Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Effects of omega-3 polyunsaturated fatty-acid supplementation on neuropathic pain symptoms and sphingosine levels in Mexican-Americans with type 2 diabetes
Authors Durán AM, Salto LM , Câmara J, Basu A, Paquien I, Beeson WL , Firek A, Cordero-MacIntyre Z , De León M
Received 12 September 2018
Accepted for publication 13 November 2018
Published 8 January 2019 Volume 2019:12 Pages 109—120
DOI https://doi.org/10.2147/DMSO.S187268
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Alfonso M Durán,1 Lorena M Salto,1 Justin Câmara,1 Anamika Basu,1 Ivette Paquien,1 W Lawrence Beeson,1,2 Anthony Firek,3 Zaida Cordero-MacIntyre,1,2 Marino De León1
1Center for Health Disparities and Molecular Medicine, Department of Basic Sciences, Loma Linda University School of Medicine, Loma Linda, CA, USA; 2Center for Nutrition, Healthy Lifestyle and Disease Prevention, School of Public Health, Loma Linda University, Loma Linda, CA, USA; 3Comparative Effectiveness and Clinical Outcomes Research Center, Riverside University Health System Medical Center, Moreno Valley, CA, USA
Purpose: To determine whether dietary supplementation with omega-3 polyunsaturated fatty acids (PUFAs) reduces neuropathic pain symptoms in Mexican-Americans with type 2 diabetes.
Methods: Forty volunteers with type 2 diabetes enrolled in the “En Balance-PLUS” program, which provided weekly nutrition–diabetes education and daily supplementation with 1,000 mg docosahexaenoic acid (DHA)–200 mg eicosapentaenoic acid over 3 months. The study assessed self-reported neuropathic pain symptoms pre/postintervention using the short-form McGill Pain Questionnaire (SF-MPQ), monitored clinical laboratory values at baseline and 3 months, and performed baseline and 3-month metabolomic analysis of plasma samples.
Results: A total of 26 participants self-reported neuropathic pain symptoms at baseline. After 3 months of omega-3 PUFA supplementation, participants reported significant improvement in SF-MPQ scores (sensory, affective, and visual analogue scale; P<0.001, P=0.012, and P<0.001, respectively). Untargeted metabolomic analysis revealed that participants in the moderate–high SF-MPQ group had the highest relative plasma sphingosine levels at baseline compared to the low SF-MPQ group (P=0.0127) and the nonpain group (P=0.0444). Omega-3 PUFA supplementation increased plasma DHA and reduced plasma sphingosine levels in participants reporting neuropathic pain symptoms (P<0.001 and P<0.001, respectively). Increased plasma DHA levels significantly correlated with improved SF-MPQ sensory scores (r=0.425, P=0.030). Improved SF-MPQ scores, however, did not correlate with clinical/laboratory parameters.
Conclusion: The data suggest that omega-3 PUFAs dietary supplementation may reduce neuropathic pain symptoms in individuals with type 2 diabetes and correlates with sphingosine levels in the plasma.
Keywords: lipotoxicity, painful diabetic neuropathy, health disparities, community intervention, neuroprotection, Latinos
Introduction
Diabetic neuropathy (DN), also known as distal symmetrical polyneuropathy, affects up to 50% of patients with type 2 diabetes and is a major cause of morbidity and increased mortality.1 Of those patients experiencing DN symptoms, about half report neuropathic pain.2 Glycemic control is known to prevent or delay the progression of neuropathy in patients with type 1 diabetes.3 However, tight glycemic control in patients with type 2 diabetes exhibiting neuropathy shows mixed outcomes, and intense glycemic control treatment can cause higher mortality.4,5 Successful implementation of interventions and treatments targeting dyslipidemia (hypercholesterolemia and/or hypertriglyceridemia), nutrition and lifestyle modifications, and weight loss have shown positive improvement in DN symptoms and nerve function without associated increased morbidity or mortality.6,7 Furthermore, targeted nutritional modifications are of increasing importance in mitigating pain syndromes.8,9 For example, targeting lipid-metabolism dysregulation in animal models with omega-3 polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA), shows a protective effect and potentially reverses DN.10 DHA can protect neurons and Schwann cells from oxidative stress and inflammation, and show promising effects against nociception-associated neuropathic pain.11–15
Diabetic neuropathic pain is multifactorial, with neuronal dysfunction and axonal damage as featured pathological processes.16 This cellular dysfunction can be triggered in part by the oversupply of fat to tissue not suited for lipid storage, termed lipotoxicity-associated overnutrition.17,18 Nerve cells are particularly sensitive to lipotoxicity-mediated oxidative stress.19,20 Lipotoxic stress initiates an extensive injury cascade, marked by loss of ATP production triggering cellular dysfunction and death.21,22 DHA, an omega-3 PUFA, inhibits neuronal cell death caused by palmitic acid cellular overload, supporting its neuroprotective role.14,19 In addition, lipotoxic stress activates sphingolipid metabolism, a cellular pathway important in the pathogenesis of DN.14,19,20,23–25 Research that advances our understanding of levels of dietary omega-3 PUFAs and sphingolipid metabolism may help in our understanding of the etiology and treatment of painful DN. Therefore, further studies are needed to evaluate the use of omega-3 PUFAs in diabetes health-education programs as a complementary therapy for neuropathic pain associated with type 2 diabetes.
