01.004
12. Li M, Hu H, Tong SX, et al. The therapeutic efficacy of pulsed radiofrequency alone versus a dexamethasone and pulsed radiofrequency combination in patients with trigeminal postherpetic neuralgia: a double-blind, randomized controlled trial. Pain Physician. 2022;25(4):E543–E549.
13. Andrade JG, Macle L, Bennett MT, et al. Randomized trial of conventional versus radiofrequency needle transseptal puncture for cryoballoon ablation: the CRYO-LATS trial. J Interv Card Electrophysiol. 2022;65(2):481–489. doi:10.1007/s10840-022-01277-y
14. Uematsu H, Osako S, Hakata S, et al. A double-blind, placebo-controlled study of ultrasound-guided pulsed radiofrequency treatment of the saphenous nerve for refractory osteoarthritis-associated knee pain. Pain Physician. 2021;24(6):E761–E769.
15. Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12(1):37–41. doi:10.1007/s11916-008-0008-3
16. Elawamy A, Abdalla EEM, Shehata GA. Effects of pulsed versus conventional versus combined radiofrequency for the treatment of trigeminal neuralgia: a Prospective Study. Pain Physician. 2017;20(6):E873–E881.
17. Fang L, Jia Z. In response to comments on ”long-term follow-up of pulsed radiofrequency treatment for trigeminal neuralgia: Kaplan-Meier Analysis in a Consecutive Series of 149 Patients”. Pain Physician. 2022;25(2):E409.
18. Fang L, Ying S, Tao W, Lan M, Xiaotong Y, Nan J 3D. CT-guided pulsed radiofrequency treatment for trigeminal neuralgia. Pain Pract. 2014;14(1):16–21. doi:10.1111/papr.12041
19. Luo F, Meng L, Wang T, Yu X, Shen Y, Ji N. Pulsed radiofrequency treatment for idiopathic trigeminal neuralgia: a retrospective analysis of the causes for ineffective pain relief. Eur J Pain. 2013;17(8):1189–1192. doi:10.1002/j.1532-2149.2012.00278.x
20. Wang B, Du Z, Xia J, Zhang H. Efficacy of high-voltage pulsed radiofrequency for the treatment of elderly patients with acute herpes zoster neuralgia. Rev Assoc Med Bras. 2021;67(4):585–589. doi:10.1590/1806-9282.20201124
21. Wan CF, Song T. Comparison of two different pulsed radiofrequency modes for prevention of postherpetic neuralgia in elderly patients with acute/subacute trigeminal herpes zoster. Neuromodulation. 2021;24(6):1121–1126. doi:10.1111/ner.13288
22. Luo F, Wang T, Shen Y, Meng L, Lu J, Ji N. High voltage pulsed radiofrequency for the treatment of refractory neuralgia of the infraorbital nerve: a Prospective Double-Blinded Randomized Controlled Study. Pain Physician. 2017;20(4):271–279.
23. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349(jan02 1):g7647. doi:10.1136/bmj.g7647
24. Jia Y, Wang Z, Ma Y, et al. Efficacy and safety of high-voltage versus standard-voltage pulsed radiofrequency ablation for patients with neuropathic pain: protocol for a systematic review and meta-analysis. BMJ open. 2022;12(7):e063385. doi:10.1136/bmjopen-2022-063385
25. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142. doi:10.1002/14651858.ED000142
26. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi:10.1016/j.jclinepi.2010.04.026
27. Luo F, Wang T, Lu J, Ji N. Comparison of high-voltage- with standard-voltage pulsed radiofrequency of gasserian ganglion in the treatment of idiopathic trigeminal neuralgia. Pain Pract. 2015;15(7):595–603. doi:10.1111/papr.12227
28. Han Z, Hong T, Ding Y, Wang S, Yao P. CT-guided pulsed radiofrequency at different voltages in the treatment of postherpetic neuralgia. Front Neurosci. 2020;14:579486. doi:10.3389/fnins.2020.579486
29. Li H, Ding Y, Zhu Y, Han Z, Yao P. Effective treatment of postherpetic neuralgia at the first branch of the trigeminal nerve by high-voltage pulsed radiofrequency. Front Neurol. 2021;12:746035. doi:10.3389/fneur.2021.746035
30. Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–935. doi:10.1016/S1474-4422(14)70102-4
31. Torrance N, Ferguson JA, Afolabi E, et al. Neuropathic pain in the community: more under-treated than refractory? Pain. 2013;154(5):690–699. doi:10.1016/j.pain.2012.12.022
32. Shi Y, Wu W. Treatment of neuropathic pain using pulsed radiofrequency: a meta-analysis. Pain Physician. 2016;19(7):429–444.
