Back to Journals » Clinical Interventions in Aging » Volume 17
Effects of Immersive Virtual Therapy as a Method Supporting Recovery of Depressive Symptoms in Post-Stroke Rehabilitation: Randomized Controlled Trial
Authors Kiper P , Przysiężna E, Cieślik B , Broniec-Siekaniec K, Kucińska A, Szczygieł J, Turek K, Gajda R, Szczepańska-Gieracha J
Received 23 May 2022
Accepted for publication 25 October 2022
Published 23 November 2022 Volume 2022:17 Pages 1673—1685
DOI https://doi.org/10.2147/CIA.S375754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Pawel Kiper,1 Ewa Przysiężna,2 Błażej Cieślik,1 Katarzyna Broniec-Siekaniec,3 Aleksandra Kucińska,3 Jarosław Szczygieł,4,5 Katarzyna Turek,6 Robert Gajda,7,8 Joanna Szczepańska-Gieracha2
1Healthcare Innovation Technology Lab, IRCCS San Camillo Hospital, Venezia, Italy; 2Faculty of Physiotherapy, University School of Physical Education in Wroclaw, Wroclaw, Poland; 3“REPTY” Upper Silesian Rehabilitation Centre, Tarnowskie Góry, Poland; 4Humanitas University in Sosnowiec, Sosnowiec, Poland; 5Neurorehabilitation Department, District Hospital, Zawiercie, Poland; 6The Karol Lipiński Academy of Music in Wroclaw, Wrocław, Poland; 7Gajda-Med District Hospital in Pultusk, Pułtusk, Poland; 8Department of Kinesiology and Health Prevention, Jan Dlugosz University in Częstochowa, Częstochowa, Poland
Correspondence: Pawel Kiper, IRCCS San Camillo Hospital, Alberoni 70, Venezia, 30126, Italy, Tel +390412207157, Email [email protected]
Purpose: Depressive symptoms constitute an important group of mental problems that alter the course of post-stroke rehabilitation by reducing quality of life, physical activity, social functioning, and interpersonal relationships. Although several studies have shown the efficacy of virtual reality (VR) in the motor treatment of poststroke patients, there is a lack of studies that would also evaluate the impact of VR on psychological aspects. Thus, we investigated the effectiveness of immersive VR therapy on both functional activity and depressive symptoms in stroke survivors.
Patients and Methods: We conducted a single blind, randomized controlled trial comparing VR therapy with Schultz’s Autogenic Training (SAT). Patients randomized to the VR group received treatment in an immersive VR therapeutic garden with elements of psychotherapy and physical activity of the upper extremities, whereas patients in the control group received SAT. Additionally, patients in both groups received standard neurological rehabilitation. The full research cycle lasted six weeks. We used Geriatric Depression Scale, Generalized Self-Efficacy Scale, Acceptance of Illness Scale, Visual Analogue Scale of pain, Hospital Anxiety and Depression Scale, Barthel Index, Lawton Instrumental Activities of Daily Living Scale and Rivermead Motor Assessment for outcome assessment. This trial was registered with ClinicalTrials.gov (NCT03830372).
Results: We assessed 60 patients and randomly assigned to the VR or control group. The VR group showed a significant reduction in depressive symptoms (ηp2 = 0.13, p < 0.01) compared to SAT. The applied VR therapy significantly increased the sense of self-efficacy and the level of acceptance of the illness; however, this effect was similar to that obtained with the standard intervention. We did not observe statistically significant changes in the functional parameters of post-stroke patients.
Conclusion: The use of VR therapy combined with neurological rehabilitation had a positive effect on improving mood and reducing depressive symptoms in post-stroke patients.
Keywords: stroke, depressive symptoms, virtual reality, mental health, neurorehabilitation
Introduction
One out of six stroke patients (ie, about five million people a year worldwide) suffer from permanent disability and up to 75% of them require assistance from others in daily activities.1,2 For this reason, post-stroke rehabilitation focuses mainly on motor improvement, not necessarily focusing on activities related to treating psychological aspects and behavioural disorders. It should be emphasized that stroke survivors must overcome difficulties related not only to their physical but also to their cognitive, behavioural, or psychological impairment. Therefore, an important aspect that can improve the health conditions of stroke patients is their level of psychological well-being. Post-stroke depression (PSD) is recognized as the most common neuropsychiatric complication following stroke. Its symptoms develop within three to six months after a stroke event and affect 20–65% of stroke patients.3 Depression adversely affects quality of life and daily activity, but more importantly it is also associated with increased mortality in this patient group.4 Furthermore, a high level of depression is a predictor of worse effects of rehabilitation.5 Participants with post-stroke depression have less independence in activities of daily living and motivation for rehabilitation.6–8 Furthermore, depression is associated with a worse functional outcome.9 To date, PSD has typically been treated with pharmacological and psychotherapeutic therapies. On the one hand, antidepressants generate side effects that can ultimately limit the patient’s ability to take them.10 On the other hand, access to psychotherapy can be limited.