The purpose of this single-arm pilot study without a control group was to test the efficacy of a 3-month group-based omega-3 PUFA dietary intervention on Mexican-Americans with type 2 diabetes. The analysis compared neuropathic pain symptoms pre- and postintervention. Additionally, plasma metabolites were measured using an untargeted metabolomic approach. Bioinformatics to analyze metabolomic data was used to identify promising metabolites associated with omega-3 supplementation and neuropathic pain symptoms.
Methods
En Balance-Plus cohort
En Balance-Plus is an interventional study that used a single-group design to assess the efficacy of dietary omega-3 PUFA pre- and postsupplementation in participants previously diagnosed with type 2 diabetes. Participants completed a 3-month group-based intervention that consisted of taking dietary omega-3 PUFA capsules and attending weekly diabetes-education classes conducted in Spanish. Participants were self-reported Mexican-Americans living in the Inland Empire of southern California and recruited from Spanish-speaking Seventh-Day Adventist churches. Initial screening and selection was done via scripted telephone interviews. Participants were excluded if they reported a clinical history of drug or alcohol abuse, steroid use, or psychological or other major systemic disease that could affect program compliance, such as end-stage renal disease. Each participant was interviewed in person to obtain diabetes history, medication use, diet, and physical activity habits. The study was conducted in accordance with the Declaration of Helsinki.
A total of 40 Hispanic adults diagnosed with type 2 diabetes between the ages of 33 and 74 years completed the 3-month study. The program included 12 hours of healthy-lifestyle classes taught over a 3-month period, as reported in Salto et al.26 We previously determined that 31 participants were necessary to have at least 80% power to detect a 13% reduction in fasting blood glucose, allowing for type I (α) error of 5%. We used the short-form McGill Pain Questionnaire (SF-MPQ) to obtain self-reported information from the participants about having neuropathic pain symptoms. All participants completed the SF-MPQ at the start of the study and after 3 months.
Three participants were excluded: one due to a lab reporting error, and two excluded due to missing (SF-MPQ) data. Participants were instructed to consume a daily intake of 2,000 mg omega-3 fish-oil supplements (provided by study) containing 1,000 mg DHA and 200 mg eicosapentaenoic acid (EPA) for the duration of the study. In addition to the health-education classes, each participant was contacted weekly by phone to monitor them and encourage study compliance.
Data collection
Data were collected for all participants at baseline and after 3 months, and included fasting blood plasma samples, anthropometric measurements for weight, height, and waist and hip circumferences, and dual-energy X-ray absorptiometry (Discovery A fan beam; Hologic, Marlborough, MA, USA). Plasma samples were tested at the Loma Linda University Medical Center laboratory to determine fasting blood glucose, HbA1c, and lipid profiles (high-density lipoprotein [HDL], low-density lipoprotein [LDL], total cholesterol, and triglycerides). All anthropometric measurements were taken twice for reliability, using Lohman et al’s standardized techniques.27 Weight and height were assessed using a balance scale (Detecto, Webb City, MO, USA) and a wall-mounted stadiometer (Holtain, Crymych, England), respectively.
Blood sampling
Blood samples were collected at baseline and 3 months from each participant into EDTA-treated (lavender-top) tubes between 8 and 10:30 am after a 12-hour fast. For plasma collection, blood was centrifuged at 2,000 g at 15°C for 15 minutes and immediately aliquoted into sterile polypropylene tubes. Plasma aliquots were sent to Loma Linda University Medical Center for clinical lab analysis, with the remaining aliquots stored in liquid nitrogen at –80°C until metabolomic analyses.
