Back to Journals » Cancer Management and Research » Volume 16
Effects of Metformin on JNK Signaling Pathway and PD-L1 Expression in Triple Negative Breast Cancer
Received 14 December 2023
Accepted for publication 27 March 2024
Published 2 April 2024 Volume 2024:16 Pages 259—268
DOI https://doi.org/10.2147/CMAR.S454960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Ruibin Wang,1,* Yanjie Zhao2,*
1Department of Emergency, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Medical Oncology, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanjie Zhao, Beijing Shijitan Hospital, Capital Medical University, Tieyi Road 10, Haidian District, Beijing, 100038, People’s Republic of China, Tel/Fax +86-10-63926299, Email [email protected]
Background: Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer. Metformin has been shown to have the potential to inhibit the proliferation of malignant cells. This study aimed to investigate the regulatory effect of metformin on the expression of programmed death protein ligand 1(PD-L1) and mechanisms in TNBC.
Methods: Mouse breast cancer cell line 4T1 was co-cultured with metformin, and the effect of metformin on cell proliferation was detected by MTT assay. The effect of metformin on the expression of JNK, RSK2 and CREB was detected by MAPK pathway protein chip. BALB/c mice were inoculated with 4T1 cells with knockdown/overexpression of C-Jun N-terminal kinase (JNK), and administered with metformin. The weight of tumor tissue was observed at the end of the experiment. The expression of PD-L1 in tumor cells was observed by immunofluorescence staining and the level of INF-γwas quantitatively determined by ELISA.
Results: Metformin inhibited the viability of 4T1 cells and increased the phosphorylation of JNK to reduce the phosphorylation of RSK2 and CREB. Metformin and JNK knockdown reduced the expression of PD-L1 in tumor cells, but there was no significant difference in the weight of tumor tissue. Metformin can reduce the level of INF-γ in tumor tissues, but JNK has no effect.
Conclusion: Metformin can inhibit the expression of PD-L1 in triple-negative breast cancer mice and improve the tumor microenvironment, but does not reduce the size of the tumor.
Keywords: triple negative breast cancer, metformin, cell programmed death ligand 1, C-Jun amino-terminal kinase
Introduction
Breast cancer (BC) has become the most common malignant tumor in women in the world, accounting for 30% of new malignant cases and 15% of deaths in women.1 Chinese BC patients have special clinicopathological features, namely low expression of hormone receptor and high expression of human epidermal growth factor receptor 2 (HER2).2 Triple-negative breast cancer (TNBC) is characterized by negative expression of estrogen receptor (ER), progesterone receptor (PR), and HER2. It is a more aggressive subtype of breast cancer with the worst prognosis.3 TNBC in China accounts for approximately 15% to 20% of all breast cancer pathologies.3 Tumor immune microenvironment is closely related to the prognosis, recurrence and metastasis of breast cancer.4 Programmed death protein ligand 1(PD-L1) is a negative costimulatory molecule, which is highly expressed on the surface of tumor cells. It binds to its receptor Programmed cell death-1 (PD-1) to inhibit the activation of T lymphocytes and enable tumor cells to escape from the body’s immune killing.5 The expression of PD-L1 in TNBC patients was significantly higher than that in non-TNBC patients (59% vs 33%), suggesting that the immune microenvironment of TNBC is inhibited.6 Metformin is one of the commonly used oral hypoglycemic drugs in clinical practice. Recent studies have found that metformin can reduce the recurrence of cancer patients and improve the survival rate of cancer patients.7 The anti-tumor mechanism of metformin may be related to its regulation of the immune system. Metformin reduced the expression of PD-L1 in endometrial cancer cell lines,8 and promote the activation and proliferation of T cells through JNK signaling pathway.9 C-Jun N-terminal kinase (JNK) is an important component of the mitogen-activated protein kinase (MAPK) signaling pathway, which participates in a variety of biological reactions such as cell proliferation, differentiation, and apoptosis.10 At present, there are few studies on the relationship between JNK signaling pathway and PD-L1. This study investigated whether metformin can affect the expression of PD-L1 in TNBC cells through the JNK signaling pathway through mouse breast cancer cells and animal models.
Methods
Ethics Approval
Animal experiments were approved by the Medical Ethics Committee of Beijing Shijitan Hospital, Capital Medical University and were carried out in accordance with the Animal Welfare Guidelines of the Medical Ethics Committee of Beijing Shijitan Hospital, Capital Medical University, in line with the guidelines for the care and use of laboratory animals by the Chinese Institute of Health, and in compliance with all regulatory guidelines.