In the meta-analysis by Hackett et al comprising 61 studies, the authors observed the incidence of depression in 33% of patients one year post stroke, decreasing to 25% within one to five years’ post stroke and to 23% five years after a stroke event.11 People with PSD have over three times the risk of death within ten years after stroke compared to those who did not suffer from depression after stroke.12 Other researchers have also highlighted the impact of mood disorders, as key factors, on the quality of life of people with disabilities.13 Recent studies confirm the link between depression and a significantly increased risk of death, lower post-stroke physical activity and a higher level of disability in stroke survivors.12,14,15 However, epidemiological studies of PSD are discrepant, and the lack of consistency may be due to certain methodological issues related to diagnosis, time of assessment and patient attitude.
Consequently, the last decade has seen an increase in the number of studies on the use of virtual reality (VR) therapy and the assessment of its efficacy in the rehabilitation of stroke survivors. The development of technology has contributed to the adoption of modern tools, including virtual reality, which improves opportunities for comprehensive rehabilitation of stroke patients. The various areas in which this technology has found useful application include reduction of anxiety;16 treatment of post-traumatic stress disorder, depression and paranoid delusions;17,18 reduction of discomfort in cancer patients undergoing chemotherapy;19,20 reduction of acute pain during wound treatment and physical therapy in patients with burn injuries;21 functional ability training and motor rehabilitation in patients with central nervous system dysfunctions (including stroke patients);22 and rehabilitation of cognitive and linguistic functions.23–25 The authors of the above-cited studies emphasize the lack of VR-based technological solutions that, in addition to the physical rehabilitation of stroke patients, would offer therapeutic tools that could alleviate mental disorders and improve the mood and motivation of patients.26
Therefore, the aim of this study was to assess the effectiveness of added immersive VR intervention to conventional rehabilitation on functional activity and mental state of stroke survivors. Thus, we evaluated the effect of VR therapy on the severity of post-stroke depressive symptoms, acceptance of illness and the sense of self-efficacy. We also analysed the efficacy of VR rehabilitation on the patient’s functional state, activities of daily living and motor functions.
Materials and Methods
Study Design
The study design was set as a randomized controlled trial with a blinded outcome assessor. The study was carried out in the Upper-Silesian Rehabilitation Centre (REPTY) at Tarnowskie Góry, Poland. The design followed recommendations for the third phase (VR3) of VR clinical trials in health care, focusing on the effectiveness of the proposed treatment modality with respect to the control group.27 This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study protocol was reviewed and approved (Ref. No. 30/2017) by the Institutional Review Board of the University School of Physical Education in Wroclaw (Poland) and registered in the ClinicalTrials.gov repository (NCT03830372). All participants gave their informed written consent to participate in the study.
Participants
After an initial eligibility assessment, 60 participants were included in the study as shown in Figure 1. The inclusion criteria were 55–75 years of age, a history of ischaemic stroke and a 30-item Geriatric Depression Scale (GDS-30) score of ≥10. The age range was restricted due to differences in mental state of post-stroke survivors as it differs between young adults and the elderly. Young adults (25–54), on average, could have significantly higher scores of depressive symptoms.28,29 On the other hand, older people, due to less exposure to new technologies, may have a different reaction to virtual reality. The exclusion criteria were epilepsy; vertigo; a Mini-Mental State Examination (MMSE) score of <24, aphasia and a serious loss of sight or hearing that made it impossible to assess cognitive functions based on the MMSE; refusal to participate; intellectual disabilities and disturbances of consciousness. All participants gave informed written consent to participate in the study.
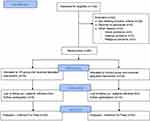 |
Figure 1 Flow diagram of the study procedure. |
Randomization
Participants were randomly assigned (1:1) into two groups using the block randomization method: 30 individuals were included in the experimental group (VR group) and 30 served as the control group. The randomization sequence was created using a computer software program. The allocation sequence was concealed from the principal investigator, enrolling patients in sequentially numbered, opaque, sealed envelopes. The researcher responsible for randomization was independent from the assessors, ensuring blindness to treatment allocation and randomization procedures. Due to the treatment modality, it was not possible to blind patients with respect to the treatment received.
Interventions
For each patient, the entire research cycle took six weeks as shown in Figure 2. The first 3 weeks represent the period in which the patient underwent functional rehabilitation combined with VR intervention (or Schultz’s Autogenic Training in the control group). This is the standard length of neurological rehabilitation in Polish hospitals. After this time, the outcome evaluation was performed (T1). Subsequently, an additional 3 weeks of rehabilitation were offered for a total of 6 weeks that included only functional rehabilitation. Subsequently, the follow-up assessment was performed (T2). In both groups, upper and lower limb exercises were performed in the same manner to maintain comparable exercise content in both treatments. However, each training program was personalized to the individual patient’s motor capacity, with a progressive increase in complexity. The patients were treated to restore functionality of the upper and lower limbs. In depth, patients in both groups performed 30 min of aerobic training, 30 min of balance exercises and 60 min of individual rehabilitation (according to NDT Bobath concept and proprioceptive neuromuscular facilitation). This protocol is the standard procedure for stroke rehabilitation at the Upper-Silesian Rehabilitation Center (REPTY).
