Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Effects of Clinical and Tumor Characteristics on Survival in Patients with Hepatocellular Carcinoma with Bone Metastasis
Authors Ozer M, Goksu SY, Lin RY, Ayasun R, Kahramangil D, Rogers SC, Fabregas JC, Ramnaraign BH, George TJ , Feely M, Cabrera R, Duarte S, Zarrinpar A, Sahin I
Received 27 April 2023
Accepted for publication 10 July 2023
Published 19 July 2023 Volume 2023:10 Pages 1129—1141
DOI https://doi.org/10.2147/JHC.S417273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mohamed Shaker
Muhammet Ozer,1 Suleyman Yasin Goksu,2 Rick Y Lin,3 Ruveyda Ayasun,4 Doga Kahramangil,5 Sherise C Rogers,5,6 Jesus C Fabregas,5,6 Brian H Ramnaraign,5,6 Thomas J George,5,6 Michael Feely,7 Roniel Cabrera,8 Sergio Duarte,9 Ali Zarrinpar,9 Ilyas Sahin5,6
1Department of Medical Oncology, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA, USA; 2Division of Hematology/Oncology, Department of Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA; 3Department of Medicine, University of Florida Health Cancer Center, Gainesville, FL, USA; 4Laura and Isaac Perlmutter Cancer Center, New York University Langone Medical Center, New York, NY, USA; 5Division of Hematology/Oncology, Department of Medicine, University of Florida, Gainesville, FL, USA; 6University of Florida Health Cancer Center, Gainesville, FL, USA; 7Department of Pathology, Immunology, and Laboratory Medicine, University of Florida, Gainesville, FL, USA; 8Division of Gastroenterology, Hepatology, and Nutrition, University of Florida, Gainesville, FL, USA; 9Department of Surgery, College of Medicine, University of Florida, Gainesville, FL, USA
Correspondence: Ilyas Sahin, Department of Medicine, University of Florida, Gainesville, FL, 32608, USA, Email [email protected]
Background: Advanced hepatocellular carcinoma (HCC) generally has a dismal prognosis. Bone metastases from HCC are infrequent, with a poorer prognosis. However, the survival influencing factors are not yet well understood.
Aim: The aim of the present study was to assess the clinical features and tumor characteristics of HCC patients with bone metastasis.
Methods: A cohort of 170,576 adult patients with HCC was studied using the National Cancer Database (NCDB) spanning from 2010 to 2019, and within this group, 5285 patients (3.1%) were diagnosed with bone metastasis. We performed the Kaplan–Meier method to calculate the median overall survival (OS). We included demographics (age at diagnosis, gender, race, insurance status), comorbidity score, and treatment characteristics.
Results: Of a total of 5285 HCC patients with bone metastasis, 86.2% were male and 61.2% were non-Hispanic white. Most patients (55.1%) were below 65, and 89% had a total Charlson-Deyo comorbidity score of under 3. Among patients with known tumor grade, 24.8% had well-differentiated tumors, and 36.1% had poorly differentiated tumors. Chemotherapy was administrated to 39.5% of patients. In univariate analysis, patients with well-differentiated tumors had better OS compared to poorly differentiated tumors (5.4 months vs 3.0 months, p = 0.001). Patients who received single or multiagent chemotherapy were significantly associated with improved OS compared to patients who did not receive chemotherapy (7.0 and 8.5 months vs 1.94 months, respectively). We also found mortality difference between age, comorbidity scores, facility types and race groups.
Conclusion: In this cohort analysis of NCDB data, we found better OS in treatment receipt, lower tumor grade, younger age, non-Hispanic Black and Hispanic race, treatment at academic facility and lower comorbidity score in HCC patients with bone metastasis. The study results may have a consequential impact on the treatment decisions for HCC patients with bone metastasis.
