Back to Journals » Journal of Inflammation Research » Volume 14
Effect of Vitamin D Deficiency and Supplementation in Lactation and Early Life on Allergic Airway Inflammation and the Expression of Autophagy-Related Genes in an Ovalbumin Mouse Model
Authors Zhou Y, Xue Y, Bao A, Han L, Bao W, Xia C, Tian X, Zhang M
Received 26 May 2021
Accepted for publication 13 August 2021
Published 24 August 2021 Volume 2021:14 Pages 4125—4141
DOI https://doi.org/10.2147/JIR.S321642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Yan Zhou,1,* Yishu Xue,1,* Aihua Bao,1,* Lei Han,1 Wuping Bao,1 Chao Xia,2 Xue Tian,1 Min Zhang1
1Department of Respiratory and Critical Care Medicine, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200081, People’s Republic of China; 2Department of Gerontology, Xin Hua Hospital Affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, 200092, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Min Zhang
Department of Respiratory and Critical Care Medicine, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, 100 Haining Road, Hongkou District, Shanghai, 200080, People’s Republic of China
Tel +86 21 63071428
Email [email protected]
Background and Objective: Vitamin D is involved in various physiological and pathological processes, including inflammation and autophagy. We aimed to investigate the effects of dietary vitamin D deficiency or supplementation initiated in lactation and early life on inflammation and autophagy in an ovalbumin (OVA) mouse model.
Methods: Female BALB/c were fed with vitamin D-deficient, sufficient or supplemented diets throughout lactation and their offspring followed the same diet after weaning. Offspring were then sensitized and challenged with OVA, airway resistance (RL) was measured, and their serum, bronchoalveolar lavage fluid (BALF), and lung tissue were collected. Alveolar macrophages (AMs) were isolated from lung tissue and cultured with different concentrations of 1,25(OH)2D3. The expressions of autophagy-related (ATG) proteins including light-chain 3 (LC3), Beclin-1, and ATG5, and NF-κB p65 in lung tissue and AMs were measured.
Results: OVA sensitization and challenge induced dramatic allergic airway inflammation and higher RL in the vitamin D-deficient group compared with vitamin D-sufficient or the supplemented group. The expression of ATGs including LC3, Beclin-1, and ATG5, and NF-κB p65 in lung tissue in the vitamin D-deficient OVA-mediated group was increased compared with vitamin D-supplemented OVA-mediated group. There was correlation between the expression of LC3 mRNA and inflammatory cell numbers and cytokines in BALF. In vitro, 1,25(OH)2D3 also regulated the expression of LC3, Beclin-1, ATG5, and NF-κB p65 mRNA in AMs in a time- and dose-dependent manner.
Conclusion: Deficiency of vitamin D in early life may aggravate allergic airway inflammation, and maintaining sufficient vitamin D during early life is necessary for lung health. Vitamin D may modulate autophagy in lungs of OVA sensitized/challenged mice, thus playing a protective role in OVA-induced allergic airway inflammation.