Animal Administration
Thirty SPF BALB/c mice (Cavens Experimental Animal Co. Ltd.), aged 6 weeks, female, weighing 26–28 g. Animal production license number: SCXK (Su) 2021-0016, animal use license number: SYSK (Beijing) 2021-0025. The mouse model establishment and material sampling were carried out in the Animal Experiment Building of Beijing Shijitan Hospital affiliated to Capital Medical University. All procedures in animal experiments are in accordance with the 3R principle and have been approved by the Scientific Research Ethics Committee of Beijing Shijitan Hospital, Capital Medical University (sjtky11-lx-2021-100). The mice were divided into metformin administration group and non-administration group with 15 mice in each group. Wild-type 4T1 cells, JNK knockdown 4T1 cells, and JNK overexpression 4T1 cells were inoculated subcutaneously into the abdomen of 5 mice in each group, respectively. The rats in the metformin group were given metformin (500mg/kg·d) by gavage for 4 weeks. When tumors reached 1cm in diameter, all mice were anesthetized, and tumors were aseptically removed and fixed in formalin.
4T1 Cell Line Culture
Mouse breast cancer cell line 4T1 (Nanjing Kebai Biotechnology Co., LTD.). The frozen 4T1 cell lines were removed in liquid nitrogen, thawed in a water bath at 37°C, added to a centrifuge tube with 4–6 mL complete medium (DMEM+ 10% FBS+ 1% P/S), mixed, centrifuged at 1000RPM for 3min, and the cells were resuspended in complete medium after abandoning the supernatant. The cells were added to culture flasks containing 6–8mL of complete medium and cultured overnight at 37 ° C, passaged once for 2–3 days, and passed to the third passage for experiments.
Reagents and Instruments
Metformin (batch number: 20,210,841) was purchased from Sino-American Shanghai Bristol-Myers Squibb Pharmaceutical Co., LTD. MAPK pathway phosphorylation array kit (item number: AAH-MAPK-1-2) was purchased from RayBiotech (USA). Fetal bovine serum, 1640 medium, EDTA, MTT, Trypsin, INF-γ detection kits (product numbers were A3161001, 10,564,011, 61,870,036, MA5405, BMS603HS and PMC4016, respectively) were purchased from Thermo Fisher Scientific Co., LTD. Rabbit anti-mouse PD-L1 antibody (product number: Ab213480), sheep anti-rabbit fluorescent antibody (product number: Ab150080) (Abcam Company, UK). Inverted microscope and imaging system (Nikon Ci-S, Nikon DS-U3, Nikon, Japan). JNK lentivirus overexpression and interference vector plasmid construction (Jiman Biotech).
Cell Viability Assays
Cell viability was determined by MTT assay. The density of 4T1 cells after mixing was diluted to 1x105/mL in a 96-well plate, 2.0mL per well, and the cells were cultured for 24h at 37 ° C in 5%CO2. The next day, every hole to join metformin concentration respectively (0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 20.0 mmol/l), 37 °C 5% CO2 for 24 h. An additional 20μL (5g/L) MTT was added to each well, and the reaction was terminated after 4h at 37 ° C. The supernatant was discarded, and 100μL DMSO was added to each well and allowed to stand for 15 min. The absorbance was measured at 570 nm to calculate the inhibition rate of cell proliferation.
MAPK Pathway Analysis
The effect of metformin on MAPK signaling pathway in 4T1 cells was determined by protein microarray (Raybiotech, Inc., Raybio C1). 4T1 cells were lysed post metformin treatment (10.0mmol/L). The membrane chip was incubated with diluted protein lysates followed by incubation with 1mL biotin labeled antibody. The membrane was then incubated with HRP-streptavidin. ImageQuant LAS4000 chemiluminescence imaging analysis system (General Electric Company) was used for scanning. The experiment was repeated twice. The scanned data were normalized by EXCEL formula, including subtracting background and averaging. The normalized data were analyzed using R language. The selection criteria of potential signal protein were set as upregulation more than 20.0% and downregulation more than 16.7%.