Metabolite profiling
The plasma samples from participants were measured by Metabolon (Durham, NC, USA). Metabolic profiling was performed as previously described.28 Briefly, untargeted semiquantitative metabolomic analysis was performed on three independent platforms: ultra-HPLC/tandem mass spectrometry (MS2) optimized for basic species, UHPLC/MS/MS2 optimized for acidic species, and gas chromatography (GC) MS. Metabolites were identified by comparing the ion features in the experimental samples to a reference library of chemical standards that included retention time, molecular weight (m/z), preferred adducts, and in-source fragments, as well as associated MS. Biochemical features were curated by visual inspection for quality control using the software developed at Metabolon.29
Metabolomic analyses
As reported, the estimate of the false discovery rate was calculated considering the multiple comparisons that normally occur in metabolomic-based studies, using q value of<0.10 as an indication of high confidence in a result.11 We further analyzed the metabolomic data using random forest (RF), a supervised classification technique based on an ensemble of decision trees.30 This machine-learning classification method was performed using the R RF package, in an attempt to identify the most important metabolites for separating baseline and 3-month groups. We used the default values in the package, which build trees until the nodes are pure. Further, for bootstrapping, we sampled without replacement. The number of trees used for RF analysis was 50,000. We used 50% of the group size to cross-validate. However, since groups were uneven, we used 50% of the smaller group for cross-validation of both groups. RF analysis is unbiased, since the prediction for each sample is based on trees built from a subset of samples that do not include that sample. When the full forest is grown, the class predictions are compared to the true classes, generating the “out-of-bag error rate” as a measure of prediction accuracy. Therefore, the prediction accuracy is an unbiased estimate of how well one can predict sample class in a new data set. RF has several advantages: it makes no parametric assumptions, variable selection is not needed, it does not overfit, and it is invariant to transformation.31
Short-form McGill Pain Questionnaire
Every participant in the diabetes-education program completed the SF-MPQ form at the start and at the end of the 3 months. The SF-MPQ is a well-established pain-assessment tool that has been tested and validated in many languages, including Spanish.32 The SF-MPQ consists of 15 descriptors (eleven sensory, four affective) rated on a 4-point scale of 0 (none) to 3 (severe), a visual analogue scale measured in millimeters, and a present pain-intensity index (score ranging from 0 to 5). The present pain-intensity index was not recorded consistently by participants; therefore, this qualitative measurement was not used in the data analysis. The SF-MPQ has also been validated for use in patients with type 2 diabetes experiencing neuropathic pain symptoms and for assessing interventions for pain-improvement outcomes.33 We did not perform a focused neurological exam or nerve-conduction study to confirm a diagnosis of painful DN. Participants reporting symptoms consistent with neuropathic pain and also indicating radiating pain in stocking distribution on the body diagram of the SF-MPQ were classified as the group exhibiting neuropathic pain symptoms. Conversely, participants not reporting neuropathic pain symptoms were grouped as the nonneuropathic pain group.
Statistical analysis
Statistical analyses were performed using SPSS version 25 (IBM, Armonk, NY, USA), Prism 6 (GraphPad Software, San Diego, CA, USA), the R program (http://cran.r-project.org), and MetaboAnalyst 4.0.34 One-way ANOVA followed by Bonferroni post hoc comparisons were used to evaluate relevant metabolites that differed significantly between tested groups. All other data were assessed by paired-sample t-tests for normally distributed continuous variables and Wilcoxon signed-rank tests for abnormally distributed continuous variables to assess if there were statistically significant differences from baseline vs 3 months postsupplementation. Kolmogorov–Smirnov and Shapiro–Wilk normality tests, together with the Grubbs’s test, also known as extreme “studentized” deviate (www.graphpad.com), were used to investigate outliers and spread. Pearson correlation tests were used to explore associations between detected metabolites and SF-MPQ scores. Data are presented as mean ± SD. Statistical differences were considered significant at α=0.05, unless otherwise specified.
Ethics statement
All participants provided informed written consent in their preferred language prior to beginning the study. The En Balance-Plus study was approved by the Loma Linda University Institutional Review Board.