Keywords: hepatocellular carcinoma, HCC, bone metastasis
Introduction
Recent developments in diagnostic and therapeutic methods have improved the overall survival (OS) of HCC patients.1 However, most HCC patients are diagnosed at the locally advanced stage. Although extrahepatic metastasis in HCC is uncommon during the initial diagnosis, it is becoming more significant as advancements in the management of intrahepatic lesions have led to improved OS.2 Given the dismal prognosis of advanced HCC, early diagnosis, and screening become crucial to obtain favorable outcomes. The 5-year survival rate of advanced HCC is less than 20%.3 At the time of HCC diagnosis, 14–36.7% of the patients were found to have distant metastasis.4,5
Bone metastases from HCC are infrequent, with a poorer prognosis with a median survival time of 2 to 4.6 months.6,7 Until recently, bone metastases from HCC were rarely described, and many studies were limited to small sample sizes. Santini et al observed that 5–25% of HCC patients developed bone metastasis.8 In another study, Natsuizaka et al reported that 16.1% to 38.5% of HCC patients had bone metastasis at the time of diagnosis and 11.7% developed bone metastasis later.9 The most common bone metastasis sites in HCC patients are the spine, pelvis, and ribs.10 Among all, spinal metastasis accounts for approximately 40% of bone metastasis in HCC patients.9,11
It is widely accepted that bone metastasis is one of the most important factors that affect quality of life and survival in patients with HCC. Bone metastases from HCC were found to be associated with severe pain, spinal cord compression, pathological fractures, ambulatory dysfunctions, malignant hypercalcemia, the requirement for surgery or radiotherapy, and lower quality of life.1,12 Thus, there is an urgent need to identify risk factors and prognostic variables in HCC patients with bone metastasis. To date, the factors influencing their survival are not well understood.
In the present study, we evaluated the clinical features and tumor characteristics of HCC patients with bone metastasis. Furthermore, we performed survival analyses and reported prognostic factors among the HCC patients who presented with bone metastasis.
Materials and Methods
The National Cancer Database (NCDB) is a comprehensive collection of clinical oncology information in the United States. It covers more than 70% of newly diagnosed cancer cases and contains detailed data on patient demographics, socioeconomic status, tumor features, and treatment modalities. The NCDB is a de-identified database from over 1500 hospitals accredited by the Commission on Cancer (CoC).
Study Population
We evaluated 170,576 adult patients (≥18 years) diagnosed with HCC between 2010 and 2019 using the ICD-O-3/WHO 2008 site recode “C22.0” and ICD-O-3 histologic codes “8170–8175.” Patients with bone metastasis were included. We excluded patients with secondary tumors and those who did not receive the first line of treatment at the reporting center (N: 165,291) (Figure 1).
 |
Figure 1 Study flow chart. Patient selection criteria and the distribution based on year of diagnosis. |
Variables
This study focused on bone metastasis identified at the time of cancer diagnosis, and the data was collected by the NCDB from 2010 onwards. Chemotherapy administration was classified into three categories: none, single-agent, and multi-agent. The year of diagnosis was stratified into five groups: 2010–2011, 2012–2013, 2014–2015, 2016–2017, and 2018–2019. The study considered demographic variables such as age (<49, 50–64, ≥65 years), race and ethnicity (non-Hispanic white, non-Hispanic black, and Hispanic), and gender (male, female). Socioeconomic factors were defined as median income quartiles (< $40,227, $40,227–50,353, $50,354–63,332, ≥ $63,333), an education level (percentage of patients with no high school level ≥17.6%, 10.9–17.5%, 6.3–10.8%, <6.3%), rurality (metropolitan, urban, rural), insurance status (uninsured, private insurance, Medicaid, Medicare, other government insurance). The facility type was categorized as academic/research and non-academic. The Charlson-Deyo Comorbidity Score is a scoring system that considers several medical conditions to predict the likelihood of mortality within one year of hospitalization. The score is calculated by assigning weights to various comorbidities and is categorized into four groups ranging from 0 to 3. Additionally, the cancer grade was categorized as well-differentiated (grade I), moderately differentiated (grade II), and poorly differentiated (grade III).
Statistical Analysis
In this study, we provided a comprehensive account of the baseline characteristics, which included demographic and socioeconomic factors (such as age at diagnosis, gender, race, insurance status, rurality, median income, education level), facility type, comorbidity score, cancer grade, and treatment characteristics. The percentages were used to present the findings. The median OS was calculated using the Kaplan–Meier method. Cox proportional hazards regression analysis was employed for multivariable analysis to adjust for confounding factors such as age, gender, race/ethnicity, comorbidity score, facility type, and chemotherapy. The statistical analyses were conducted using SPSS version 22.0. A p-value less than 0.05 was considered statistically significant. As the data was de-identified, the Institutional Review Board at the University of Florida deemed this study exempt from further review.
