Back to Journals » Journal of Pain Research » Volume 12
Effects of extracorporeal shock waves on neuralgia in diabetic rats
Authors Zhou Y, Dai H, Long J , Kang XG, He CJ
Received 18 June 2018
Accepted for publication 13 December 2018
Published 17 January 2019 Volume 2019:12 Pages 387—394
DOI https://doi.org/10.2147/JPR.S177585
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Yue Zhou,1 Hong Dai,2 Juan Long,1 Xin-Guo Kang,1 Chun-Jing He1
1Department of Pain, Guizhou Provincial People’s Hospital, Guiyang 550002, China; 2Department of Neurology, Guizhou Provincial People’s Hospital, Guiyang 550002, China
Objective: The aim of this study was to observe the effects of extracorporeal shock waves (ECSWs) on neuralgia in diabetic rats.
Materials and methods: Diabetic neuralgia model was established in rats via injection of streptozotocin. The rats were divided into diabetic neuralgia group (Group A, n=6) and ECSW treatment group (Group B, n=6). Another six rats were taken as control group (Group C, n=6). The mechanical withdrawal threshold (MWT) and thermal withdrawal latencies (TWLs) were measured at specific points throughout the experiment, and the sciatic nerve was bluntly severed under anesthesia after the last measurement. The protein expressions of Sphk1 and TNF-α were detected by Western blot, and the mRNA expressions of Sphk1 and TNF-α were detected by reverse transcription PCR. The structure of the sciatic nerve was observed by electron microscopy.
Results: Compared with Group C, MWT and TWLs were decreased significantly in Groups A and B (P<0.05). The protein expressions of TNF-α and Sphk1 in Groups A and B were both significantly higher than those in Group C (P<0.05), with higher expression in Group A than in Group B (P<0.05). The mRNA expressions of TNF-α and Sphk1 were similar. Electron microscopy showed the intact structure of the myelin sheaths of the sciatic nerve fibers in Group C, whereas the structure of the nerve fibers was damaged, with a large number of vacuoles in the myelin sheath in Group A. In Group B, the vacuoles were occasionally formed on the sciatic nerve myelin sheath, with more compact and tidy layer arrangement compared with Group A.
Conclusion: ECSWs can relieve neuralgia in diabetic rats. Sphk1 and TNF-α may be involved in the occurrence and development of diabetic peripheral neuralgia.
Keywords: extracorporeal shock waves, diabetic neuralgia, Sphk1, TNF-α, rats, ECSWs
Introduction
Diabetic peripheral neuralgia (DPN) is one of the chronic complications of diabetes, which has a high rate and is difficult to treat, seriously affecting the quality of life of patients.1 The early manifestations of DPN mainly include pain (burning pain, electric shock, or acupuncture-like pain), paresthesia (such as hyperalgesia), and numbness. In the late stage, motor function is impaired, with weakness spreading from feet to the lower legs and even ankles. With the development of DPN, both lower legs further lose sensation and develop motor dysfunction (dull feelings, ataxia, etc), which can then develop into foot ulcers or gangrene and even result in amputation. Currently, the pathogenesis of DPN has not been fully elucidated. The majority of studies have suggested that the occurrence of DPN is related to metabolic disturbances caused by long-term sustained hyperglycemia in vivo. Various factors mediating the vascular inflammatory response led to endothelial dysfunction and resulted in nerve injury.2 Treatment methods are mainly drug therapy, physical therapy, and minimally invasive treatment. Drug therapy mainly involves tricyclic antidepressants,3 antiepileptic drugs, opioids, gabapentin, and pregabalin.4 Gabapentin can also improve sleep quality and relieve anxiety,5,6 but it has side effects such as dizziness, drowsiness, peripheral edema, and gait disorders, as well as severe drug resistance.7 Minimally invasive treatment methods such as lumbar sympathetic nerve damage and spinal cord electrical stimulation are invasive and expensive, limiting the clinical application in a wide range. Physical therapies such as single-frequency infrared light therapy, electroacupuncture, and hyperbaric oxygen therapy are now popular, non-invasive, and have no side effects, but their efficacy is not yet satisfactory. Therefore, finding a non-invasive, safe, and effective treatment method without side effects is currently a prevalent research topic. Extracorporeal shock waves (ECSWs) have been used to treat urinary tract stones, chronic Achilles tendinitis, and myofascial pain syndrome,8,9 and they can attain satisfactory clinical effect. However, whether ECSWs can improve pain symptoms in patients with DPN has not been studied. At present, a large number of studies have illustrated that ECSWs can inhibit inflammatory cell responses, oxidative stress, and apoptosis.10,11 Tumor necrosis factor (TNF-α) is one of the most essential inflammatory factors, while another inflammatory factor Sphk1 interacts with TNF-α to mediate the inflammatory responses. In this study, the rat model of DPN was established to observe the changes in TNF-α and Sphk1 so as to explore the possible mechanism of ECSWs in alleviating DPN.