Results
Characteristics of En Balance-Plus participants presupplementation (baseline)
The self-reported data obtained from the SF-MPQ at baseline were used to segregate the participants into two distinct groups: participants reporting significant neuropathic pain symptoms (n=26), and those not reporting neuropathic pain symptoms (n=11). Characteristics of these two subgroups are shown in Table 1. Both the neuropathic pain-symptom group and the nonneuropathic pain-symptom group had similar age ranges and distribution. In the neuropathic pain-symptom group, there was a higher number of female participants, overall higher body mass index (BMI), and longer duration of type 2 diabetes, all suggested risk factors for developing painful DN.35,36 Overall clinical outcomes exhibited by these two groups at baseline (BMI, LDL, HDL, total cholesterol, triglycerides, A1c, and fasting blood glucose) did not show significant differences (Table 1). The group reporting neuropathic pain symptoms actually had lower A1c and fasting glucose, although this was not statistically significant. The two groups exhibited differences in terms of medication-usage rates. Self-reported antiglycemic usage, not including metformin, was higher for the neuropathic pain vs nonneuropathic pain-symptom group (26.9% vs 7.7%, respectively). There were insufficient numbers to determine any relationship with pain or specific diabetes mellitus medications. As expected, self-reported usage rates of analgesics and gabapentin were higher in the neuropathic pain-symptom group compared to the nonneuropathic pain-symptom group at baseline (Table 1). Self-reported statin-usage rate was higher in the nonneuropathic pain group than the neuropathic group presupplementation (23.1% vs 11.5%, respectively), but despite this difference, there was no difference in LDL cholesterol. Neither group reported any alcohol or tobacco usage (Table 1).
The data obtained using the SF-MPQ were further analyzed based on the severity of the neuropathic pain symptoms reported. We based pain-severity categories on previously reported sensory mean scores of patients reporting painful neuropathic pain on the SF-MPQ.37 Participants with a sensory score <7 were denominated as the low SF-MPQ score group (n=14) and participants with sensory >7 designated into the moderate–high SF-MPQ score group (n=12). Under these criteria, at baseline these two groups self-reported significantly different mean sensory scores: the low SF-MPQ group reported an 3.4 and the moderate–high sensory group reported 12.8.
Characteristics of En Balance-Plus participants postsupplementation (3 months)
Table 2 shows that participants self-reporting neuropathic pain symptoms at baseline (n=26) had a significant reduction in both SF-MPQ sensory and affective scores at the final evaluation after 3 months of supplementation (change from baseline –5.3 [P<0.001] and –1.3 [P=0.012], respectively). Further, a significant decrease in the SF-MPQ sensory score were also observed in the low and moderate–high neuropathic pain-symptom groups (change from baseline –2.1 [P=0.014] and –9.2 [P=0.002], respectively). Interestingly, none of the clinical parameters measured (HbA1c, triglycerides, BMI, total body fat, and visceral fat) had changed significantly at the end of the 3 months for any of the groups. We observed only a small decrease in LDL for participants reporting moderate-high SF-MPQ scores at 3 months.
Metabolomic analysis before and after 3 months of omega-3 PUFA supplementation
Untargeted metabolomic analysis was performed as described in the Methods section on participants’ plasma samples at baseline and after 3 months of omega-3 PUFA supplementation. First, we investigated relative DHA and EPA levels to confirm participants’ compliance. As expected, relative EPA and DHA levels in plasma were significantly higher after 3 months of omega-3 PUFA supplementation in all participants reporting neuropathic pain symptoms (P<0.001). The only exception was that EPA concentration did not change in the moderate–high SF-MPQ pain subgroup (Table 3). Untargeted metabolomic analysis of participants’ plasma resulted in the identification of 659 metabolites (data not shown). We proceeded to analyze all 659 metabolites using RF for unbiased identification of prominent species, differentiating data collected at baseline (presupplementation) vs 3 months (postsupplementation). The RF analysis allowed us successfully to classify metabolites pre- and postsupplementation (Figure 1) with predictive accuracy of 92%. As expected, 3-carboxy-4-methyl-5-propyl-2-furanpropanoate and DHA were among the most prominent metabolites segregating baseline and 3-month metabolome features (Figure 1). Of particular interest, the RF analysis identified sphingosine among the top five prominent metabolites differentiating these groups (Figure 1). Furthermore, there is evidence that omega-3 PUFAs stimulate hepatic lipogenesis increasing the production of glycerolipids.38 Our results support this effect, as several of the top features of the RF were metabolites of glycerolipid metabolism (eg, glycerol 3-phosphate, 1-linoleoly-GPA [18:2] and linoleoyl-docosahexaenoyl-glycerol [18:2/22:6]). Next, we observed a significant improvement in participants’ omega-6 (linoleic acid [LA 18:2n6 and arachidonic acid [AA], 20:4n6) to omega-3 [DHA]ratio after 3 months of supplementation (change from baseline –1.29 [P=<0.0001] and –1.24 [P=<0.0001]; data not shown).