Results
Baseline Characteristics
In our cohort of 170,576 patients diagnosed with HCC, 3.1% (5285) had bone metastasis. The highest proportion of patients with bone metastasis occurred in 2016–2017 and 2018–2019, followed by 2014–2015, 2012–2013, and 2010–2011.
The majority of patients with bone metastasis were male (86.2%), non-Hispanic White (61.2%), and under 65 years of age (55.1%). More than one-third of them had a median income of less than $40,227 (30.5%) and were less educated (35%). Most of these patients resided in metropolitan areas (85.4%), while a smaller percentage lived in urban and rural areas (12.7% and 1.9%, respectively). Medicare insurance was the most common form of insurance (46.7%), followed by private insurance (26.1%). Most of the patients received treatment at non-academic facilities (58.6%) (Table 1).
 |
Table 1 Baseline Characteristics of Hepatocellular Carcinoma (HCC) Patients with Bone Metastasis |
In terms of comorbidity, over half of the patients had a Charlson-Deyo comorbidity score of 0 (52.2%), while 15.1% had a score of ≥3. Among patients with known tumor grade, 24.8% had well-differentiated tumors, 39.1% had moderately differentiated tumors, and 36.1% had poorly differentiated tumors.
Of the total patient population, 39.5% (2043) received chemotherapy (NCDB classifies multityrosine kinase inhibitors as chemotherapy), with the majority receiving single-agent chemotherapy (91.7%) and the remaining receiving multi-agent chemotherapy (8.3%).
Subgroup analyses demonstrated that patients below 50 years were most likely to have a comorbidity score of zero (64.5%, compared with 53.1% and 50.1% for ages 50–64 and 65+, respectively, p < 0.001). In addition, they had a greater prevalence of chemotherapy use than those in the 50–64 and 65+ age groups (47.4% vs 41.5% vs 36.6%, respectively, p < 0.001) (Supplementary Table 1). Regarding gender, although males had a higher tendency to undergo chemotherapy than females (40.1% vs 35.9%, p = 0.03), females were more likely to receive multi-agent chemotherapy than males (12.0% vs 7.8%, p = 0.03) (Supplementary Table 2). Lastly, as for facility type, patients treated at academic institutions were more likely to undergo chemotherapy than those at non-academic facilities (41.8% vs 37.8%, p = 0.004) (Supplementary Table 3).
Survival Analysis
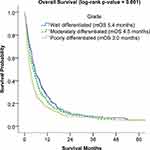
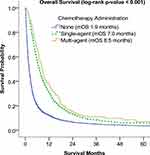
In this study, univariate survival analyses showed that patients with well-differentiated tumors had significantly better OS than those with poorly differentiated tumors (5.4 months vs 3.0 months, p = 0.001) (Figure 2). Additionally, both single- and multi-agent chemotherapy as first-course therapy significantly improved OS compared to patients who did not receive chemotherapy (7.0 months vs 1.94 months and 8.5 months vs 1.94 months, respectively) (Figure 3).
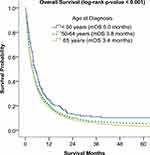
Younger patients (<50 years) had a better OS compared to those in the average age group (50–64 years) and older adults (≥65 years) (5.0 months vs 3.8 months vs 3.4 months, respectively, p < 0.001) (Figure 4). Non-Hispanic White patients had a decreased median survival time compared to non-Hispanic Black and Hispanic patients (3.4 months vs 4.0 months vs 4.0 months, respectively, p < 0.001). Patients treated at academic facilities had a favorable survival compared to those treated at non-academic facilities (4.3 months vs 3.2 months, p < 0.001). Patients with a total Charlson-Deyo score of 0 had better survival than those with a total Charlson-Deyo score of 3 (4.1 months versus 2.1 months, p < 0.001). There were no significant differences in survival based on gender (Supplementary Table 4).
Discussion
In the current study, we reported that 3.1% (5285) of the HCC patients had bone metastasis. Similarly, a study from Surveillance, Epidemiology, and End Results (SEER) database between 2010 and 2014 reported that 4.29% of HCC patients were found to have bone metastasis.11, They reported the estimated incidence of 2–25% in HCC patients with bone metastasis. Previously, the incidence of bone metastasis in HCC was reported to increase from 4.5% during 1978–1987 to 12.9% during 1988–1997.13 Although bone metastasis is not common in HCC, the detection of bone metastasis is increasing because of the development of survival and diagnostic modalities. In our large cohort, when we stratified the HCC patients with bone metastasis over the years, we found that the number of bone metastasis increased over time, with a peak of 24.4% cases in 2019 (Figure 1).