Materials and methods
Reagents and instruments
The reagents included acrylamide, methylene bisacrylamide, ammonium sulfate, tetramethylethylenediamine, glacial acetic acid, methanol, ethanol, isopropanol, EDTA, Tris base, sodium lauryl sulfate, glycine, glycerol, β-mercaptoethanol, phenylmethylsulfonyl fluoride (PMSF), sodium orthovanadate, aprotinin, bromophenol blue, dithiothreitol, BSA, skim milk powder, NaHCO3, KHCO3, NaCl, deionized water, concentrated hydrochloric acid, Coomassie brilliant blue fast stain, sodium acetate, RIPA lysate, Tween-20, Ponceau, diethyl pyrocarbonate (DEPC), TRNzol, chloroform, ReverTra Ace Reverse Transcription Kit, Sepharose, 2× PCR Mix Taq, 2× SYBR Green, DL2000 Marker, and ethidium bromide. The instruments included a Full-Automatic Plantar Analgesia Tester BME-410 (Institute of Biomedical Engineering, Chinese Academy of Biomedical Sciences), a cryogenic centrifuge (Sigma-Aldrich, St.Louis, MO, USA), a Bio Rad 3,000 UV Viscometer (Bio-Rad Laboratories Inc., Hercules, CA, USA), a CFX PCR instrument (Bio-Rad Laboratories Inc.), and an H-1500 transmission electron microscope (Hitachi Ltd., Tokyo, Japan).
Experimental animals and grouping
Twenty healthy 8-week-old Sprague-Dawley rats weighing 180–200 g were provided and fed by the Experimental Center of the Third Military Medical University (animal use license number SCXK(R) 2012-0005). Twenty rats were prepared for the establishment of diabetes model. During the modeling period, two rats died and one rat failed to become diabetic. After successful preparation of the diabetes model, the mechanical withdrawal threshold (MWT) of another two rats did not reach 50% and hence these two rats were excluded from the experiment. We randomly selected 12 out of the remaining 15 rats and divided them into two groups, 6 rats per group. Another six rats were selected as the normal control group. The animals were randomly divided into the normal control group (Group C, n=6), diabetic neuralgia group (Group A, n=6), and ECSWs treatment group (Group B, n=6). The rats were housed in cages, with free access to water and a standardized diet, with room temperature at 21°C–23°C and alternating conditions of 12 hours of light and 12 hours of dark cycle.
All experiment protocols were approved by the Institutional Animal Care and Use Committee of the Guizhou Provincial People’s Hospital in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No 80-23), revised in 1996. The research was also approved by the ethical committee of Guizhou Provincial People’s Hospital. All efforts were made to minimize the number of animals used and their suffering.