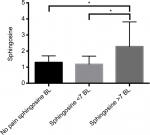
Sphingosine has been reported to be increased in rats with neuropathic pain; therefore, we focused the analysis on this metabolite. First, we compared sphingosine levels at baseline for the self-reported neuropathic or nonneuropathic pain-symptom groups. One-way ANOVA showed significant differences in relative sphingosine-metabolite concentrations between groups at baseline (F2,33=4.857, P=0.0142; Figure 2). Post hoc analyses demonstrated that the moderate–high SF-MPQ pain subgroup had significantly higher plasma sphingosine levels when compared to the low SF-MPQ pain and nonpain subgroup (P=0.0127 and P=0.0444, respectively; Figure 2). Further, Wilcoxon signed-rank tests of participants experiencing neuropathic pain (n=26) at baseline showed significantly lower relative plasma sphingosine (change from baseline –0.91, P<0.001) levels after 3 months of omega-3 PUFA supplementation compared to baseline (Table 3).
Lastly, we examined relative plasma concentrations of analgesic medications taken by participants before and after 3 months of omega-3 supplementation. Several common analgesic medications were detected by the untargeted metabolomic analysis presupplementation in participants’ plasma, including the following: gabapentin, naproxen, ibuprofen, acetaminophen, and salicylate. None of the analgesic concentrations changed significantly postsupplementation (Table 4). Also, there was no self-reported change in analgesic use by the participants.
DHA supplementation was correlated with improved SF-MPQ scores
We performed linear regression analysis to determine the relationship between omega-3 PUFA supplementation and improvement in SF-MPQ sensory scores. We found a significant correlation between DHA levels measured in participants’ plasma and their self-reported improvement in SF-MPQ sensory score (n=26, r=0.425; P=0.030; Figure 3A) from baseline to 3 months. Interestingly, we did not find a significant correlation between EPA levels and improvement in SF-MPQ scores (n=26, r=0.219; P=0.282; Figure 3B). These results suggest that the DHA component of supplementation may be more important for improvement in SF-MPQ sensory scores in this cohort of participants compared to EPA. Next, we examined significant interactions between DHA and sphingosine levels pre- and post supplementation in participants reporting most severe sensory symptoms at baseline (SF-MPQ sensory >7). We found that relative plasma DHA change postsupplementation was predictive of lower relative levels of sphingosine at 3 months in moderate–high SF-MPQ sensory pain group (r=0.698, P=0.017; Figure 3C). Furthermore, we found a significant but moderate association using data from all participants (n=26) reporting neuropathic pain symptoms (r=0.4716, P=0.0173; Figure 3D).
Discussion
Lipotoxic stress is a proposed major contributor to the development of painful DN. Several studies have suggested that exposure to chronic lipotoxic stress causes axonal injury and neuronal dysfunction in experimental animals. For instance, the overconsumption of saturated FAs, such as palmitic acid, results in excess substrates for β-oxidation/tricarboxylic acid cycles and provides nerve cells with an overabundance of NADH and FADH2 electron donors.21 This surplus of electron donors can cause uncoupling of the normal proton gradient, which interferes with ATP production, leading to a marked increase in reactive oxygen species.21 This metabolic insult can overwhelm the neuron’s antioxidant capacity, resulting in widespread bioenergetic dysregulation and nerve-cell dysfunction.22 Schwann cells that are exposed to palmitic acid-induced lipotoxicity are particularly susceptible to metabolic stress, dysfunction, and cell death.14,39 Studies show lipotoxic stress increases sphingolipid levels in metabolic diseases, such as type 2 diabetes.40 In agreement with our results, plasma samples of patients with type 2 diabetes exhibiting DN showed higher levels of sphingolipid-related metabolites.23 These findings suggest that interventions that reverse lipotoxicity-mediated metabolic stress and reduce sphingolipid-mediated apoptosis may be potential therapeutic targets to address painful DN. Omega-3 PUFAs are known to protect tissue from lipotoxicity-induced metabolic stress. DHA, a well-known omega-3 PUFA, protects from and reverses palmitic acid-induced lipotoxicity in neuronal cell models by inhibiting mitochondrial membrane depolarization, a major biochemical feature in the development of DN.19,21 Further, omega-3 PUFA inhibition of metabolic pathways related to oxidative stress during traumatic spinal cord injury in rats reduces nerve-tissue damage and ameliorates neuropathic pain.11
Omega-3 PUFAs are known to regulate sphingolipid metabolism, as DHA specifically upregulates transcription of the enzymes GCS and SphK1.41 Upregulation of these enzymes decreases the level of sphingosine in retinal cells and prevents sphingosine-induced apoptosis. Interestingly, inhibition of SphK1 abolishes DHA protection, suggesting that a decrease in sphingosine levels through its phosphorylation to S1P may be required for DHA’s therapeutic effects.42 As reported in this study, participants with moderate–high SF-MPQ sensory scores presented with markedly increased plasma sphingolipid-metabolite levels at baseline. However, after 3 months of omega-3 PUFA supplementation, toxic sphingosine levels had lowered to the measured levels of the nonneuropathic pain group (Table 3). The participants reported no change in the amount of antiglycemic medication usage. There was a difference in statin use between the groups, but there is no evidence that statins may regulate sphingosine levels in humans. Our findings support a potential, clinically relevant role of omega-3 PUFAs in mitigating increases in toxic sphingolipid metabolites. These findings may be particularly clinically important, as the effects of glycemic control on DN is variable and often difficult to achieve in type 2 diabetes.