Participants randomized to the VR group were treated for 20 min with the use of VR TierOne software, which represents a virtual therapeutic garden. VR TierOne consists of original software integrated with HTC VIVE PRO Goggles and a controller that allows for precise registration of the patient’s hand movements and is capable of haptic touch simulation. The controller was needed for the activity of colouring the mandala. If the upper limb mobility was not sufficient to complete movement task, the use of two hands was permitted to support the paretic upper limb. The therapeutic process used in the VR group, based on the assumptions of Erickson’s psychotherapy, relies mainly on metaphorical communication, and uses specific symbolism for this purpose hypnosis.30–32
It is assumed that the patient will see the high-walled garden of revival, which at the initial stage of therapy is weakened and colourless, as the symbol of the patient’s health, which at the beginning of therapy is weak and requires support as shown in Figure 3.
Due to the patient’s involvement in the garden cultivation, the virtual world begins to buzz with life and energy, symbolizing the treatment and rehabilitation process. The power of metaphorical communication lies in bypassing patient resistance, as therapy does not refer to the patient’s state of health or life situation directly, but shows an analogous process that unfolds before the eyes of the user. Engaging all the user’s senses and using hypnotic communication further increases the chances of success of VR therapy. During each session, in the central place of the virtual garden appears a mandala, which symbolizes various traits and emotions crucial to the process of treatment and rehabilitation (vitality, joy, optimism, diligence, creativity, inner wisdom and trust). Emotions associated with specific colours and specially selected music become an illustration of subsequent sessions, accompanying the patient as he or she becomes immersed deeper and deeper in the virtual world. Repeating the same motif during each session (ie, the activity of colouring the mandala, where the degree of difficulty, colours and metaphorical meaning vary but the action itself remains the same) creates a sense of security, as the patient always knows what to expect the next day. Such a pattern also reflects the process of rehabilitation, which is, by nature, repetitive and predictable. Thus, VR therapy strengthens the traits essential for successful rehabilitation, such as patience and perseverance in pursuing a goal. Cognitive and kinaesthetic engagement in performing the tasks of colouring subsequent mandalas deepens the sense of immersion in the virtual world. Separating the patient from stressful stimuli and transferring him or her to a virtual world filled with beautiful plants and sounds of nature relates to the patient’s previous positive experiences and makes it easier to put the patient into a state of psychophysical relaxation. The entire experience is complemented with natural, spatial sound of the wind, birds and calm, relaxing music. In addition to the friendly and soothing environment, messages spoken by the narrator play an important role in each session, as the patient is asked to pay attention to his or her breathing and to gently slow down its rhythm. It is worth noting that the therapy is not meant solely as an escape from stressful stimuli but as a means for patients to recover mental balance, tap into their internal resources and draw on the inner strength and faith in their own abilities, all of which is so crucial in the rehabilitation process. The content of the VR therapy was developed by a certified psychotherapist of the European Association of Psychotherapy, and with collaboration of the Polish Milton Institute, as well as the Scientific Department of Psychotherapy of the Polish Psychiatric Association.
Participants randomized to the control group received 20 min of Schultz’s Autogenic Training (SAT), which is a desensitization-relaxation technique. The therapeutic process of relaxation took place in the supine position. At the beginning, it was suggested that patients try to feel the weight of their body and then the body temperature, heart rate and breath. During the SAT, an autogenic training is played from the CD. The patient’s task is to duplicate the body relaxation exercises he hears through headphones. The purpose of SAT is generally to calm down and relax to reduce feelings of anxiety, sadness and depression.33 SAT is universal in approach and can be used in any case where patient calm and tranquillity are recommended. SAT was used in the form of audio recording, which was similar in nature to VR therapy. It is not possible to use cognitive behavioural therapy (CBT) in the form of an audio recording, as a therapist is needed to conduct CBT, and then the therapeutic relationship established between the patient and the therapist is of great importance.
Outcomes
Outcome assessments were conducted at three time points: on the second day of the patient’s stay in the ward (T0, before the intervention), after three weeks of rehabilitation (T1, after the intervention) and after six weeks of rehabilitation (T2, follow-up). Primary outcome measures included the 30-item Geriatric Depression Scale (GDS-30), the Generalized Self-Efficacy Scale (GSES), the Acceptance of Illness Scale (AIS), the Visual Analogue Scale of pain (VAS) and the Hospital Anxiety and Depression Scale (HADS). Secondary outcome measures were the Barthel Index (BI), the Lawton Instrumental Activities of Daily Living Scale (IADL), and the Rivermead Motor Assessment (RMA).
Statistical Analysis
We calculated the sample size by assuming minimum significance (α = 0.05), effect size (f = 0.20) and statistical power (1−β = 0.95), as no previous studies have been performed using a similar design with the use of immersive VR. Therefore, a total of 80 participants were needed to reach statistical significance with 20% dropout. However, after the completion of 60 participants, we have rerun the power analysis, using our primary outcome measure, ie, GDS results (np2 = 0.13; effect size f = 0.38). The analysis showed sufficient statistical power on the primary outcome; thus, we stopped further enrolment of participants. Reversed power analysis was necessary due to the COVID-19 outbreak and the inability to continue research.