Knockdown/Overexpression of JNK in 4T1 Cells
Lentiviral-mediated JNK overexpression vector and shRNA vectors were obtained from GeneChem (Shanghai, China). Target sequences were shown in (Table 1). Cells were seeded at 70–80% confluency in medium and transfected with lentivirus and polybrene A reagent according to the manufacturer’s instructions. The empty vector served as a negative control. After 12h, the medium was removed and replaced with fresh culture medium. Three days later, green fluorescent protein gene expression was observed under a fluorescence microscope, and the experiment was repeated twice; then, the cells were collected for subsequent culture.
 |
Table 1 The Sequences of shRNA for JNK and PCR Primer |
Plasmid Transfection
Polybrene to 6μg/mL and appropriate amount of virus were added to 2×105/mL suspension cells, mixed thoroughly, and incubated at 37 ° C. After 4h, an equal volume of fresh medium was added to dilute polybrene. After 3 days of continuous culture, the total RNA of cells was extracted for qRT-PCR detection to verify the knockdown effect, and the cells were collected for subsequent culture.
PD-L1 Detection
PD-L1 markers were stained and visualized by immunofluorescence (IF) staining. Paraffin embedded tumor tissue sections with a thickness of 4μm were transferred to a roasting machine and baked for 4h at 60 ° C. After exfoliation, the slides were washed 3 times with PBS solution for 5min each time. After the second wash, the slides were slightly dried and the tissue area to be tested was marked with an immunohistochemical pen. After incubation with blocking solution for 2 hours, press 1: The primary antibody working solution was removed and rinsed 3 times with PBS solution. The secondary antibody working solution was prepared and added at a ratio of 1:200. The tablets were incubated at 37°C for 2 hours, thawed with DAPI, stained for 10min, washed 5 times with PBS solution, dried in an oven at 60°C, and sealed with anti-fluorescence quenching solution. Images were acquired with a Nikon inverted microscope at 100 × and 400 × field of view.
Detection of INF-γ
INF-γ levels were examined by ELISA Kit. Mouse breast cancer tissues were collected to prepare homogenate, which was treated with Polytron-type reagent and centrifuged, and the supernatant was taken for testing. The solid-phase antibody was prepared by coating the microplate with the purified antibody. The standard, the tested sample, biotinylated INF-γ and HRP-labeled avidin were added to the solid-phase antibody coated microwells at one time, and then the color was developed with the substrate TMB after thorough washing. TMB is converted to blue color by peroxidase catalysis and to the final yellow color by acid. There was a positive correlation between the depth of color and INF-γ in the sample. The sample concentration was calculated by measuring the absorbance (OD) at a wavelength of 450±2nm using a microplate reader.
qRT-PCR
Mouse Mapk8 gene shRNA vector and negative control vector were co-transfected into 4T1 cells with the overexpression vector, respectively. After 72 hours, the cells were collected and total RNA was extracted for qRT-PCR. After the cell culture medium was removed, the lysate was added, the cells were lysed by repeated suction, and then added to the DNA removal column. The supernatant was collected and added to the buffer, then transferred to the RNA extraction column, and the RNA solution was obtained after washing and purification. Three repeat Wells were made when qRT-PCR was loaded on the machine. The reaction conditions for cDNA synthesis were 37 ° C for 15min→85 ° C for 5s→4 ° C for termination. The PCR reaction conditions were as follows: (95 °C for 30 min→95 °C for 5 s→60 °C for 31s) ×40 cycles. The 2-ΔΔCt analysis method was used to calculate the relative expression of each sample based on the reference gene.
Statistical Analysis
GraphPad Prism 7.0 software was used for statistical analysis. All data were presented as median and interquartile range (IQR). Differences between groups were estimated using the Kruskal–Wallis test. Wilcoxon test and Bonferroni adjustment were used for pairwise comparisons. All tests were two-sided, and p<0.05 was considered statistically significant.
Results
Metformin Inhibited the Viability of 4T1 Cells
Compared with the control group, metformin at low concentrations (0.1–5.0 mmol/L) had no significant effect on the viability of 4T1 cells (Figure 1), while metformin at high concentrations (10.0 mmol/L and 20.0 mmol/L) significantly inhibited the viability of 4T1 cells in a concentration-dependent manner (Figure 1). The IC50 of the drug was 15mmol/l.