An important finding in this study is that while the omega-3 supplementation significantly reduced neuropathic pain symptoms in the postsupplementation cohort, it did not alter such clinical parameters as BMI, LDL, HDL, total cholesterol, triglycerides, or HbA1c. These findings suggest that dietary omega-3 PUFAs can reverse lipotoxicity-induced sphingolipid-metabolite overload and may be sufficient to improve neuropathic pain symptoms. Our results indicate that sphingolipid-metabolite levels might serve as potential molecular targets for the identification of at-risk patients.
There is growing evidence suggesting that targeted nutritional interventions may improve pain syndromes.8,9 Specifically, reversal of a high omega-6 (AA and LA) to omega-3 ratio is thought to mitigate pain syndromes by increasing circulating antinociceptive mediators and decreasing pronociceptive lipid derivatives.9 Our participants reporting neuropathic pain symptoms at baseline were found to have high plasma LA/AA to DHA ratios. However, after 3 months of omega-3 supplementation, LA/AA to DHA ratios had improved dramatically on average to < 0.7. This decrease in LA/AA to DHA ratios suggests a change in circulating mediators to an antinociceptive lipid-derivative profile. Taken together, both reversal of elevated sphingosine-induced lipotoxicity and high omega-6 to omega-3 ratio could be potential therapeutic targets for patients suffering from painful DN.
Omega-3 PUFAs are known to have anti-inflammatory effects through the production of both resolvins and protectins, which can improve neuropathic pain symptoms.43–46 Specifically, DHA is the substrate for D-series resolvins, which display potent anti-inflammatory actions.45 Supplementing the participants with high doses of DHA may increase circulating levels of 17S and 17R, D-series resolvins, that inhibit TNFα-induced IL1β transcription levels. IL1β is a proinflammatory cytokine implicated in the development of pain.46–49 Furthermore, protectins derived from DHA are also known to have potent protective activity in inflammatory and neural systems.50–52 Together, these lipid mediators may have played an important role in the improved neuropathic pain symptomatology of our participants. Our reporting of low analgesic usage by the neuropathic pain-symptom group supports similar findings that up to 39% of patients with painful DN had never received treatment for their painful symptoms.53 Additionally, not only is painful DN undertreated, it has been reported that painful DN is underdiagnosed.54 These findings are of significant importance, since early interventions can improve pain symptoms and may slow disease progression.
The present study has several limitations. It was not designed as a clinical trial; rather it was set up as an educational diabetes program that monitored the impact of omega-3 supplementation on type 2 diabetes outcomes, including neuropathic pain symptoms. Volunteers participated in an interactive and supportive environment with their peers, which may have introduced the Hawthorne or placebo effect regarding their reporting of pain. Eliminating this effect would require further studies in a randomized trial without the effects of the interactive education program. Also, a conclusive diagnosis of painful DN was not made in our participants, nor were all other potential causes of peripheral neuropathy ruled out. However, participants in our study reported neuropathic pain symptoms consistent with painful DN. For example, all participants reported pain symptoms that included several descriptors associated with neuropathic pain (eg, numbness, prickling, burning, and throbbing) in a bilateral radiating stocking distribution. We cannot completely rule out selection bias, which affects this cohort of participants in terms of both eligibility and selection criteria. We addressed this potential issue by presenting quantitative and qualitative analysis of paired data. By assigning participants as their own controls and measuring changes from their individual baseline values, we diminish many of these extraneous sources of variation, as well as account for the effect of participating in our diabetes-intervention program. However, findings from this study need to be followed up by a large, randomized, double-blind, controlled trial to exclude categorically any hidden placebo effect.