Single-agent sorafenib or systemic chemotherapy with doxorubicin, gemcitabine, or combined regimens was reported to improve survival in HCC patients with bone metastasis.3,14 It is worth mentioning that sorafenib has been established as the primary treatment option for patients with advanced HCC ever since the FDA first granted its approval for HCC in 2007 and has largely displaced traditional chemotherapy in practice. In the current study, we identified that chemotherapy (single and multi-agent regimens including tyrosine kinase inhibitors (TKIs) such as sorafenib) as a first-course treatment was associated with improved OS in HCC patients with bone metastasis. While the median survival rate among chemotherapy recipients was between 7 and 8.5 months, the survival rate in the patients without systemic treatment was 1.94 months (Figure 2). Similar to our study, Hu et al showed that the absence of systemic chemotherapy in HCC patients with bone metastasis was an independent prognostic risk factor and related to poor prognosis.15 In our further analysis for the factors related to survival, we found that younger age (<50 years), lower tumor grade, receiving treatment at the academic facility, and lower comorbidity scores were associated with better OS. This could be attributed to the fact that younger patients tend to be healthier and more often receive aggressive treatments. The observed discrepancies between facilities could be explained by the differences in quality of care and the varying expertise levels across different categories of cancer centers. In their study, higher rates of bone metastasis in the male sex, higher T stage, and intrahepatic metastatic disease were found. In another study with 37 patients, Kim et al did not show a survival difference between locoregional and/or systemic chemotherapy compared to supportive care in HCC patients with bone metastasis.16 It is important to note that the study was conducted before the era of sorafenib and with a relatively small sample size between June 2000 and April 2007. This may partly explain why there was no difference between locoregional and systemic treatment for HCC patients with bone metastasis.
Although the prognosis of HCC patients with bone metastasis is dismal, early detection of bone metastasis is extremely important to provide timely treatment.17 Given the low incidence of bone metastasis in HCC patients, the optimal treatment strategies are not well defined. HCC patients with bone metastasis should be considered for systemic treatment and palliative bone-directed localized therapies. Bone metastasis-related events, including severe pain, spinal cord compression, pathological fractures, and ambulatory dysfunctions, significantly impact the patients.1 Currently, there are no widely accepted bone metastasis screening strategies in HCC patients. Therefore, it is crucial to identify the risk factors to predict the patients at high risk of bone metastasis and spare them from subsequent complications. Bone scintigraphy is the most utilized imaging tool for skeletal metastases. Primarily, it has a high sensitivity for osteosclerotic or mixed metastases.18,19 On the other hand, FDG-PET has the ability to detect 80% of bone metastases in HCC and may be easier to obtain in clinical practice.20,21 Although The National Comprehensive Cancer Network (NCCN) guidelines do not currently recommend routine PET/CT due to its limited sensitivity but high specificity, it is suggested that PET/CT may be considered in cases where there is an equivocal finding. Specifically, when an HCC is detected by CT or MRI and exhibits increased metabolic activity on PET/CT, a higher intralesional standardized uptake value may indicate biologic aggressiveness and predict a less optimal response to locoregional therapies. NCCN does recommend a bone scan and/or additional bone imaging if there is suspicious bone pain or if cross-sectional imaging suggests the possibility of bone metastases.