Diabetes model preparation
The rats in Groups A and B were intraperitoneally injected with streptozotocin (STZ), at a dose of 60 mg/kg. The concentration of STZ was 1%, and it was dissolved in a citric acid–sodium citrate buffer solution with a pH value of 4.5. In Group C, the same dose of saline was injected into the abdominal cavity, and then the rats were bred separately after modeling. Fasting blood glucose concentration was measured before injection (after fasting for 12 hours) and 2 days after injection (after fasting for 12 hours). Diabetes was determined when the fasting blood glucose concentration was >16.7 mmol/L at 2 days after STZ injection. The non-diabetic rats were excluded from the experiment.12
To prepare the diabetic neuralgia model, the right hind paws of the rats were stimulated with von Frey filaments of different strengths on the 7th, 14th, 21st, and 28th day after STZ injection. The mechanical paw withdrawal response was induced, and the MWT of the right hind paw of the rat was measured by the up and down methods.12 A rat model of DPN was successfully established when MWT was reduced by >50% at 28th day.
Measurement of MWT and thermal withdrawal latencies (TWLs)
MWT and TWLs of all three groups were measured separately in the morning of the day successfully modeling (T0), the 7th day after successfully modeling (T1), the 14th day after successfully modeling (T2), the 21st day after successfully modeling (T3), and the 28th day after successfully modeling (T4). The rats were placed in a reticular flat and covered with a transparent glass cage for 30 minutes before measurement so that the rats could adapt to the experimental environment. When the measurements began, we vertically stimulated the right hind paw of the rats by von Frey filaments and recorded the data. The strength of von Frey fibers was 0.7 g, 2.5 g, 3.5 g, 4.0 g, 6.0 g, 10.0 g, 12.0 g, 16.0 g, and 25.0 g, respectively. The initial stimulus strength was set at 6 g, and the duration was controlled to be ≤4 seconds. Once the rats presented foot-lifting or foot-licking behaviors, we determined that the consequence was positive, otherwise negative. If the result was positive (negative), a stimulus with a lower (higher) intensity would be applied to the exact experimental subject with an interval of 15 seconds. The experiments should be conducted continuously in this method until one negative (or positive) result occurred. The strength of the last (first) positive stimulus would be taken and measured for four times with an interval of 15 seconds. If the number of times of foot contraction was ≥3, the strength would be regarded as MWT. If the foot contraction was <3 times, then the next higher level of stimulation was supposed to be selected and the process would be repeated until the number of times of foot contraction turned out to be ≥3, when the intensity should be MWT. TWLs were measured using a BME-410 thermo-pain stimulator. After the rats were also acclimated to the environment for 30 minutes, the rats were irradiated at the plantar part on a glass plate with a thermo-pain stimulator (10 V and 30 W), and the diameter of the irradiated spots was 0.5 cm. In order to avoid the burn on the sole of the rats, the upper limit of the length of thermal stimulation was set to be 30 seconds and the interval of thermal stimulation to be 5 minutes. The abovementioned measurements were repeated three times, and the mean values were accepted as the final results. The duration of the recorded automatic foot-shortening reaction was used as the thermal pain threshold.
ECSW treatment
Rats in Group B received ECSW at T1, T2, T3, and T4 after MWT and TWL measurements, with a frequency of ECSW treatment of 10–15 Hz and the energy of 2.0 bar.17 Before treatment with 6,000 ECSWs per treatment, the right hind limb sciatic nerve distribution area underwent skin preparation and iodophor conventional skin disinfection.
Detection of indicators
At seventh day after the last ECSW treatment (T4, 16-week old), the sciatic nerve was bluntly severed under 10% chloral hydrate-induced anesthesia, and each was cut to ~2 cm. The left normal sample was rinsed in saline and fixed with 2.5% glutaraldehyde. The specimens were rinsed three times with phosphate buffer and embedded in epoxy resin. Conventional ultrathin pellets were stained with uranyl acetate and lead citrate and observed with an H-1500 transmission electron microscope (Hitachi Ltd.). The right side was rinsed with normal saline on ice and was placed in a –80°C freezer for subsequent Western blot detection.