In summary, these findings provide preliminary evidence that a health-education diabetes program using omega-3 PUFA supplementation may be a potential complementary approach to address the lipotoxicity-driven sphingolipid-metabolism dysregulation associated with DN. We found a robust improvement in neuropathic pain symptoms that was significantly associated with increased DHA levels in participants’ plasma. Furthermore, key metabolites associated with neuropathic pain, specifically sphingosine, may be susceptible to regulation by omega-3 supplementation. Since omega-3 FA supplementation is known to be safe in patients with type 2 diabetes, future studies are warranted to define its role further in the possible improvement of painful DN symptoms.55
Conclusion
The major findings of this study are as follows. Omega-3 PUFA supplementation is associated with a significant reduction in self-reported neuropathic pain symptoms on the SF-MPQ in Mexican-Americans diagnosed with type 2 diabetes. This reduction in neuropathic pain symptoms was significantly correlated with an increase in plasma DHA levels for all participants with neuropathic pain symptoms. Reduction in neuropathic pain symptoms was not associated with significant changes in such clinical values as BMI, LDL, HDL, total cholesterol, triglycerides, HbA1c, or fasting blood glucose. Untargeted metabolomic and RF analysis showed that sphingosine levels in participants with highest SF-MPQ sensory scores presupplementation were significantly elevated. Following omega-3 supplementation, sphingosine levels were markedly reduced and approached those of nonpain participants.
Acknowledgments
The authors thank all investigators, study teams, and volunteers for participating in this study. This study was funded by NIH grants 5P20MD006988 and 5R25GM060507. The content of this article is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health.
The authors report no conflicts of interest in this work.
References
Boulton AJ. Treatment of symptomatic diabetic neuropathy. Diabetes Metab Res Rev. 2003;19(Suppl 1):S16–S21. | ||
Spallone V, Lacerenza M, Rossi A, Sicuteri R, Marchettini P. Painful diabetic polyneuropathy: approach to diagnosis and management. Clin J Pain. 2012;28(8):726–743. | ||
Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14(9):528. | ||
Calles-Escandón J, Lovato LC, Simons-Morton DG, et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(4):721–727. | ||
Callaghan BC, Kerber K, Smith AL, Fendrick AM, Feldman EL. The evaluation of distal symmetric polyneuropathy: a physician survey of clinical practice. Arch Neurol. 2012;69(3):339–345. | ||
Davis TM, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2008;51(4):562–566. | ||
Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. | ||
Tick H. Nutrition and pain. Phys Med Rehabil Clin N Am. 2015;26(2):309–320. | ||
Ramsden CE, Ringel A, Majchrzak-Hong SF, et al. Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids. Mol Pain. 2016;12:174480691663638. | ||
Coste TC, Gerbi A, Vague P, Pieroni G, Raccah D. Neuroprotective effect of docosahexaenoic acid-enriched phospholipids in experimental diabetic neuropathy. Diabetes. 2003;52(10):2578–2585. | ||
Figueroa JD, Cordero K, Serrano-Illan M, et al. Metabolomics uncovers dietary omega-3 fatty acid-derived metabolites implicated in anti-nociceptive responses after experimental spinal cord injury. Neuroscience. 2013;255:1–18. | ||
Huang CT, Tsai YJ. Docosahexaenoic acid confers analgesic effects after median nerve injury via inhibition of c-Jun N-terminal kinase activation in microglia. J Nutr Biochem. 2016;29:97–106. | ||
Almaguel FG, Liu JW, Pacheco FJ, De Leon D, Casiano CA, De Leon M. Lipotoxicity-mediated cell dysfunction and death involve lysosomal membrane permeabilization and cathepsin L activity. Brain Res. 2010;1318:133–143. | ||
Descorbeth M, Figueroa K, Serrano-Illán M, De León M. Protective effect of docosahexaenoic acid on lipotoxicity-mediated cell death in Schwann cells: Implication of PI3K/AKT and mTORC2 pathways. Brain Behav. 2018;8(11):e01123. | ||
Sloan G, Shillo P, Selvarajah D, et al. A new look at painful diabetic neuropathy. Diabetes Res Clin Pract. 2018;144:177–191. | ||
Tesfaye S, Kempler P. Painful diabetic neuropathy. Diabetologia. 2005;48(5):805–807. | ||
Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol. 2003;65:333–347. | ||
Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. | ||
Almaguel FG, Liu JW, Pacheco FJ, Casiano CA, De Leon M. Activation and reversal of lipotoxicity in PC12 and rat cortical cells following exposure to palmitic acid. J Neurosci Res. 2009;87(5):1207–1218. | ||
Ulloth JE, Casiano CA, De Leon M. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J Neurochem. 2003;84(4):655–668. | ||