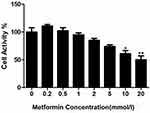 |
Figure 1 The effects of metformin on 4T1 cell viability, *P<0.05, **P<0.01. |
Effect of Metformin on the MAPK Pathway in 4T1 Cells
Metformin increased the expression of JNK from 2462.0 (IQR=265.02) to 2991.7 (IQR=264.86), an increase of 21.4% (p<0.05, Figure 2A–D). The expression of RSK2 and CREB decreased from 1111.5 (IQR=233.16) and 2397.0 (IQR= 252.06) to 923.5 (IQR=234.45) and 1867.7 (IQR=250.05), respectively (p<0.05, Figure 2A–D).
Quantitative Values and Analysis of MAPK8 Gene Knockdown Effect
Compared with the 4T1 cells transfected with the negative control vector (Table 2), MAPK8 mRNA expression level in the shRNA1 vector transfected 4T1 cells was 52% of the negative control group, that is, the MAPK8 gene was knocked down by 48%; The mRNA level of MAPK8 gene in shRNA2 vector transfected group was 31% of that in negative control group, that is, the MAPK8 gene was knocked down by 69%. The mRNA level of MAPK8 gene transfected with shRNA3 vector was 90% of that of the negative control, that is, the MAPK8 gene was knocked down by 10%. The expression of MAPK8 gene in shRNA2 vector was inhibited more significantly than that in the control group, and was selected as the interference sequence for the mouse triple-negative breast cancer model.
 |
Table 2 Presents the Quantitative Assessment and Analysis of the Impact of MAPK8 Gene Knockdown |
No Change of the Weight of Xenograft Tumors in Mice
Metformin did not affect the weight of xenograft tumors in mice between groups (p>0.05, Figure 3).
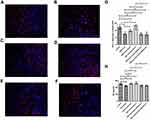
Effects of Metformin and JNK Pathway on PD-L1 Expression and INF-γ
Compared with the control group, JNK knockdown group and JNK overexpression group, the expression of PD-L1 in the metformin group was decreased (p<0.05, Figure 4). The expression of PD-L1 was decreased in the JNK knockdown group compared with the control group (p<0.05, Figure 4). Compared with the control group, the expression of PD-L1 in the JNK knockdown + metformin group and the JNK overexpression+metformin group was decreased (p<0.05, Figure 4), but there was no significant difference between the JNK knockdown+metformin group and the metformin group (p>0.05, Figure 4). The level of INF-γ in tumor tissues in the metformin group was lower than that in the control group and in the JNK knockdown and JNK overexpression groups, respectively (p<0.05, Figure 4). The levels of INF-γ in tumor tissues were significantly lower in the JNK knockdown+metformin group and the JNK overexpression + metformin group than in the control group and the JNK knockdown and JNK overexpression groups, respectively (p<0.05, Figure 4). However, there was no significant difference between the metformin group and the JNK knockdown+metformin group or the JNK overexpression+ metformin group (p>0.05, Figure 4).
Discussion
TNBC is a subtype with poor immune microenvironment among all breast cancer molecular phenotypes.11 Both metformin and JNK pathway have regulatory effects on the immune microenvironment of triple-negative breast cancer. As a first-line drug for the treatment of diabetes, metformin has good efficacy and high safety,10 and as a candidate drug for the prevention and treatment of tumors, it also plays a role in affecting the effect of immunotherapy.12 The results of the present study also confirmed that high concentrations of metformin could significantly inhibit the activity of mouse breast cancer cells in vitro. The effect of metformin on the immune microenvironment is mainly through AMP-activated protein kinase (AMPK) signaling pathway and mitogen-activated protein kinase (MAPK) signaling pathway. Our previous study demonstrated that metformin increased the functional phenotype of TILs and associated with JNK pathway, and suppressed the exhausted phenotype of TILs independently to JNK pathway in TNBC microenvironment.13 Other studies have confirmed that MAPK signaling pathway could also improve the tumor microenvironment by regulating intestinal flora.14 MAPK signaling pathway is one of the important pathways involved in the regulation of innate immunity.15 In the present study, it was found that metformin increased the phosphorylation of JNK, but decreased the phosphorylation of RSK2 and CREB. JNK is a component of the MAPK signaling pathway that inhibits malignant cell differentiation and induces apoptosis.16 CREB is frequently overexpressed in hematopoietic and solid tumors.17 CREB activation is closely associated with tumor growth, proliferation, increased angiogenesis and metabolism, and apoptosis.17 ERK/MAPK/RSK signaling pathway is activated by the oncogenic effects of EGFR, Ras and BRAF in a variety of tumors.18 Overactive RSK signaling has been shown to induce cell transformation and promote tumor growth.19 However, no study has confirmed the correlation between the phosphorylation levels of CREB and RSK and the tumor immune microenvironment. The anti-proliferative effect of metformin may also be related to the down-regulation of CERB and RSK2. However, probably due to the short study time, this study did not find significant differences in tumor tissue tumors in each group of mice.