Data distribution analysis was performed using the Shapiro–Wilk test. Except for VAS, BI, IADL and RMI, all data had a near-normal distribution. The study applied the intention-to-treat (ITT) analysis method.34 In the case of missing data, we applied the last observation carried forward (LOCF) method.35 According to the ITT assumptions, statistical analysis was performed for all participants included in the study. Baseline data between the groups were compared using the unpaired t-test (continuous variables) and the chi-squared test (categorical variables). The impact of the intervention at specific time points was determined using two-way repeated measures analysis of variance (ANOVA) with Bonferroni correction (p value adjusted for comparing a family of 15 comparisons). Both the p and f values for ANOVA were adjusted using the Greenhouse–Geisser correction. For pain assessment (VAS), the efficacy of the intervention was assessed using Friedman’s ANOVA. Because the number of participants in the groups differed, comparison of differences between those who dropped out and those who completed the study was performed using the Mann–Whitney U-test. In all analyses, the statistical significance was established at p < 0.05.
Results
Participants
Of 102 individuals screened for eligibility, 60 patients were randomly assigned to the VR (n = 30) or control (n = 30) group between 6 May 2019 and 3 April 2020. Admission to the rehabilitation ward was mainly from home (56; 93.33%) or from another hospital (4; 6.67%). Regarding marital status, most of the patients were married (35; 58.33%), 22 were widowed (36.67%) and three (5%) were single. No statistically significant baseline characteristics of participants were included in either group. The baseline characteristics are shown in Table 1.
 |
Table 1 Baseline Characteristics of the Intention-to-Treat Population |
Dropout Analysis
A total of 21 participants dropped out post-treatment at T1 examination (8 from the VR group and 13 from the control group). Participants who dropped out from the study had a 64% higher GDS-30 score (11.48 vs 7.23; p = 0.0006). No significant differences were found between the remaining studied psychological and functional parameters (Table 2).
 |
Table 2 Dropout Analysis Post-Treatment (at T1) |
Effectiveness of Interventions on Psychological Parameters
In the VR group, the intervention led to a statistically significant reduction of the GDS-30 score post-treatment (T1, 46%; 13.77 vs 7.43) and at follow-up (T2, 48%; 13.77 vs 7.17; p < 0.0001). For the total HADS, we observed a significant decrease of 26% (14.15 vs 10.40) at T1 and 27% (14.15 vs 10.30) at T2 (p < 0.0001): HADS-A (anxiety) decreased by 32% (7.05 vs 4.80; T1) and 25% (7.05 vs 5.25; T2; p = 0.0018) and HADS-D by 21% at both T1 (7.10 vs 5.60) and T2 (7.10 vs 5.05; p < 0.0057). The AIS score increased by 12% (23.23 vs 26.03) and 16% (23.23 vs 26.97) at T1 and T2, respectively (p = 0.007), resulting in improved well-being of patients. Regarding the VAS and GSES results, no statistically significant change was demonstrated at any time point. In the control group, a statistically significant improvement was shown on the GDS-30, HADS, HADS-A and AIS (Figure 4). Considering the GDS-30 results, after ten interventions, we obtained a reduction of 25% (13.40 vs 20.23) with respect to T1 and 22% (13.40 vs 10.23) with respect to T2 (p < 0.0001). For the total HADS, a statistically significant decrease of 18% was observed at both T1 (15.11 vs 12.26) and T2 (15.11 vs 12.21; p = 0.003). Considering the HADS-A results, after the intervention we obtained reductions of 25% (8.32 vs 6.21) at T1 and 27% (8.32 vs 6.11) at T2. For the AIS score, statistically significant increases of 17% (22.07 vs 25.90) and 18% (22.07 vs 26.20) were observed at T1 and T2, respectively (p < 0.0001). The differences obtained in the VAS, HADS-D and GSES scores were not statistically significant. A within–between analysis (Time*Group, Table 3) indicates that the only statistically significant difference was observed in the GDS-30 (p < 0.0001). There was no statistically significant difference between the groups in the other parameters analysed.
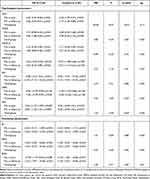 |
Table 3 Effects of Virtual Reality (VR) Therapy in Terms of Mean Differences (95% CI) |
 |
Figure 4 Mean values of the psychological and functional parameters results at individual time points. |
Effectiveness of Interventions on Functional Parameters
Analysing the results of the repeated measures ANOVA, we observed a significant effect of the applied intervention on all the functional parameters in both groups. In the VR group, the mean BI score increased by 6% (89.00 vs 94.50) at T1 and by 8% (89.00 vs 96.00) at T2 (p < 0.0001). In the IADL, the increases amounted to 7% (19.93 vs 21.43) and 8% (19.93 vs 21.60) at T1 and T2, respectively (p < 0.0001), and in the RMA by 7% (9.23 vs 9.93) at T1 and by 13% (9.23 vs 10.47) at T2 (p < 0.0001) (Figure 4). The control group also demonstrated a significant effect of the interventions applied. In the case of the BI, the mean score at T1 was 6% (86.33 vs 91.67) higher and at T2 it was 7% (86.33 vs 92.83) higher (p < 0.0001). The IADL score increased by 7% (19.30 vs 20.70) and 8% (19.30 vs 20.87) at T1 and T2, respectively (p < 0.0001). The RMA score at T1 was 12% higher than at T0 (8.63 vs 9.73), and at T2 it was 15% higher (8.63 vs 9.97; p < 0.0001) (Figure 4). The Time*Group analysis did not show statistically significant differences in any of the functional parameters studied, which was also confirmed by post hoc tests (Table 3).