Western blot showed that for protein sample preparation, RIPA lysate and PMSF were mixed at a volume ratio of 100:1. The tissues were fully dissolved and placed at 4°C in a centrifuge at 12,000 × g for 15 minutes, and the supernatant was saved. The protein concentration of the sample was measured according to the BAC protein concentration measurement kit. After vertical electrophoresis, the membrane was transposed for 1.5 hours and blocked for 2 hours. The primary antibody was incubated overnight at 4°C (diluted 1:500; Abcam), and the secondary antibody was incubated at room temperature for 1 hour (dilution 1:1,000). Enhanced chemiluminescence was used before film exposure. The gray scale values of the bands were measured using Quantity-one image analysis software.
The mRNA expressions of Sphk1 and TNF-α were detected by reverse transcription PCR (RT-PCR). Samples of the right sciatic nerve were collected and transferred to a 1.5 mL Eppendorf (EP) tube. One milliliter of TRNzol was added, and the solution was completely shaken to completely lyse the solution. Chloroform of 1/5 vol (0.2 mL) was added, stirred, homogenized, and centrifuged at 15,000 × g for 5 minutes at 4°C. The upper aqueous phase (~400 µL) was transferred to another 1.5 mL EP tube and an equal volume of isopropanol (~400 µL) was added. Then, the solution was being shaken, mixed, and centrifuged at 15,000 × g for 20 minutes at 4°C. The supernatant was discarded, and 1 mL of pre-chilled 75% ethanol was added, and then the solution was centrifuged at 15,000 × g for 5 minutes at 4°C. The supernatant was discarded, and 1 mL of absolute ethanol was added, and then the solution was further centrifuged at 15,000 × g for 5 minutes at 4°C. Then, the supernatant was discarded and dried in air for 5–10 minutes, dissolved in 40 µL of 1 DEPC water, and stored at –70°C for use. After that, 2 µL of RNA was diluted for 50 times, and ultraviolet spectrophotometer was used to determine the OD value ratio at UV 260 nm/280 nm. cDNA was synthesized and reverse transcribed (20 µL system), and the sample loading system was as follows: RNA 2 µg, 5× RT buffer 4.0 µL, RTase 0.5 µL, RNase inhibitor 0.5 µL, dNTPs 2.0 µL, oligo(dT) 1.0 µL, and 1% DEPC water to 20.0 µL; the reaction conditions were at 42°C for 10 minutes → at 30°C for 20 minutes → at 99°C for 5 minutes → at 4°C for 5 minutes. Quantitative PCR was used to compare the expression of Sphk1 of TNF-α mRNA in the cells. The primers were synthesized by Chongqing Jinmai Biotechnology Co., Ltd (Chongqing, China). The primers were diluted to 10 µm and stored at –20°C. Three replicate wells were used for each sample. The reaction conditions were as follows: at 96°C for 5 minutes → (at 96°C for 30 seconds → at 57°C for 30 seconds → at 72°C for 30 seconds) × 40 cycles → at 72°C for 10 minutes, and the data were calculated according to the 2–ΔΔct method.
Statistical analysis
The SPSS statistical software package (version 20.0) was used for statistical analysis. Measurement data were processed using a one-way ANOVA for the groups and least significant difference for the pairwise comparisons. P<0.05 was considered statistically significant.
Results
Changes in MWT and TWLs
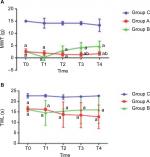
MWT and TWLs were measured before treatment (T0) in all 18 rats and used as the basic threshold. The MWT and TWL values of Group B were measured at T1, T2, T3, and T4 after ECSW treatment. The results showed that there was no significant change in MWT or TWLs at each time point in Group C (P>0.05). The MWT and TWL values of Groups A and B were significantly lower than the baseline threshold (Figure 1A: P=0.000, F=360.438; Figure 1B: P=0.000, F=50.661). Meanwhile, both MWT and TWLs in Group B began to increase from (0.250±1.282) g and (14.554±5.952) seconds at T1 to (4.750±2.121) g and (15.990±6.471) seconds at T4, which was significantly higher than Group A from T2 to T4 (in Group A, T2: (1.457±0.709) g and (13.716±6.070) seconds; T3: (1.314±0.682) g and (13.445±5.952) seconds; T4: (171±0.616) g and (12.687±5.613) seconds; in Group B: T2: (3.000±1.852) g and (15.366±1.053) seconds, T3: (4.250±1.982) g and (15.929±6.447) seconds, and T4: (4.750±2.121) g and (15.990±6.471) seconds; Figure 1), indicating that the ECSWs indeed inhibited hyperalgesia of the right hind paw.