Keywords: vitamin D, allergic airway inflammation, asthma, autophagy, LC3, Beclin-1, ATG5
Introduction
Asthma is a chronic airway disease that is increasing globally. It is characterized by airway hyper-responsiveness (AHR) and inflammation as a result of molecular and cellular responses.1 Increasing evidence indicates that both genetic and environmental factors are involved in the pathogenesis and development of allergic airway inflammation and that perinatal exposures are highly influential.2 Vitamin D deficiency has been implicated in various kinds of disorders, including infectious or allergic lung disease.3 Epidemiological and experimental research has also demonstrated that a lower serum vitamin D level in early life was associated with poorer lung function and higher sensitivity to aeroallergens,4 and supplementation of vitamin D during pregnancy as shown in the VDAART trial plays a role in averting less persistent forms of wheezing in infants with a parental history of asthma but has no effect in preventing asthma in schoolchildren.5,6 Thus, studies need to confirm the effect of different levels of vitamin D on allergic airway inflammation in early life. However, few studies have established murine models with vitamin D deficiency or supplementation in early life to study allergic airway inflammation and the relevant mechanisms.7,8
Vitamin D is synthesized by the skin in response to sunlight and available through diet in humans. Circulating 25-hydroxyvitamin D (25(OH)D) is a typical form used to evaluate vitamin D levels, and 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) is the most active vitamin D metabolite. Although 1,25(OH)2D3 is most commonly thought to regulate calcium homeostasis that affects bone metabolism, the wide distribution of vitamin D receptor (VDR) suggests that vitamin D may have much broader functions, including playing roles in apoptosis, inflammation, immunomodulation, and autophagy.9–12 The relationship between vitamin D and autophagy in innate immunity, inflammatory bowel diseases, infection, and cancer13–16 has been the subject of recent investigation. Studies have shown that vitamin D suppressed experimental autoimmune encephalomyelitis-induced LC3 in the brain13 or angiotensin II–induced LC3 in the kidney,14 while it increased LC3 II and Beclin-1 in enteroids15 and human peritoneal mesothelial cells.16 Nevertheless, it is still unclear how vitamin D regulates autophagy, especially in different vitamin D concentrations in early life in the mouse model of allergic airway inflammation.
Therefore, we speculated that allergic airway inflammation may be attributed to vitamin D deficiency in early life. In this study, we tried to verify our assumptions in vivo using vitamin D-deficient,7,8 vitamin D-sufficient,17,18 and vitamin D-supplemented19 mouse models of allergic airway inflammation, which were mediated with ovalbumin (OVA) initiated in lactation and early life. NF-κB is a key transcription factor that also regulates immune and inflammatory responses in asthma,20–22 and LL-37, as an antimicrobial peptide, can be induced by physiological concentrations of 1,25(OH)2D3 in established cell lines.23,24 Thus, NF-κB and LL37 were also studied to evaluate the allergic airway inflammation attributed to vitamin D deficiency in early life. Since LC3, Beclin-1, and ATG5 are the most well-known ATG proteins for autophagosome formation and closely related to allergic inflammation,12,25,26 the expressions of ATG proteins were studied in the presence of different levels of vitamin D in early life in the OVA-mediated mouse model and alveolar macrophages isolated from bronchoalveolar lavage fluid (BALF) to verify the relationship of vitamin D and autophagy.
Methods
Mice and Diets
Pathogen-free BALB/c mice were obtained from SLAC Laboratory Animal Co. Ltd. (Shanghai, China) and housed in controlled conditions of temperature (21–25°C) and humidity (40–60%), with ad libitum access to water (free from OVA) and food (details of diets are described below). Before beginning the experiments, mice were acclimatized for 1 week with a 12-h light-dark cycle. Female mice were randomly divided into three groups. At 8 weeks old, they were placed with male BALB/c mice of the same age for up to 2 weeks for breeding. During this time, all the mice were provided standard food containing 1,000 IU vitamin D/kg.17,18,27 Once the offspring were born, during lactation28 the maternal mice were fed with vitamin D-deficient (LVD) (0 IU vitamin D/kg), vitamin D-sufficient (NVD) (1,000 IU vitamin D/kg), and vitamin D-supplemented (HVD) diets (2,280 IU vitamin D/kg), respectively (Jinpan Laboratories, Shanghai, China). The LVD diets were also supplemented with 1.2% calcium and vitamins A, E, and K as in the NVD and HVD diets. The offspring were weaned on the same diet as their mothers and were primary experimental subjects for the rest of our research (see schematic diagram of mouse experiment in Appendix 1 of Supplementary Information). The numbers of female and male offspring studied in this study are shown in Table 1 of Supplementary Information.