The activation of PD-1/PD-L1 pathway is one of the main mechanisms of tumor immune escape. In solid tumors, tumor cells can silence the immune system by increasing PD-L1 expression on the cell surface.19 PD-L1 increases tumorigenesis and invasion and makes tumor cells less sensitive to specific CD8+T cells.20 PD-L1, as a ligand of the checkpoint, can deliver negative signals to T cells through its inherent reverse signaling upon binding to PD-1, leading to dysfunction and ultimately apoptosis. Constitutive expression of PD-L1 in tumor cells is caused by dysregulation of oncogenic or tumor suppressor gene signaling pathways, activation of aberrant transcription factors, genomic aberrations, or gene amplification.21 The regulation of PD-L1 is very complex. The Ras/MAPK and PI3K-AKt signaling pathways can be activated by the epidermal growth factor (EGFR) receptor, and then the signal is transmitted to the nucleus by transcription factors such as nuclear activator protein 1 and nuclear factor κB(NF-κB) to increase the transcription level of PD-L1.22 In addition to EGFR, interferon -γ(IFN-γ) can also directly affect PD-L1 expression.23 IFN-γ, produced by activated T cells and NK cells infiltrating the tumor, is one of the inflammatory cytokines often used as an inducer to study PD-L1.24,25 This study confirmed that JNK knockdown in mouse 4T1 cells reduced PD-L1 expression, similar to a recent study that used JNK inhibitor to reduce PD-L1 expression in renal cancer cells.26 The present study also confirmed that metformin reduced PD-L1 expression in mouse 4T1 cells regardless of the effect of JNK expression and that metformin reduced IFN-γ content in mouse tumor tissues. This also explains the fact that although the present study found that metformin increased JNK phosphorylation, it decreased PD-L1 expression. Because IFN-γ also plays an important role in the regulation of PD-L1 expression. IFN-γ up-regulated the expression of PD-L1 in tumor cells through the activator of JAK-STAT signal transduction and transcription pathway.27 Metformin inhibits the secretion of cytokine-induced pro-inflammatory cytokines (TNF-α, IFN-γ and IL-6) by activating AMP-activated protein kinase (AMPK) and then inhibiting the activity of NF-κB. Therefore, the inhibitory effect of metformin on INF-γ in mouse breast cancer tissues may lead to the reduction of PD-L1 expression. Although metformin did not significantly inhibit the tumor growth in our mouse model, clinical studies have shown that long-term treatment with metformin can improve the survival rate of diabetic breast cancer patients.28
In conclusion, metformin increased the phosphorylation of JNK in mouse 4T1 cells, inhibited the expression of PD-L1, and improved the tumor microenvironment in triple-negative breast cancer mice, but did not reduce tumor size. The results of this study provide an adjuvant treatment option for triple-negative breast cancer.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Funding
This study was financially supported by Beijing Hospital Scientific Research Training Program (PX2020032). Open project of Beijing Key Laboratory of Tumor Therapeutic Vaccine (2019-KF04). The supporting organizations had no role in study design, data collection, analysis and interpretation.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Zheng S, Bai JQ, Li J, et al. The pathologic characteristics of breast cancer in China and its shift during 1999–2008: a national-wide multicenter cross-sectional image over 10 years. Int J Cancer. 2012;131(11):2622–2631. doi:10.1002/ijc.27513
3. Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: a population-based study. Int J Cancer. 2015;136(8):1921–1930. doi:10.1002/ijc.29227
4. Verma R, Hanby AM, Horgan K, et al. Levels of different subtypes of tumour-infiltrating lymphocytes correlate with each other, with matched circulating lymphocytes, and with survival in breast cancer. Breast Cancer Res Treat. 2020;183(1):49–59. doi:10.1007/s10549-020-05757-5