33. Erdine S, Ozyalcin NS, Cimen A, Celik M, Talu GK, Disci R. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur J Pain. 2007;11(3):309–313. doi:10.1016/j.ejpain.2006.04.001
34. Teixeira A, Sluijter ME. Intradiscal high-voltage, long-duration pulsed radiofrequency for discogenic pain: a preliminary report. Pain Med. 2006;7(5):424–428. doi:10.1111/j.1526-4637.2006.00138.x
35. Keller SD, Majkut TC, Kosinski M, Ware JE Jr. Monitoring health outcomes among patients with arthritis using the SF-36 health survey: overview. Med Care. 1999;37(5 Suppl):Ms1–9. doi:10.1097/00005650-199905001-00001
36. Tan CY, Shahrizaila N, Goh KJ. Clinical characteristics, pain, and quality of life experiences of trigeminal neuralgia in a Multi-Ethnic Asian Cohort. J Oral Facial Pain Headache. 2017;31(4):e15–e20. doi:10.11607/ofph.1793
37. van den Beukel TO, Siegert CE, van Dijk S, Ter Wee PM, Dekker FW, Honig A. Comparison of the SF-36 five-item mental health inventory and beck depression inventory for the screening of depressive symptoms in chronic dialysis patients. Nephrol Dial Transplant. 2012;27(12):4453–4457. doi:10.1093/ndt/gfs341
38. Vissers MM, Bussmann JB, Verhaar JA, Busschbach JJ, Bierma-Zeinstra SM, Reijman M. Psychological factors affecting the outcome of total hip and knee arthroplasty: a systematic review. Semin Arthritis Rheum. 2012;41(4):576–588. doi:10.1016/j.semarthrit.2011.07.003
39. Silva V. Comments on ”long-term follow-up of pulsed radiofrequency treatment for trigeminal neuralgia: Kaplan-Meier analysis in a consecutive series of 149 patients”. Pain Physician. 2022;25(2):E408.
40. Sam J, Catapano M, Sahni S, Ma F, Abd-Elsayed A, Visnjevac O. Pulsed radiofrequency in interventional pain management: cellular and molecular mechanisms of action - an update and review. Pain Physician. 2021;24(8):525–532.
41. Wu CY, Lin HC, Chen SF, et al. Efficacy of pulsed radiofrequency in herpetic neuralgia: a meta-analysis of randomized controlled trials. Clin J Pain. 2020;36(11):887–895. doi:10.1097/AJP.0000000000000867
42. Vanneste T, Van Lantschoot A, Van Boxem K, Van Zundert J. Pulsed radiofrequency in chronic pain. Curr Opin Anaesthesiol. 2017;30(5):577–582. doi:10.1097/ACO.0000000000000502
43. Luo F, Lu J, Ji N. Treatment of refractory idiopathic supraorbital neuralgia using percutaneous pulsed radiofrequency. Pain Pract. 2018;18(7):871–878. doi:10.1111/papr.12687
44. Ren H, Shen Y, Luo F. Treatment of supraorbital neuralgia using ultrasound-guided radiofrequency thermocoagulation of the supraorbital nerve: a Retrospective Study. J Pain Res. 2020;13:251–259. doi:10.2147/JPR.S228720
45. Chen MJ, Gu LX, Zhang WJ, Yang C, Dong MJ. Electromagnetic navigation-guided radiofrequency thermocoagulation in trigeminal neuralgia: technical note with three case reports. J Neurol Surg a Cent Eur Neurosurg. 2013;74(4):251–257. doi:10.1055/s-0032-1330953
46. Simopoulos TT, Kraemer J, Nagda JV, Aner M, Bajwa ZH. Response to pulsed and continuous radiofrequency lesioning of the dorsal root ganglion and segmental nerves in patients with chronic lumbar radicular pain. Pain Physician. 2008;11(2):137–144. doi:10.36076/ppj.2008/11/137
47. Vigneri S, Sindaco G, Gallo G, et al. Effectiveness of pulsed radiofrequency with multifunctional epidural electrode in chronic lumbosacral radicular pain with neuropathic features. Pain Physician. 2014;17(6):477–486. doi:10.36076/ppj.2014/17/477
48. Vigneri S, Sindaco G, La Grua M, et al. Electrocatheter-mediated high-voltage pulsed radiofrequency of the dorsal root ganglion in the treatment of chronic lumbosacral neuropathic pain: a Randomized Controlled Study. Clin J Pain. 2020;36(1):25–33. doi:10.1097/AJP.0000000000000766
49. Tanaka N, Yamaga M, Tateyama S, Uno T, Tsuneyoshi I, Takasaki M. The effect of pulsed radiofrequency current on mechanical allodynia induced with resiniferatoxin in rats. Anesth Analg. 2010;111(3):784–790. doi:10.1213/ANE.0b013e3181e9f62f
50. Ding Y, Hong T, Li H, Yao P, Zhao G. Efficacy of CT guided pulsed radiofrequency treatment for trigeminal postherpetic neuralgia. Front Neurosci. 2019;13:708. doi:10.3389/fnins.2019.00708
51. Jia Y, Shen Y, Meng L, Wang T, Luo F. Efficacy, safety, and predictors of response to pulsed radiofrequency therapy for acute zoster-related trigeminal neuralgia patients: a Multicenter Retrospective Study. Pain Physician. 2022;25(4):E523–E530.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.