Sensitization and Challenge of Ovalbumin in Mice
The offspring were sensitized and challenged with allergens as previously described.29 Offspring were sensitized by intraperitoneal injection (IP) with 10 μg chicken OVA (Grade V, Sigma Aldrich Co., St. Louis, MO, USA) emulsified in 2 mg of alum (Shanghai No. 4 Reagent & H.V. Chemical Industries Ltd., Shanghai, China) in a total volume of 100 μL of 0.9% sterile saline at 8 weeks of age (Day 0) and boosted 14 days later (Day 14). Non-sensitized mice received phosphate buffered saline (PBS) in the same volume. For the OVA challenge, mice were placed in a plastic box (50 × 30×40 cm3) and inhaled 1% OVA in a saline (10 mg/mL) aerosol delivered using an ultrasonic nebulizer (PARI BOY, Starnberg, Germany) for 30 min on 5 successive days from Day 21 to Day 25. Each group consisted of 7 to 9 mice.
Measurement of Airway Responsiveness
At 24 h after the last challenge, mice were anesthetized with Zoletil 50 (tiletamine hydrochloride and zolazepam hydrochloride, 25 mg/kg, Virbac S.A., France) and xylazine hydrochloride (10 mg/kg, Chang Sha Best Biological Technology Institute Co., Ltd, China) via IP injection,30 and an intratracheal cannula FTC 101 (1.12-mm inner diameter, 1.33-mm outer diameter) was inserted via tracheotomy. The tracheostomized mice were connected to a ventilator (MiniVent, Hugo Sach Electronic, Germany) and ventilated at 180 breaths/min and tidal volume of 210 μL in a whole-body plethysmograph with a pneumotachograph connected to a transducer (EMMS, Hants, UK)30,31 24 h after the last OVA challenge. Pulmonary airway resistance (RL) to inhaled acetylcholine chloride (ACh, Sigma, USA) was recorded for 3 min at each level as the concentration gradually increased (4, 8, 16, 32, 64, 128, and 256 mg/mL). RL was expressed as percentage change from baseline RL (measured following nebulized PBS). The ACh concentration needed to elevate RL by 100% from baseline was calculated (PC100). The -log PC100 was taken as an indication of airway hyper-responsiveness.30,31
Bronchoalveolar Lavage Collection and Measurements
After RL was recorded, the trachea was exposed and three aliquots of 0.6 mL sterilized saline were instilled through a PE-60 tube (0.72-mm inner diameter, 1.22-mm outer diameter). Return volume was recorded and the recovery rate was above 70%. The BALF was centrifuged at 1000 × g for 10 min at 4°C. The supernatant was stored at −80°C for further assay. The total cells in the pellet were resuspended in 1 mL of PBS solution and enumerated by counting with a hemocytometer. Differential cell counts were performed on cytospin slides with Wright–Giemsa stain by counting 200 cells under 400 times magnification from two independent, blinded investigators. Cells were identified by standard morphology and differentiated into neutrophils, eosinophils, lymphocytes, and macrophages.
ELISA
Serum 25(OH)D levels were analyzed by ELISA following the manufacturer’s instructions (R&D Systems China Co. Ltd., Shanghai, China). All the cytokines, including IL-4, IL-5, and IL-10 in supernatants of BALF were measured by commercial kits (R&D Systems China Co. Ltd., Shanghai, China), according to the manufacturer’s protocol.
Histological Analysis, Immunohistochemical Analysis, and Double Immunofluorescence Staining
After the mice were sacrificed, the lobes of left lungs were removed and fixed with 10% formaldehyde and embedded in paraffin. Tissues were cut into 4-μm sections and placed onto Fisher PLUS slides. After deparaffinization and rehydration, the sections were stained with hematoxylin-eosin (H&E) and observed under a microscope by two independent observers in a blinded manner using a reproducible scoring system.30 Each tissue section was scored with a value from 0 to 3 to document the pulmonary inflammation based on the percentage of the area containing cellular inflammation by two criteria: peribronchial inflammation and perivascular inflammation. A value of 0 was given when no inflammation was detectable, a value of 1 indicated occasional cuffing with inflammatory cells and less than one-third bronchi or vessels surrounded by thin layer, a value of 2 indicated more than one-third of the bronchi or vessels were surrounded by thin layer (one to five cells) or less than one-third surrounded by thick layer (more than five cells) of inflammatory cells, and a value of 3 indicated more than one-third of the bronchi or vessels were surrounded by a thick layer or more than two-thirds were surrounded by thin layer of inflammatory cells. The inflammation score was defined as the mean of scores of each area for two to three tissue sections per mouse.