Feldman EL, Nave KA, Jensen TS, Bennett DLH. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron. 2017;93(6):1296–1313. | ||
Fernyhough P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep. 2015;15(11):89. | ||
Dohrn MF, Othman A, Hirshman SK, et al. Elevation of plasma 1-deoxy-sphingolipids in type 2 diabetes mellitus: a susceptibility to neuropathy? Eur J Neurol. 2015;22(5):806–814, e55. | ||
Alecu I, Tedeschi A, Behler N, et al. Localization of 1-deoxysphingolipids to mitochondria induces mitochondrial dysfunction. J Lipid Res. 2017;58(1):42–59. | ||
Hube L, Dohrn MF, Karsai G, et al. Metabolic Syndrome, Neurotoxic 1-Deoxysphingolipids and Nervous Tissue Inflammation in Chronic Idiopathic Axonal Polyneuropathy (CIAP). PLoS One. 2017;12(1):e0170583. | ||
Salto LM, Cordero-MacIntyre Z, Beeson L, Schulz E, Firek A, De Leon M. En Balance participants decrease dietary fat and cholesterol intake as part of a culturally sensitive Hispanic diabetes education program. Diabetes Educ. 2011;37(2):239–253. | ||
Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. | ||
Figueroa JD, Cordero K, Llán MS, De Leon M. Dietary omega-3 polyunsaturated fatty acids improve the neurolipidome and restore the DHA status while promoting functional recovery after experimental spinal cord injury. J Neurotrauma. 2013;30(10):853–868. | ||
Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform. 2010;2(1):9. | ||
Breiman L. Random Forests. Mach Learn. 2001;45(1):5–32. | ||
Gromski PS, Muhamadali H, Ellis DI, et al. A tutorial review: metabolomics and partial least squares-discriminant analysis – a marriage of convenience or a shotgun wedding. Anal Chim Acta. 2015;879:10–23. | ||
Escalante A, Lichtenstein MJ, Ríos N, Hazuda HP. Measuring chronic rheumatic pain in Mexican Americans: cross-cultural adaptation of the McGill Pain Questionnaire. J Clin Epidemiol. 1996;49(12):1389–1399. | ||
Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456–2465. | ||
Chong J, Soufan O, Li C, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46(W1):W486–W494. | ||
Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35(3):206–213. | ||
Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220–2224. | ||
Satoh J, Yagihashi S, Baba M, Suzuki M, Arakawa A, Yoshiyama T. Efficacy and safety evaluation of pregabalin treatment over 52 weeks in patients with diabetic neuropathic pain extended after a double-blind placebo-controlled trial. J Diabetes Investig. 2011;2(6):457–463. | ||
Ikeda I, Cha JY, Yanagita T, et al. Effects of dietary alpha-linolenic, eicosapentaenoic and docosahexaenoic acids on hepatic lipogenesis and beta-oxidation in rats. Biosci Biotechnol Biochem. 1998;62(4):675–680. | ||
Padilla A, Descorbeth M, Almeyda AL, Payne K, de Leon M. Hyperglycemia magnifies Schwann cell dysfunction and cell death triggered by PA-induced lipotoxicity. Brain Res. 2011;1370:64–79. | ||
Rodriguez-Cuenca S, Pellegrinelli V, Campbell M, Oresic M, Vidal-Puig A. Sphingolipids and glycerophospholipids - the “ying and yang” of lipotoxicity in metabolic diseases. Prog Lipid Res. 2017;66:14–29. | ||
Miranda GE, Abrahan CE, Politi LE, Rotstein NP. Sphingosine-1-phosphate is a key regulator of proliferation and differentiation in retina photoreceptors. Invest Ophthalmol Vis Sci. 2009;50(9):4416–4428. | ||
Rotstein NP, Miranda GE, Abrahan CE, German OL. Regulating survival and development in the retina: key roles for simple sphingolipids. J Lipid Res. 2010;51(6):1247–1262. | ||
Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. | ||
Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197–1204. | ||
Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196(8):1025–1037. | ||
Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278(17):14677–14687. | ||
Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60(1):57–64. | ||
Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115(7):1265–1275. | ||
Zelenka M, Schäfers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116(3):257–263. | ||
Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278(44):43807–43817. | ||
Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115(10):2774–2783. | ||
Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101(22):8491–8496. | ||
Daousi C, Macfarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21(9):976–982. | ||
Sadosky A, Hopper J, Parsons B. Painful diabetic peripheral neuropathy: results of a survey characterizing the perspectives and misperceptions of patients and healthcare practitioners. Patient. 2014;7(1):107–114. | ||
Hartweg J, Perera R, Montori V, Dinneen S, Neil HA, Farmer A. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;(1):CD003205. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.