Cut-Off Analysis
All participants included in the study had a GDS-30 score of ≥10, which is the first depression cut-off in this scale. In the VR group, 67% (n = 20) of participants were below this threshold immediately after the intervention and 73% (n = 22) were below the threshold in the follow-up study. In the control group, 43% (n = 13) of participants were below this cut-off at both T1 and T2 (Table 4). No adverse events were reported to the trial team (ie, complaints such as dizziness, nausea or cyber sickness) and no negative emotional effects were recorded, such as anxiety, discomfort or inconvenience associated with using the device itself.
 |
Table 4 Geriatric Depression Scale (GDS-30) Cut-off Frequency Differences Among the Groups |
Discussion
This study is the first to assess the impact of an added immersive original VR therapy to conventional rehabilitation on the mental state of stroke survivors. As most studies that used VR in post-stroke rehabilitation focused on improving functional state, here we provide a specific immersive VR therapy to reduce post-stroke depressive symptoms. The developed therapeutic programme is the first attempt to implement Erickson’s psychotherapy in a virtual environment.
The findings of this study indicate a reduction of depressive symptoms that was significantly greater in the VR group, along with improvement of the patients’ mental state. When considering the severity of depressive symptoms, a statistically significant reduction was found in the primary outcome measure (GDS-30) both in the VR and in the control group. Both groups had similar pre-intervention severity of depressive symptoms; however, at the post-intervention measurement (T1), the difference between the means was 2.57 points, which increased to 3.07 points at follow-up (T2). This may indicate the positive medium-term effects of this specific VR therapy on the well-being of the participants. In the present study, patients in both groups demonstrated a significant impact of the applied interventions on parameters such as BI, IADL and RMA. At the same time, no evidence of superiority of this specific VR intervention over the standard motor post-stroke rehabilitation model was demonstrated in the studied functional ability areas. Although we assumed that the improvement of mental state under the influence of VR therapy would also improve post-stroke motricity, the results did not confirm this hypothesis. This is probably related to the lack of improvement observed in self-efficacy, as a low GSES level may reduce the rehabilitation effect for stroke patients.36,37 Importantly, our objective was to determine whether any of the groups differs in the increase/decrease of the parameters examined and not the effectiveness of rehabilitation in individual groups. Therefore, we did not interpret the individual increase in the BI, the IADL and the RMA scores because we were searching for differences between groups. Due to this, we were able to check whether the conversion of SAT to VR would be effective. Apart from the GDS, we found no significant differences; therefore, we did not interpret individual changes in the groups.
A patient’s attitude towards his or her own illness is important in the rehabilitation process because it can either advance the improvements in functional state and quality of life or have a negative impact in these areas.38 In the present study, the level of acceptance of illness increased in both groups after the intervention and remained at this increased level until the follow-up assessment. This may indicate both a positive influence of the VR treatment that was applied or it is a result of natural adjustment to illness, and it can be time-dependent. Song and Park assessed the differences between rehabilitation based on VR games and rehabilitation on a cycle ergometer in patients after stroke.39 After the intervention, a reduction in depressive symptoms was observed in both groups but, as with our study, the reduction was greater in the VR group. Moreover, a meta-analysis by Fodor et al found that interventions using VR were statistically significantly more effective than standard interventions for reducing anxiety and depressive symptoms.40 It is also worth noting that the literature is dominated by a syndrome-focused approach to treating stroke patients and concentrates primarily on motor treatment methods, leaving the theoretical and methodological justification of the psychological aspect of post-stroke rehabilitation insufficiently represented.
VR therapy (ie, applied to motor impairment) has shown to increase motivation of post-stroke patients (REF) and may increase variability of neurorehabilitation programmes.41,42 Finding motivation for undertaking activity and increasing the sense of self-efficacy both constitute an important goal of therapy based on the VR TierOne medical device system. Indeed, in this study, applied VR therapy stimulated the transformation process, from grey and opaque reality to a naturally coloured environment. In this process, the patient was followed to feel co-responsible for his/her result.