The identification of biomarkers potentially contributes to timely diagnosis and could improve survival rates through prognostication and treatment response monitoring. Therefore, previous studies evaluated various prognostic factors. Most importantly, He et al have shown that higher alpha-fetoprotein (AFP) levels were significantly related to poorer survival in HCC patients with bone metastasis.12 In addition to AFP, previous studies suggested various risk factors, including Child-Pugh class, tumor size, Tomita scoring, BMI, marital status, skeletal-related events, and treatment history.10,11,22–25 Hu et al developed a diagnostic nomogram for predicting bone metastasis. They also reported a prognostic nomogram in HCC patients with bone metastasis.15 They identified that T and N stage, sex, and tumor grade were significant predictors for bone metastasis in HCC patients. Also, various molecular expressions, including LncRNA34a, stromal cell-derived factor-1 (SDF-1), connective tissue growth factor (CTGF), interleukin-11 (IL-11), chemokine receptor CXCR4, MicroRNA-34a were found to be associated with bone metastasis in HCC patients.26–30 Similarly, Zhang et al reported higher expression levels of Lnc34a in HCC patients with bone metastasis than those without bone metastasis.29
Consistent with previous reports, the axial bones were the most common sites of bone metastasis in HCC patients in the current study. Studies hypothesized that portal hypertension-related collateral formations around the vertebral vein system promote the metastasis.1 Although the steps of bone metastasis from solid tumors are similar, the precise pathophysiology of early bone metastasis from HCC remains elusive. The development of bone metastasis in HCC involves a complex mechanism influenced by multiple factors. Vascular endothelial growth factor (VEGF), recognized as a crucial driver of angiogenesis in both the primary HCC lesion and its metastasis to bone, is thought to have a critical role in promoting bone resorption and facilitating tumor growth in the bone microenvironment.31 The molecular mechanisms, such as the development of the extracellular matrix, stemness, metabolic and epigenetic changes, dysregulated non-coding RNAs, and activation of the p38 stress-induced pathway, have the potential to activate dormant cancer cells and drive their progression towards the formation of micro-metastases.32 This multi-step process starts with detaining cancer cells from the primary tumor site and invading the regional blood vessels and lymph nodes. Once cancer cells reach distal capillaries, they extravasate and disseminate to adjacent tissues and bone marrow to further progress to bone metastasis.33,34 With the progression of metastasis, dormant cells start to proliferate extensively and lead to the modification of the bone structure. Adhesive molecules interact with cancer cells with bone matrix and increase bone resorbing pro-angiogenic molecules production. Moreover, resorption of the bone induces the release of platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), insulin-like growth factors (IGF), and bone morphogenetic proteins.35,36 Although advancements have enabled the study of rare cells, including dormant cells in the bone microenvironment, enhancing our understanding of early bone metastasis development, the precise pathophysiological mechanisms that underlie the crucial early events in HCC-related bone metastasis are not yet fully understood.37 Current mouse models do not accurately represent clinical progression, hindering the development of therapies targeting dormancy.38 Targeting dormancy could be key to reducing bone metastasis incidence. Further molecular-based studies are needed to illuminate the precise mechanisms further.
Most of the treatment modalities for bone metastases aim to palliate symptoms. Radiotherapy has been used for palliative purposes, however, with limited effect on survival. A study from Seong et al reported relieved pain in 73% of the HCC patients with bone metastasis with palliative radiotherapy with a median dose of 30 Gy.39 Chow et al reported complete pain response in 33% of the patients.40 In another study with 192 HCC patients with bone metastasis, Choi et al reported a complete pain response in 21.4% of the patients.41 Recently, ablative radiotherapies (higher doses in few fractions) gained popularity with a potentially positive impact on survival rates.42 Most recently, Kim et al demonstrated improved OS with ablative radiotherapy.43 Some studies showed that combination modalities with transarterial chemoembolization (TACE) and radiotherapy provided a higher percentage of permanent pain relief than TACE or radiotherapy alone.44,45
Strengths and Limitations
Our study has individual strength in reporting the tumor and clinical characteristics on survival in HCC patients with bone metastasis in a large cohort that is not studied well in the current literature. Also, our study has several limitations, particularly with being a retrospective study; it inevitably carries the risk of selection bias. As the NCDB is not a population-based database, the HCC patients with bone metastasis included in this study may not be representative of the entire US population. The information collected in the NCDB database is open to potential coding errors. Bone metastasis was recorded at the first diagnosis of HCC; therefore, the patients who developed bone metastasis in the latter stage cannot be recorded. Also, NCDB does not have information regarding certain variables that could affect OS, including Karnofsky performance status, Child-Pugh classification, Skeletal-related events, treatment details and the etiology of the HCC. Another limitation of the study is the underrepresentation of women in the study population, with men comprising approximately 86% of the cohort. Lastly, the NCDB is limited to documenting only the first course of treatment with lack of details on subsequent therapeutic interventions, their regimens, or the specific dosages involved. Also, the current study did not include immunotherapies as part of systemic treatments, which have been increasingly utilized for HCC patients following FDA approvals in recent years.