All the primary and secondary antibodies used for immunohistochemical examinations were obtained from Servicebio (Wuhan, China). The primary antibodies included anti-LC3 protein antibody (GB13431) with 1/150 dilution and anti-Beclin-1 antibody (GB11228) with 1/1500 dilution. For each section stained, 10 high-power fields (400×) were randomly selected for analysis. Immunoreactivity of LC3 and Beclin-1 in lung tissue was quantified by histochemistry score (H-score),32,33 which incorporates both the intensity of the specific staining and the percentage of positive cells. The relative intensity of specific staining was defined as not present (0), weak but detectable above control level (1+), distinct (2+), and very strong (3+). The final score was the sum of the relative intensity of specific staining multiplied by the percentage of positive cells. The H-score analysis was carried out independently by two experienced pathologists who were blinded to the final clinical diagnosis of all cases studied. A third pathologist reviewed the score when there was an inconsistency between the two pathologists.
LC3 and Beclin-1 expression was also examined by double immunofluorescence staining of LC3 and Beclin-1 (GB13431 and GB11228, respectively, rabbit, Servicebio, Wuhan, China) on slides of lung tissues. Slides were incubated overnight with primary LC3 (1:1000) and Beclin-1 (1:2000) antibodies, followed by incubation with the FITC-515-555 (green) and CY3-590 (red) secondary antibodies (GB23303, GB21303, respectively, Servicebio, Wuhan, China) for 1 hr. The sections were then mounted with Vectashield (G1012, Servicebio, Wuhan, China) containing DAPI (blue). Cells were imaged and counted using Eclipse fluorescent microscopy (NIKON ECLIPSE C1, Japan). For each experiment, the percentage of LC3- and Beclin-1-positive cells of 100 cells were counted in 10 different randomly selected areas of each section of the lung. The merged signals of LC3 and Beclin-1 in the immunofluorescence images were labeled and the percentage of co-expression cells was also examined.
Isolation and Stimulation of Alveolar Macrophages (AMs)
The trachea of OVA sensitized/challenged mice with sufficient dietary vitamin D were surgically exposed and BALF collected with prewarmed (37°C) calcium- and magnesium-free Dulbecco’s PBS containing 0.5 mM EDTA three times in succession. The above steps were repeated in several mice and lung fluid pooled into a 50-mL conical centrifuge tube and centrifuged 10 min at 400 × g at 4°C. The precipitated cells were suspended in RPMI 1640 supplemented with penicillin (100 units/mL), streptomycin (100 mg/mL), and 5% fetal bovine serum to prepare a cell suspension at a concentration of 2 × 106/mL. Then, 250 μL (5 × 105 cells) was added to each well of a 24-well plate and incubated at 37°C in a humidified atmosphere of 5% CO2 for 2 h to promote adhesion to the cell walls. After washing the plates with RPMI 1640 three times, the adherent cells, mostly alveolar macrophages, were gently scraped with a curette and then fresh RPMI 1640 containing 10% FBS was added. The cultures were further incubated with or without different concentrations of 1,25(OH)2D334 (Calbiochem, San Diego, CA, USA) for 12 hr or 24 hr.
RNA Isolation, Reverse Transcription, and RT-qPCR
Total RNA was isolated from lung tissue and AMs using the TRIzol® Reagent (Invitrogen, Calif. US) and reverse-transcribed to cDNA using PrimeScriptTM RT Master Mix (TaKaRa, Japan). Real-time quantitative PCR (RT-qPCR) was performed with a TB Green Premix Ex TaqTM II (TaKaRa, Japan). Transcript levels were determined by the Applied Biosystems ViiA™ 7 System (Applied Biosystems, CA, USA) (See thermal cycling program and primer sequences of qPCR in Appendix 2.)