Protein expressions of Sphk1 and TNF-α in the right sciatic nerves of the three groups of rats
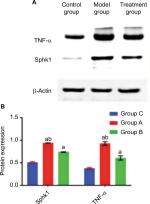
The Western blot technique was used to detect the protein expressions of Sphk1 and TNF-α (Figure 2A). Compared with Group C, the expressions of Sphk1 and TNF-α in Groups A and B were significantly increased (Figure 2B; Sphk1: P=0.000, F=3,109.072; TNF-α: P=0.000, F=325.844). This confirmed that the Sphk1 and TNF-α would be elevated in rats with DPN. Compared with Group A at T4, the expressions of Sphk1 and TNF-α in Group B were decreased significantly (Figure 2B; Sphk1: P=0.000, F=10,832.555; TNF-α: P=0.000, F=162.568), indicating that the ECSWs can reduce the content of Sphk1 and TNF-α.
Sphk1 mRNA and TNF-α mRNA expression
RT-PCR showed that the mRNA expressions of Sphk1 and TNF-α in Groups A and B were significantly increased (Sphk1: P=0.000, F=58.740; TNF-α: P=0.000, F=20.089) compared with Group C (Figure 3), further indicating the continuous secretion of Sphk1 and TNF-α in rats with DPN. Compared with Group A, the mRNA expression of Sphk1 was significantly decreased in Group B (Sphk1: P=0.002, F=16.464) (Figure 3).
  | Figure 3 The mRNA expression of Sphk1 and TNF-α in the right sciatic nerve in three groups of rats (six rats per group). Compared with Group C, aP<0.05; compared with Group B, bP<0.05. |
Changes in sciatic nerve structure
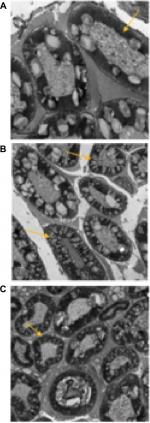
Electron microscopy showed that the myelin sheaths of the sciatic nerve fibers were intact in Group C (Figure 4A). In Group A, the nerve fiber structure of the rat sciatic nerve was impaired, with looser layer structure of the myelin sheath than Group C. The arrangement was irregular, and the myelin edema was severe. Transmission electron microscopy observed a large number of vacuoles, some of which were bulged toward the axons (Figure 4B). In Group B, the vacuoles formed by the myelin sheath of the sciatic nerve were occasionally observed, with neatly and compactly arranged layers. Individual axons were compressed, but the Schwann cells were not significantly swollen (Figure 4C).
Discussion
ECSWs have been used to treat urinary calculi in the past, but increasing numbers of relevant medical reports have confirmed that ECSWs have also been used to effectively treat chronic Achilles tendinitis and myofascial pain syndrome and can obtain outstanding efficacy.8,13 In recent years, it has been reported that low-energy ECSWs could promote nerve regeneration to treat DPN.14 Study by Chen et al15 demonstrated that ECSWs effectively protected the sciatic nerve in leptin-deficient rats by inhibiting inflammatory and oxidative stress. In this study, the rats with successful diabetic neuropathic pain showed decreased activity, apathy, yellowing of the coat, and typical diabetic symptoms, such as polydipsia, polyphagia, and polyuria. Both MWT and TWLs in rats were significantly decreased compared to their baseline thresholds. The duration of the reaction was significantly shorter than the baseline threshold. Both the MWT and the TWL values of the ECSW treatment group at T4 were significantly higher than those in the diabetic group. In this study, the structural integrity of the sciatic nerve fibers was damaged in Group A, with a large number of vacuoles formed in the myelin sheaths. In Group B, the vacuolation in the myelin sheath of the sciatic nerve was reduced, and the arrangement of the layers was more compact and tidy than Group A, indicating that ECSW treatment could effectively alleviate the neuropathic pain of diabetic rats.