Conclusion
Our study found better OS in treatment receipt, lower tumor grade, younger age, non-Hispanic Black and Hispanic race and lower comorbidity score in HCC patients with bone metastasis. As the first-line treatment, both single and multi-agent chemotherapies improved OS compared to patients who did not receive chemotherapy. Small sample sizes and a lack of nationwide oncology outcome data have limited previous studies of HCC patients with bone metastasis. Therefore, future prospective studies should be conducted to determine the risk factors and the survival characteristics of HCC patients with bone metastases with therapies designed to optimize clinical outcomes.
Until recently, tyrosine kinase inhibitors were the primary treatment option for unresectable liver cancer. However, there has been a rapid emergence of new data that has significantly reshaped the treatment paradigm for HCC. The combination of atezolizumab and bevacizumab, which involves immunotherapy along with anti-VEGF therapy, as well as the combination of durvalumab and tremelimumab, two immunotherapy drugs, have now become the preferred first-line treatment for unresectable or metastatic HCC. These novel therapies have demonstrated promising effects. Nevertheless, challenges such as high rates of metastasis and drug resistance still remain. Currently, ongoing clinical trials are primarily focused on assessing the efficacy of immune checkpoint blockade using antibodies against programmed cell death 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). These trials evaluate both monotherapy and combination therapy approaches for HCC patients.46 In addition to these advancements, traditional Chinese medicines (TCMs) have gained recognition for their multi-targeted and coordinated intervention effects against HCC. Accumulating evidence suggests that TCMs may have the ability to inhibit malignancy and impede the progression of HCC.47
In summary, the treatment landscape for unresectable liver cancer has undergone a notable transformation, with immunotherapy-based regimens superseding TKIs. Ongoing clinical trials continue to explore the potential of immune checkpoint blockade, while traditional Chinese medicines offer an additional avenue with their multi-faceted mechanisms in combating HCC. Further research will be required to investigate whether these therapies can enhance outcomes for patients with bone metastasis.
Study Approval Statement
This study protocol was reviewed and approved by the University of Florida Internal Review Board. This study has been granted an exemption from requiring ethics approval.
Statement of Ethics
This study has been granted an exemption from requiring ethics approval. Patient consent was not required by the IRB as all data was deidentified and the study was retrospective and involved no intervention. Patient data and confidentiality were respected in accordance with the Declaration of Helsinki.
Author Contributions
Muhammet Ozer and Ilyas Sahin designed the study. Suleyman Yasin Goksu participated in the acquisition analysis and interpretation of the data. Muhammet Ozer, Suleyman Yasin Goksu, Rick Y Lin, Ruveyda Ayasun, Doga Kahramangil and Ilyas Sahin drafted the initial manuscript. Ilyas Sahin revised the article critically for important intellectual content. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There are no funding sources.
Disclosure
Dr Sherise C Rogers is a consultant for Natera Oncology and reports grants from Robert A Winn Diversity in Clinical Trials, outside the submitted work. Dr Jesus Fabregas reports grants from BMS Foundation, during the conduct of the study; institutional research support from Natera, outside the submitted work. Dr Brian H Ramnaraign is a consultant for Pfizer and Ipsen, outside the submitted work. Dr Thomas J George is a consultant for Tempus Labs and Billion To One, outside the submitted work. The authors have no other conflicts of interest to declare in this work.