Although the literature often mentions factors that constitute motivation for the use of VR tools, we are not aware of any systematic assessment of the features of VR-based therapy or their impact on the rate of patient dropout. Presumably, factors such as the level of VR immersion, the fact that treatment content is based on a story/narrative or the relevance of this content to the patient’s daily life are important factors for sustained patient motivation.43 In the present study, 21 people dropped out of the project, of which 13 dropped out of the control group. The GDS-30 score obtained by the participants who dropped out from the study was 64% higher than those who remained. Our results suggest that the patients’ poorer mental state contributed to premature discharge from the hospital ward and early termination of post-stroke therapy. McGrady et al demonstrated similar findings in their study, concluding that high levels of anxiety and depression at the start of rehabilitation are the main predictors for dropping out of therapy.44 Therefore, most often, the fact of giving up is related to the poor psychological state of the patient, namely lack of motivation and commitment as well as passivity in relation to the undertaken interventions, which in consequence leads to cessation of the rehabilitation process. This, in turn, may result in lack of functional improvement and further deterioration of their mental condition. Untreated depressive symptoms often lead to diminished engagement in the rehabilitation process, withdrawal from social relationships, increased disability, reduced quality of life, lower physical activity and higher mortality and risk of recurrence of stroke, as confirmed in recent studies.12,14,15 Numerous authors have emphasized the effectiveness of VR-based treatments in alleviating psychological and behavioural problems and psychiatric disorders.35,40 In doing so, they have also highlighted the lack of VR-based technological solutions that, in addition to motor rehabilitation of stroke patients, would offer the therapeutic tools to reduce psychological disorders and improve the patient’s mood and motivation. This gap seems to be filled by the VR programme analysed here, which was designed to support the neurological rehabilitation process by reducing levels of depression while increasing the acceptance of illness and boosting the sense of self-efficacy.45
An important issue that is influenced by the psychological state of the patient is the act of returning to work after a stroke. However, PSD can effectively hinder the resumption of work, thereby locking the patients into their illness and reducing their chances of psychosocial and functional recovery.46 For instance, pain and depression are important comorbidities. Both clinical and preclinical studies clearly indicate that pain can cause depression and that depression can worsen pain behaviours.47 In turn, depression and pain share biological pathways and neurotransmitters.48 Rehabilitation using immersive VR may provide new, positive stimuli and distracts the patient from the distressing aspects of a hospital stay. It is believed that the combination of the patient’s internal motivation and full immersion can induce a trance-like state in which the patient is able to forget his or her circumstances.49 Thus, implementation of VR therapy could expand the treatment options for patients suffering depressive symptoms and we should look for methods that can improve self-efficacy in the process of neurorehabilitation.
Despite the encouraging results obtained in this research, the main limitation was that a high percentage of patients dropped out of rehabilitation after three weeks and thus the analyses adopted ITT with the LOCF method to cover the missing data. Secondly, the study included only individuals with depressive symptoms based on the GDS results, but without a major depressive disorder diagnosis. Thirdly, we used only self-reported questionnaires and functional scales, without more objective outcome measures. Moreover, the scientific method used in the study is mainly based on total immersion, so if a person being examined is not fully “immersed” in the therapy, the effects may differ. Therefore, it would also be worth using a more objective way of measuring the stress level (eg, cortisol level test), whilst assessing the participants’ real perception of the virtual world (eg, Spatial Presence Experience Scale). Considering the above limitations, the results of our study are important but should be interpreted with caution.
Conclusion
The findings of this study showed that VR therapy may support the rehabilitation process of people suffering depressive symptoms. In particular, VR therapy combined with neurological rehabilitation was more effective at improving mood than SAT. Finally, the VR intervention with its therapeutic virtual garden did not change the functional aspects. Similar scores on the BI, IADL and RMA scales were observed in both study groups after the intervention and at follow-up measurement.
Abbreviations
VR, virtual reality; PSD, post-stroke depression; REPTY, Upper-Silesian Rehabilitation Centre; GDS-30, geriatric depression scale; MMSE, Mini-Mental State Examination; SAT, Schultz’s autogenic training; GSES, Generalized Self-Efficacy Scale; AIS, Acceptance of Illness Scale; VAS, Visual Analogue Scale of pain; HADS, Hospital Anxiety and Depression Scale; IADL, Lawton Instrumental Activities of Daily Living Scale; RMA, Rivermead Motor Assessment; ITT, intention-to-treat; LOCF, last observation carried forward.
Data Sharing Statement
The de-identified participant data intended for open access will be placed in the AZON (zasobynauki.pl) repository along with the relevant descriptions and in the right file formats, as required by the service. Data will be available “with publication”. The AZON platform provides mechanisms for rendering resources available in line with the Linked Open Data paradigm, which particularly applies to permanent identifiers, different metadata profiles, format conversion, the search feature, and the presentation layer compatible with WCAG2.0, etc. The chosen open license type will depend on the nature of the resources to be rendered available, with preference given to the CC-BY-SA licence. A unique identifier will be generated for a data set being placed in the AZON repository. The repository policy guarantees that each resource is assigned a unique, permanent, and preferable identifier, and it provides for integration with the DOI.
Acknowledgments
The authors would like to thank Mr. Tomasz Mandzyn for his support with the VR technology and creating the virtual garden.