Ceramide is a type of sphingolipids and can promote inflammatory and apoptotic reactions. It can not only act as a second messenger to activate downstream effector but also serve as a precursor of other secondary messengers, such as sphingomyelin. Sphingosine-1-phosphate (S1P) is a ceramide metabolized into sphingosine by the activating ceramidase. Sphk1 can further catalyze sphingosine to form S1P.16 S1P is a multifunctional lipid bioactive molecule that exerts a wide range of biological effects by binding to a specific receptor on the cell surface (S1PR). In the meantime, S1P participates in the development of vascular inflammation. S1P, secreted by platelets, monocytes, vascular endothelial cells, and smooth muscle cells, can regulate cell proliferation and migration and mediate pro-inflammatory responses and apoptosis.17 To date, five subtypes of G-protein-coupled S1P receptors have been discovered: S1PR 1–5.18 Janes et al19 showed that paclitaxel could induce neuropathic pain through activation of the S1P/S1PR1 signaling pathway and was associated with increased TNF-α formation. A large number of studies have shown that the occurrence of DPN is due to central sensitization and peripheral sensitization (abnormally high excitability of primary sensory neurons).20 Studies have shown that S1P also plays an important role in regulating the thermal stimulus response and the sensitivity of nociceptors. S1P can increase the sensitivity of peripheral nociceptive nerve endings, leading to increased excitability,21 whereas activation of sphk1 is key to increase S1P formation.22 At the same time, Finley et al23 found that S1P-mediated peripheral sensitization and hyperalgesia depended on neutrophil oogenesis. Alvarez et al24 found that S1P could activate the NF-κB signaling pathway, the “bridge” of the inflammatory response, and regulate the occurrence of the oxidative stress response, proving that Sphk1 could mediate the process of DPN by mediating oxidative stress. In addition, relevant experiments have shown that the expression of cyclooxygenase 2 and the production of prostaglandin E2 in the S1P-treated murine fibroblasts were increased in comparison to the ceramide-treated cells, demonstrating the specific role of S1P in inflammation.25 In this study, the expressions of Sphk1 and TNF-α in the right sciatic nerve of Group A rats were significantly higher than Group B at T4, which was also consistent with the behavioral findings after ECSW treatment in rats. At T4, the MWT and TWL values in Group A were significantly lower than those in Group B. It was shown that ECSW treatment could inhibit the formation of sphk1 and hence alleviate DPN.
TNF-α is a pro-inflammatory cytokine produced by macrophages and monocytes, which is mainly involved in inflammatory reactions and immune responses. Devaraj et al26 found that increased expression levels of microvascular inflammatory mediators in patients with type 1 diabetes mellitus could damage vascular endothelial cells. TNF-α is the most important inflammatory factor, and it can act on peripheral and central nervous tissues, causing changes in related nerve structures and functions. This study showed that the protein expression of TNF-α in Group A was significantly higher than that in Group C, suggesting that high levels of inflammatory factors might be associated with neuropathy. The increased secretion of TNF-α could increase vascular permeability, induce thrombosis, increase risk of microvascular endothelial disease, reduce blood flow, and cause nerve ischemia and hypoxia, resulting in demyelination. The expression of TNF-α in the right sciatic nerve at T4 in Group B was significantly lower than that in the diabetic group, indicating that ECSWs treatment could regulate the expression of TNF-α.