References
1. Longo V, Brunetti O, D’Oronzo S, Ostuni C, Gatti P, Silvestris F. Bone metastases in hepatocellular carcinoma: an emerging issue. Cancer Metastasis Rev. 2014;33(1):333–342. doi:10.1007/s10555-013-9454-4
2. Kanda M, Tateishi R, Yoshida H, et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008;28(9):1256–1263. doi:10.1111/j.1478-3231.2008.01864.x
3. Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1(3–4):144–158. doi:10.1159/000343828
4. Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. doi:10.1148/radiology.216.3.r00se24698
5. Shuto T, Hirohashi K, Kubo S, et al. Treatment of adrenal metastases after hepatic resection of a hepatocellular carcinoma. Dig Surg. 2001;18(4):294–297. doi:10.1159/000050155
6. Attili VS, Babu KG, Lokanatha D, Bapsy PP, Ramachandra C, Rajshekar H. Bone metastasis in hepatocellular carcinoma: need for reappraisal of treatment. J Cancer Res Ther. 2008;4(2):93–94. doi:10.4103/0973-1482.42257
7. Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20(11):1781–1787. doi:10.1111/j.1440-1746.2005.03919.x
8. Santini D, Pantano F, Riccardi F, et al. Natural history of malignant bone disease in hepatocellular carcinoma: final results of a multicenter bone metastasis survey. PLoS One. 2014;9(8):e105268. doi:10.1371/journal.pone.0105268
9. Lu Y, Hu JG, Lin XJ, Li XG. Bone metastases from hepatocellular carcinoma: clinical features and prognostic factors. Hepatobiliary Pancreat Dis Int. 2017;16(5):499–505. doi:10.1016/S1499-3872(16)60173-X
10. Lee MH, Lee SH, Kim ES, Eoh W, Chung SS, Lee CS. Survival-related factors of spinal metastasis with hepatocellular carcinoma in current surgical treatment modalities: a single institute experience. J Korean Neurosurg Soc. 2015;58(5):448–453. doi:10.3340/jkns.2015.58.5.448
11. Guo X, Xu Y, Wang X, et al. Advanced hepatocellular carcinoma with bone metastases: prevalence, associated factors, and survival estimation. Med Sci Monit. 2019;10(25):1105–1112. doi:10.12659/MSM.913470
12. He J, Zeng ZC, Tang ZY, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer. 2009;115(12):2710–2720. doi:10.1002/cncr.24300
13. Fukutomi M, Yokota M, Chuman H, et al. Increased incidence of bone metastases in hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2001;13(9):1083–1088. doi:10.1097/00042737-200109000-00015
14. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
15. Hu C, Yang J, Huang Z, et al. Diagnostic and prognostic nomograms for bone metastasis in hepatocellular carcinoma. BMC Cancer. 2020;20(1):494. doi:10.1186/s12885-020-06995-y
16. Kim SU, Kim DY, Park JY, et al. Hepatocellular carcinoma presenting with bone metastasis: clinical characteristics and prognostic factors. J Cancer Res Clin Oncol. 2008;134(12):1377–1384. doi:10.1007/s00432-008-0410-6
17. Bhatia R, Ravulapati S, Befeler A, Dombrowski J, Gadani S, Poddar N. Hepatocellular carcinoma with bone metastases: incidence, prognostic significance, and management-single-center experience. J Gastrointest Cancer. 2017;48(4):321–325. doi:10.1007/s12029-017-9998-6
18. Kanishi D. 99mTc-MDP accumulation mechanisms in bone. Oral Surg Oral Med Oral Pathol. 1993;75(2):239–246. doi:10.1016/0030-4220(93)90100-I
19. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45(1):53–64.
20. Ho CL, Chen S, Cheng TK, Leung YL. PET/CT characteristics of isolated bone metastases in hepatocellular carcinoma. Radiology. 2011;258(2):515–523. doi:10.1148/radiol.10100672
21. Sugiyama M, Sakahara H, Torizuka T, et al. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004;39(10):961–968. doi:10.1007/s00535-004-1427-5
22. Harding JJ, Abu-Zeinah G, Chou JF, et al. Frequency, morbidity, and mortality of bone metastases in advanced hepatocellular carcinoma. J Natl Compr Canc Netw. 2018;16(1):50–58. doi:10.6004/jnccn.2017.7024
23. Kim S, Choi Y, Kwak DW, et al. Prognostic factors in hepatocellular carcinoma patients with bone metastases. Radiat Oncol J. 2019;37(3):207–214. doi:10.3857/roj.2019.00136
24. Chang YS, Huang JS, Yen CL, et al. Body mass index above 24 is beneficial for the 6-month survival rate in hepatocellular carcinoma patients with extrahepatic metastases. Asia Pac J Clin Nutr. 2017;26(4):637–641. doi:10.6133/apjcn.062016.03
25. Seo HJ, Kim GM, Kim JH, Kang WJ, Choi HJ. 18F-FDG PET/CT in hepatocellular carcinoma: detection of bone metastasis and prediction of prognosis. Nucl Med Commun. 2015;36(3):226–233. doi:10.1097/MNM.0000000000000246
26. Zhang L, Niu H, Yang P, et al. Serum lnc34a is a potential prediction biomarker for bone metastasis in hepatocellular carcinoma patients. BMC Cancer. 2021;21(1):161. doi:10.1186/s12885-021-07808-6
27. Xiang ZL, Zeng ZC, Tang ZY, et al. Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer. 2009;9:176. doi:10.1186/1471-2407-9-176
28. Xiang ZL, Zhao XM, Zhang L, et al. MicroRNA-34a expression levels in serum and intratumoral tissue can predict bone metastasis in patients with hepatocellular carcinoma. Oncotarget. 2016;7(52):87246–87256. doi:10.18632/oncotarget.13531
29. Zhang L, Niu H, Ma J, et al. The molecular mechanism of LncRNA34a-mediated regulation of bone metastasis in hepatocellular carcinoma. Mol Cancer. 2019;18(1):120. doi:10.1186/s12943-019-1044-9
30. Xiang ZL, Zeng ZC, Tang ZY, et al. Potential prognostic biomarkers for bone metastasis from hepatocellular carcinoma. Oncologist. 2011;16(7):1028–1039. doi:10.1634/theoncologist.2010-0358
31. Iguchi H, Yokota M, Fukutomi M, et al. A possible role of VEGF in osteolytic bone metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2002;21(3):309–313.