Funding
This study was funded by the National Centre for Research and Development under grant number POIR.01-02.00-00-0134/16.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Drieu A, Levard D, Vivien D, Rubio M. Anti-inflammatory treatments for stroke: from bench to bedside. Ther Adv Neurol Disord. 2018;11:1756286418789854. doi:10.1177/1756286418789854
2. Jorgensen HS, Nakayama H, Pedersen PM, Kammersgaard L, Raaschou HO, Olsen TS. Epidemiology of stroke-related disability. Clin Geriatr Med. 1999;15(4):785–799. doi:10.1016/S0749-0690(18)30031-4
3. Saxena SK, Ng TP, Yong D, Fong NP, Koh G. Subthreshold depression and cognitive impairment but not demented in stroke patients during their rehabilitation. Acta Neurol Scand. 2008;117(2):133–140. doi:10.1111/j.1600-0404.2007.00922.x
4. Haghgoo HA, Pazuki ES, Hosseini AS, Rassafiani M. Depression, activities of daily living and quality of life in patients with stroke. J Neurol Sci. 2013;328(1–2):87–91. doi:10.1016/j.jns.2013.02.027
5. Park GY, Im S, Oh CH, Lee SJ, Pae CU. The association between the severity of poststroke depression and clinical outcomes after first-onset stroke in Korean patients. Gen Hosp Psychiatry. 2015;37(3):245–250. doi:10.1016/j.genhosppsych.2015.02.009
6. Oh SY, Hwang SY, Chung ML, Lennie TA. A prediction model of rehabilitation motivation in middle-aged survivors of stroke in rehabilitation facilities in Korea. J Cardiovasc Nurs. 2020;35(5):475–482. doi:10.1097/JCN.0000000000000656
7. Paolucci S. Advances in antidepressants for treating post-stroke depression. Expert Opin Pharmacother. 2017;18(10):1011–1017. doi:10.1080/14656566.2017.1334765
8. Ezema CI, Akusoba PC, Nweke MC, Uchewoke CU, Agono J, Usoro G. Influence of post-stroke depression on functional independence in activities of daily living. Ethiop J Health Sci. 2019;29(1):841–846. doi:10.4314/ejhs.v29i1.5
9. Etherton MR, Shah S, Haolin X, et al. Patterns of antidepressant therapy and clinical outcomes among ischaemic stroke survivors. Stroke Vasc Neurol. 2021;6(3):384–394. doi:10.1136/svn-2020-000691
10. Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. 2018;184:131–144. doi:10.1016/j.pharmthera.2017.11.005
11. Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9(8):1017–1025. doi:10.1111/ijs.12357
12. Cai W, Mueller C, Li YJ, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102–109. doi:10.1016/j.arr.2019.01.013
13. Janus-Laszuk B, Mirowska-Guzel D, Sarzynska-Dlugosz I, Czlonkowska A. Effect of medical complications on the after-stroke rehabilitation outcome. NeuroRehabilitation. 2017;40(2):223–232. doi:10.3233/NRE-161407
14. Blochl M, Meissner S, Nestler S. Does depression after stroke negatively influence physical disability? A systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2019;247:45–56. doi:10.1016/j.jad.2018.12.082
15. Thilarajah S, Mentiplay BF, Bower KJ, et al. Factors associated with post-stroke physical activity: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99(9):1876–1889. doi:10.1016/j.apmr.2017.09.117
16. Morina N, Ijntema H, Meyerbroker K, Emmelkamp PM. Can virtual reality exposure therapy gains be generalized to real-life? A meta-analysis of studies applying behavioral assessments. Behav Res Ther. 2015;74:18–24. doi:10.1016/j.brat.2015.08.010
17. Beidel DC, Frueh BC, Neer SM, Lejuez CW. The efficacy of trauma management therapy: a controlled pilot investigation of a three-week intensive outpatient program for combat-related PTSD. J Anxiety Disord. 2017;50:23–32. doi:10.1016/j.janxdis.2017.05.001
18. Falconer CJ, Rovira A, King JA, et al. Embodying self-compassion within virtual reality and its effects on patients with depression. BJPsych Open. 2016;2(1):74–80. doi:10.1192/bjpo.bp.115.002147
19. Rutkowski S, Czech O, Wrzeciono A, Kiper P, Szczepanska-Gieracha J, Malicka I. Virtual reality as a chemotherapy support in treatment of anxiety and fatigue in patients with cancer: a systematic review and meta-analysis and future research directions. Complement Ther Med. 2021;61:102767. doi:10.1016/j.ctim.2021.102767
20. Chirico A, Lucidi F, De Laurentiis M, Milanese C, Napoli A, Giordano A. Virtual reality in health system: beyond entertainment. A mini-review on the efficacy of VR during cancer treatment. J Cell Physiol. 2016;231(2):275–287. doi:10.1002/jcp.25117
21. Hoffman HG, Chambers GT, Meyer WJ 3rd, et al. Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Ann Behav Med. 2011;41(2):183–191. doi:10.1007/s12160-010-9248-7
22. Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2017;11:CD008349. doi:10.1002/14651858.CD008349.pub4
23. Cacciante L, Pieta CD, Rutkowski S, et al. Cognitive telerehabilitation in neurological patients: systematic review and meta-analysis. Neurol Sci. 2022;43(2):847–862. doi:10.1007/s10072-021-05770-6
24. Bogdanova Y, Yee MK, Ho VT, Cicerone KD. Computerized cognitive rehabilitation of attention and executive function in acquired brain injury: a systematic review. J Head Trauma Rehabil. 2016;31(6):419–433. doi:10.1097/HTR.0000000000000203