5. Su Z, Dhusia K, Wu Y, Wei G. A computational study of co-inhibitory immune complex assembly at the interface between T cells and antigen presenting cells. PLoS Comput Biol. 2021;17(3):e1008825. doi:10.1371/journal.pcbi.1008825
6. Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965–2970. doi:10.1158/1055-9965.EPI-14-0654
7. Mallik R, Chowdhury TA. Metformin in cancer. Diabet Res Clin Pract. 2018;143:409–419. doi:10.1016/j.diabres.2018.05.023
8. Xue J, Li L, Li N, et al. Metformin suppresses cancer cell growth in endometrial carcinoma by inhibiting PD-L1. Eur J Pharmacol. 2019;859:172541. doi:10.1016/j.ejphar.2019.172541
9. Blonska M, Lin X. CARMA1-mediated NF-kappaB and JNK activation in lymphocytes. Immunol Rev. 2009;228(1):199–211. doi:10.1111/j.1600-065X.2008.00749.x
10. Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15(10):569–589. doi:10.1038/s41574-019-0242-2
11. Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4(1):59. doi:10.1186/s40425-016-0165-6
12. Cha JH, Yang WH, Xia W, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell. 2018;71(4):606–620.e7. doi:10.1016/j.molcel.2018.07.030
13. Wang R, Li Y, Zhao Y, et al. Metformin inducing the change of functional and exhausted phenotypic tumor-infiltrated lymphocytes and the correlation with JNK signal pathway in triple-negative breast cancer. Breast Cancer. 2022;14:391–403. doi:10.2147/BCTT.S384702
14. Pollak M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia. 2017;60(9):1662–1667. doi:10.1007/s00125-017-4352-x
15. Xiao Y, Liu F, Li S, et al. Metformin promotes innate immunity through a conserved PMK-1/p38 MAPK pathway. Virulence. 2020;11(1):39–48. doi:10.1080/21505594.2019.1706305
16. Hsieh SC, Tsai JP, Yang SF, Tang MJ, Hsieh YH. Metformin inhibits the invasion of human hepatocellular carcinoma cells and enhances the chemosensitivity to sorafenib through a downregulation of the ERK/JNK-mediated NF-κB-dependent pathway that reduces uPA and MMP-9 expression. Amino Acids. 2014;46(12):2809–2822. doi:10.1007/s00726-014-1838-4
17. Steven A, Friedrich M, Jank P, et al. What turns CREB on? And off? And why does it matter. Cell Mol Life Sci. 2020;77(20):4049–4067. doi:10.1007/s00018-020-03525-8
18. Lucien F, Brochu-Gaudreau K, Arsenault D, et al. Hypoxia-induced invadopodia formation involves activation of NHE-1 by the p90 ribosomal S6 kinase (p90RSK). PLoS One. 2011;6(12):e28851. doi:10.1371/journal.pone.0028851
19. Zielinski C, Knapp S, Mascaux C, et al. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann Oncol. 2013;24(5):1170–1179. doi:10.1093/annonc/mds647
20. Mariotti FR, Quatrini L, Munari E, et al. Innate lymphoid cells: expression of PD-1 and other checkpoints in normal and pathological conditions. Front Immunol. 2019;10:910. doi:10.3389/fimmu.2019.00910
21. Ju X, Zhang H, Zhou Z, et al. Regulation of PD-L1 expression in cancer and clinical implications in immunotherapy. Am J Cancer Res. 2020;10(1):1–11.
22. Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910–923. doi:10.1097/JTO.0000000000000500
23. Moon JW, Kong SK, Kim BS, et al. IFNγ induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci Rep. 2017;7(1):17810. doi:10.1038/s41598-017-18132-0
24. Manguso RT, Pope HW, Zimmer MD, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547(7664):413–418. doi:10.1038/nature23270
25. Wei Y, Zhao Q, Gao Z, et al. The local immune landscape determines tumor PD-L1 heterogeneity and sensitivity to therapy. J Clin Invest. 2019;129(8):3347–3360. doi:10.1172/JCI127726
26. Ma G, Liang Y, Chen Y, et al. Glutamine deprivation induces PD-L1 expression via activation of EGFR/ERK/c-jun signaling in renal cancer. Mol Cancer Res. 2020;18(2):324–339. doi:10.1158/1541-7786.MCR-19-0517
27. Mimura K, Teh JL, Okayama H, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109(1):43–53. doi:10.1111/cas.13424
28. El-Benhawy SA, El-Sheredy HG. Metformin and survival in diabetic patients with breast cancer. J Egypt Public Health Assoc. 2014;89(3):148–153. doi:10.1097/01.EPX.0000456620.00173.c0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.