32. Jahanban-Esfahlan R, Seidi K, Manjili MH, Jahanban-Esfahlan A, Javaheri T, Zare P. Tumor cell dormancy: threat or opportunity in the fight against cancer. Cancers. 2019;11(8):1207. doi:10.3390/cancers11081207
33. Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6(10):2609–2617. doi:10.1158/1535-7163.MCT-07-0234
34. Zheng Y, Zhou H, Dunstan CR, Sutherland RL, Seibel MJ. The role of the bone microenvironment in skeletal metastasis. J Bone Oncol. 2013;2(1):47–57. doi:10.1016/j.jbo.2012.11.002
35. Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. doi:10.1056/NEJMra030831
36. Clezardin P, Teti A. Bone metastasis: pathogenesis and therapeutic implications. Clin Exp Metastasis. 2007;24(8):599–608. doi:10.1007/s10585-007-9112-8
37. Yuan X, Zhuang M, Zhu X, et al. Emerging perspectives of bone metastasis in hepatocellular carcinoma. Front Oncol. 2022;12:943866. doi:10.3389/fonc.2022.943866
38. Esposito M, Guise T, Kang Y. The biology of bone metastasis. Cold Spring Harb Perspect Med. 2018;8(6):a031252. doi:10.1101/cshperspect.a031252
39. Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 2005;25(2):261–265. doi:10.1111/j.1478-3231.2005.01094.x
40. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423–1436. doi:10.1200/JCO.2006.09.5281
41. Choi C, Seong J. Predictive factors of palliative radiotherapy response and survival in patients with spinal metastases from hepatocellular carcinoma. Gut Liver. 2015;9(1):94–102. doi:10.5009/gnl14009
42. Zeng KL, Tseng CL, Soliman H, Weiss Y, Sahgal A, Myrehaug S. Stereotactic Body Radiotherapy (SBRT) for oligometastatic spine metastases: an overview. Front Oncol. 2019;9:337. doi:10.3389/fonc.2019.00337
43. Kim TH, Park S, Rim CH, Choi C, Seong J. Improved oncologic outcomes by ablative radiotherapy in patients with bone metastasis from hepatocellular carcinoma. J Cancer Res Clin Oncol. 2021;147(9):2693–2700. doi:10.1007/s00432-021-03553-2
44. Carrafiello G, Laganà D, Ianniello A, et al. Radiofrequency thermal ablation for pain control in patients with single painful bone metastasis from hepatocellular carcinoma. Eur J Radiol. 2009;71(2):363–368. doi:10.1016/j.ejrad.2008.04.019
45. Uemura A, Fujimoto H, Yasuda S, et al. Transcatheter arterial embolization for bone metastases from hepatocellular carcinoma. Eur Radiol. 2001;11(8):1457–1462. doi:10.1007/s003300000792
46. Ozer M, George A, Goksu SY, George TJ, Sahin I. The role of immune checkpoint blockade in the hepatocellular carcinoma: a review of clinical trials. Front Oncol. 2021;11:801379. doi:10.3389/fonc.2021.801379
47. Li JJ, Liang Q, Sun GC. Traditional Chinese medicine for prevention and treatment of hepatocellular carcinoma: a focus on epithelial-mesenchymal transition. J Integr Med. 2021;19(6):469–477. doi:10.1016/j.joim.2021.08.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.