25. Cacciante L, Kiper P, Garzon M, et al. Telerehabilitation for people with aphasia: a systematic review and meta-analysis. J Commun Disord. 2021;92:106111. doi:10.1016/j.jcomdis.2021.106111
26. Szczepanska-Gieracha J, Cieslik B, Rutkowski S, Kiper P, Turolla A. What can virtual reality offer to stroke patients? A narrative review of the literature. NeuroRehabilitation. 2020;47(2):109–120. doi:10.3233/NRE-203209
27. Birckhead B, Khalil C, Liu X, et al. Recommendations for methodology of virtual reality clinical trials in health care by an international working group: iterative study. JMIR Ment Health. 2019;6(1):e11973. doi:10.2196/11973
28. Lyu Y, Li W, Tang T. Prevalence trends and influencing factors of post-stroke depression: a study based on the National Health and Nutrition Examination Survey. Med Sci Monit. 2022;28:e933367. doi:10.12659/MSM.933367
29. McCarthy MJ, Sucharew HJ, Alwell K, et al. Age, subjective stress, and depression after ischemic stroke. J Behav Med. 2016;39(1):55–64. doi:10.1007/s10865-015-9663-0
30. Matthews WJ. Ericksonian approaches to hypnosis and therapy: where are we now? Int J Clin Exp Hypn. 2000;48(4):418–426;discussion 433–417. doi:10.1080/00207140008410370
31. Otani A. Characteristics of change in Ericksonian hypnotherapy: a cognitive-psychological perspective. Am J Clin Hypn. 1990;33(1):29–39. doi:10.1080/00029157.1990.10402898
32. Moore MR. Ericksonian theories of hypnosis. Am J Clin Hypn. 1982;24(3):183–184. doi:10.1080/00029157.1982.10404048
33. Schultz JH. Das Autogene Training in der Allgemeinpraxis [Autogenic training in general practice]. Med Klin. 1950;45(31):945–949;contd. German.
34. Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ. 2001;165(10):1339–1341.
35. Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. Am J Psychiatry. 2009;166(6):639–641. doi:10.1176/appi.ajp.2009.09040458
36. Long Y, Ouyang RG, Zhang JQ. Effects of virtual reality training on occupational performance and self-efficacy of patients with stroke: a randomized controlled trial. J Neuroeng Rehabil. 2020;17(1):150. doi:10.1186/s12984-020-00783-2
37. Torrisi M, De Cola MC, Buda A, et al. Self-efficacy, poststroke depression, and rehabilitation outcomes: Is there a correlation? J Stroke Cerebrovasc Dis. 2018;27(11):3208–3211. doi:10.1016/j.jstrokecerebrovasdis.2018.07.021
38. van Mierlo ML, van Heugten CM, Post M, de Kort P, Visser-Meily J. Life satisfaction post stroke: the role of illness cognitions. J Psychosom Res. 2015;79(2):137–142. doi:10.1016/j.jpsychores.2015.05.007
39. Song GB, Park EC. Effect of virtual reality games on stroke patients’ balance, gait, depression, and interpersonal relationships. J Phys Ther Sci. 2015;27(7):2057–2060. doi:10.1589/jpts.27.2057
40. Fodor LA, Cotet CD, Cuijpers P, Szamoskozi S, David D, Cristea IA. The effectiveness of virtual reality based interventions for symptoms of anxiety and depression: a meta-analysis. Sci Rep. 2018;8(1):10323. doi:10.1038/s41598-018-28113-6
41. Kannan L, Vora J, Bhatt T, Hughes SL. Cognitive-motor exergaming for reducing fall risk in people with chronic stroke: a randomized controlled trial. NeuroRehabilitation. 2019;44(4):493–510. doi:10.3233/NRE-182683
42. Kayabinar B, Alemdaroglu-Gurbuz I, Yilmaz O. The effects of virtual reality augmented robot-assisted gait training on dual-task performance and functional measures in chronic stroke: a randomized controlled single-blind trial. Eur J Phys Rehabil Med. 2021;57(2):227–237. doi:10.23736/S1973-9087.21.06441-8
43. Rizzo AS, Koenig ST. Is clinical virtual reality ready for primetime? Neuropsychology. 2017;31(8):877–899. doi:10.1037/neu0000405
44. McGrady A, Burkes R, Badenhop D, McGinnis R. Effects of a brief intervention on retention of patients in a cardiac rehabilitation program. Appl Psychophysiol Biofeedback. 2014;39(3–4):163–170. doi:10.1007/s10484-014-9252-y
45. Szczepanska-Gieracha J, Cieslik B, Serweta A, Klajs K. Virtual therapeutic garden: a promising method supporting the treatment of depressive symptoms in late-life: a randomized pilot study. J Clin Med. 2021;10(9):9. doi:10.3390/jcm10091942
46. Westerlind E, Persson HC, Palstam A, Eriksson M, Norrving B, Sunnerhagen KS. Differences in self-perceived general health, pain, and depression 1 to 5 years post-stroke related to work status at 1 year. Sci Rep. 2020;10(1):13251. doi:10.1038/s41598-020-70228-2
47. Doan L, Manders T, Wang J. Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast. 2015;2015:504691. doi:10.1155/2015/504691
48. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi:10.1001/archinte.163.20.2433
49. Shin JH, Ryu H, Jang SH. A task-specific interactive game-based virtual reality rehabilitation system for patients with stroke: a usability test and two clinical experiments. J Neuroeng Rehabil. 2014;11(1):32. doi:10.1186/1743-0003-11-32
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