At the same time, in a previous study, Zhi et al27 reported that TNF-α interacted with Sphk1 in human monocytes and found that TNF-α could rapidly activate Sphk1 and further mediate the secretion of S1P. The results of Doyle et al28 showed that thermal hyperalgesia caused by intracervical injection of ceramide was associated with an increase in TNF-α in paw tissue. In addition, experiments have shown that toll-like receptor 4 (TLR4) is widely distributed in nerve cells, and its mediation of chronic inflammation not only can cause many diabetic complications, such as diabetic neuropathy, but also has obvious effects on the microcirculation of the nervous system and the body’s internal environment.29 TNF-α is an inflammatory cytokine of the downstream TLR4 signaling pathway. A large number of experiments have shown that the expression of TNF-α is low under normal conditions. However, once the TLR4 pathway is activated after nerve damage, it induces a large amount of TNF-α secretion, which causes neuronal cell damage and mediates neuronal cell death, leading to abnormal discharge of neurons and peripheral sensitization and even development of diabetic neuralgia.30 In addition, the involvement of Sphk1 is required for TNF-α-mediated pro-inflammatory reactions, adhesion, and chemotaxis. Thus, Sphk1 might mediate inflammatory responses by acting on the NF-κB signaling pathway and TNF-α. At 4 weeks after ECSW treatment of rat sciatic nerves, TNF-α expression showed a significant decrease, which was in consistent with the trend in Sphk1 protein expression, indicating that the interaction between Sphk1 and TNF-α might lead to the development of DPN and that ECSW could improve pain symptoms by inhibiting the secretion of Sphk1 and TNF-α.
However, certain limitations in this experiment do exist as follows. First of all, short observation time might lead to an ambiguity of the long-term therapeutic effect of the ECSW. Second, the difference in therapeutic effect between shock waves at different energy levels was not detected and compared in this experiment, which could also result in a less comprehensive discussion. Additionally, the small sample size is probably one of the deficiencies in this very experiment.
Conclusion
This study ultimately demonstrated that ECSWs could be used to treat DPN by interfering with Sphk1 formation, reducing TNF-α secretion, inhibiting oxidative stress and inflammatory response, alleviating endothelial dysfunction, and inhibiting peripheral sensitization. However, its clinical effects needs further studied. Meanwhile, owing to reasons such as small sample size, short observation time, and so on, this experiment has certain limitations. In addition, in the future experiments, we are supposed to continuously study the long-term effects of shock waves to understand the therapeutic difference of shock waves at different energy levels.
Ethics approval and informed consent
The experiment was approved by the ethics committee of Guizhou Provincial People’s Hospital. All rats in the experiment were provided and fed by the Experimental Center of the Third Military Medical University (animal use license number SCXK(R) 2012-0005).
Acknowledgment
Guizhou Foreign Cooperation Project, (Qian KeHeWai G[2014]7018), Effects of different energy shock waves on nerve function and structure in rats with DPN, 2015.1-2018.1.
Disclosure
The authors report no conflicts of interest in this work.
References
Tesfaye S, Vileikyte L, Rayman G, et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011;27(7):629–638. | ||
Shi XH. Study on the pathogenesis of diabetic peripheral neuropathy. Shanghai Med. 2016;37(2):3–7. Chinese. | ||
Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305. | ||
Lin XH, Chen XY, Zhou JX. Effects of pryrine in the treatment of diabetic painful peripheral neuropathy. China J Diab. 2014;22(8):707–710. | ||
Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68(15):1178–1182. | ||
Pollack MH, Matthews J, Scott EL. Gabapentin as a potential treatment for anxiety disorders. Am J Psychiatry. 1998;155(7):992–993. | ||
Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain in adults. The Cochrane Library. John Wiley & Sons, Ltd; 2009. Online. Available from: https://www.cochrane.org/CD007938/SYMPT_gabapentin-chronic-neuropathic-pain-adults. Accessed January 08, 2019. | ||
Hasselbalch L, Hölmich P. [Extracorporeal shock wave therapy in chronic Achilles tendinopathy]. Ugeskr Laeger. 2017;179(40):V08160596. Danish. | ||
Hong JO, Park JS, Jeon DG, Yoon WH, Park JH. Extracorporeal shock wave therapy versus trigger point injection in the treatment of myofascial pain syndrome in the quadratus lumborum. Ann Rehabil Med. 2017;41(4):582–588. | ||
Mariotto S, de Prati AC, Cavalieri E, Amelio E, Marlinghaus E, Suzuki H. Extracorporeal shock wave therapy in inflammatory diseases: molecular mechanism that triggers anti-inflammatory action. Curr Med Chem. 2009;16(19):2366–2372. | ||
Ciampa AR, de Prati AC, Amelio E, et al. Nitric oxide mediates anti-inflammatory action of extracorporeal shock waves. FEBS Lett. 2005;579(30):6839–6845. | ||
Jolivalt CG, Frizzi KE, Guernsey L, et al. Peripheral neuropathy in mouse models of diabetes. Curr Protoc Mouse Biol. 2016;6(3):223. | ||
Hong JO, Park JS, Jeon DG, Yoon WH, Park JH. Extracorporeal shock wave therapy versus trigger point injection in the treatment of myofascial pain syndrome in the quadratus lumborum. Ann Rehabil Med. 2017;41(4):582–588. | ||
Lohse-Busch H, Marlinghaus E, Reime U, Möwis U. Focused low-energy extracorporeal shock waves with distally symmetric polyneuropathy (DSPNP): a pilot study. Neurorehabilitation. 2014;35(2):227–233. | ||
Chen YL, Chen KH, Yin TC, et al. Extracorporeal shock wave therapy effectively prevented diabetic neuropathy. Am J Transl Res. 2015;7(12):2543. | ||
Salvemini D, Doyle T, Kress M, Nicol G. Therapeutic targeting of the ceramide-to-sphingosine 1-phosphate pathway in pain. Trends Pharmacol Sci. 2013;34(2):110–118. | ||
Mahajan-Thakur S, Böhm A, Jedlitschky G, Schrör K, Rauch BH. Sphingosine-1-phosphate and its receptors: a mutual link between blood coagulation and inflammation. Mediators Inflamm. 2015;2015:831059. | ||
Gräler MH. Targeting sphingosine 1-phosphate (S1P) levels and S1P receptor functions for therapeutic immune interventions. Cell Physiol Biochem. 2010;26(1):79–86. | ||
Janes K, Little JW, Li C, et al. The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1. J Biol Chem. 2014;289(30):21082–21097. | ||
Liu XG, Zhou LJ. Long-term potentiation at spinal C-fiber synapses: a target for pathological pain. Curr Pharm Des. 2015;21(7):895–905. | ||
Mair N, Benetti C, Andratsch M, et al. Genetic evidence for involvement of neuronally expressed S1P1 receptor in nociceptor sensitization and inflammatory pain. PLoS One. 2011;6(2):e17268. | ||
Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374(5–6):413–428. | ||
Finley A, Chen Z, Esposito E, Cuzzocrea S, Sabbadini R, Salvemini D. Sphingosine 1-phosphate mediates hyperalgesia via a neutrophil-dependent mechanism. PLoS One. 2013;8(1):e55255. | ||
Alvarez SE, Harikumar KB, Hait NC, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084–1088. | ||
Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J Immunol. 2010;185(4):2570–2579. | ||
Devaraj S, Jialal I, Yun JM, Bremer A. Demonstration of increased toll-like receptor 2 and toll-like receptor 4 expression in monocytes of type 1 diabetes mellitus patients with microvascular complications. Metabolism. 2011;60(2):256–259. | ||
Zhi L, Leung BP, Melendez AJ. Sphingosine kinase 1 regulates pro-inflammatory responses triggered by TNFalpha in primary human monocytes. J Cell Physiol. 2006;208(1):109–115. | ||
Doyle T, Chen Z, Obeid LM, Salvemini D. Sphingosine-1-phosphate acting via the S1P1 receptor is a downstream signaling pathway in ceramide-induced hyperalgesia. Neurosci Lett. 2011;499(1):4–8. | ||
Huang NQ, Jin H, Zhou SY, Shi JS, Jin F. TLR4 is a link between diabetes and Alzheimer’s disease. Behav Brain Res. 2017;316:234–244. | ||
De Paola M, Sestito SE, Mariani A, et al. Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model. Pharmacol Res. 2016;103:180–187. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.