Western Blot Analysis
The following antibodies were used: LC3B (1:2000; 14600-1-ap; rabbit; Proteintech group, Wuhan, China), Beclin-1 (1:2000; GB112053; rabbit; Servicebio, Wuhan, China), ATG5 (1:2000; ab108327; rabbit; Abcam, MA, USA); and NF-κB p65 (1:2000; ab32536; rabbit; Abcam, MA, USA). Nuclear and cytoplasmic protein fractions were obtained from lung tissues by the addition of radioimmune precipitation assay (RIPA) lysates (Servicebio, China). The frozen mouse-lung tissue (20–100 mg) was homogenized using ice-cold cytoplasmic extraction reagents A and B with the addition of PMSF containing protease and phosphatase inhibitor (Servicebio, Wuhan, China) and centrifuged at 16,000 × g at 4°C for 5 min. Lung lysate was prepared separately for each mouse. The supernatant was transferred (cytoplasmic extraction) to a clean tube and kept at −80°C until used. The cell pellet was suspended and vortexed for 15 s every 10 min for 60 min on ice-cold nuclear extraction reagent (NER). The sample was centrifuged at 16,000 ×g for 10 min and supernatant (nuclear extraction) was immediately transferred into a clean pre-chilled tube at −80°C. For analysis of protein expression, the protein concentration was determined by a BCA Protein Assay Kit (Servicebio, China), and 50 μg samples were loaded onto 12% SDS-PAGE. The proteins were transferred to a PVDF membrane (Millipore, USA). The membranes were then blocked with 5% BSA in Tris-buffered saline (TBS) solution (20 mM Tris and 500 mM NaCl, pH 7.5) for 2 h at room temperature and probed with primary antibody for overnight incubation at 4°C with gentle rocking. After five to six washes (10 min each) with washing buffer (0.05% Tween 20 in PBS), the membrane was incubated with HRP-conjugated secondary antibodies (1:2000) (GB12001; rabbit; Servicebio, Wuhan, China) for 2 hr at room temperature. The membrane was washed three times and the immunoreactive bands were visualized using ECL chemiluminescence detection reagents (Millipore, USA).
Statistical Analysis
The results are expressed as mean ± SEM, unless otherwise specified. The Kolmogorov–Smirnov test with Dallal-Wilkinson-Lilie for p value was used to test if the values came from a Gaussian distribution. The Kruskal–Wallis one-way analysis of variance (ANOVA) with Bonferroni’s post-hoc test (for equal variance) or Dunnett’s T3 post-hoc test (for unequal variance) was used to evaluate the differences of variances between multiple groups. To determine the percentage of positive cells, results were compared using the Mann–Whitney U-test. For Spearman correlation analysis and statistical charting, SPSS 21.0 (SPSS Inc. Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA) were used. The significance level was considered to be p ≤ 0.05.
Results
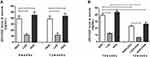
Dietary Vitamin D Supplementation Increased Circulating 25(OH)D Levels
Serum 25(OH)D levels were <20 or ≥40 ng/mL in offspring fed a vitamin D-deficient or supplemented diet, respectively (Figure 1A). The concentration of 25(OH)D in the three groups with different diets did not change in 8-week-old and 12-week-old mice (p > 0.05, Figure 1A). After OVA sensitization/challenge, the serum levels of 25(OH)D in the three groups were all decreased in comparison to their corresponding control group, and its level in the LVD+OVA group was lower than in the NVD+OVA and HVD+OVA groups (p < 0.05, Figure 1B).
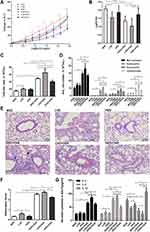
OVA Sensitized/Challenged Mice Exhibited Airway Hyperresponsiveness
There were no significant differences in the RL level at baseline following buffered PBS nebulization among six groups (p > 0.05, Figure 2A). Mice in the LVD control group exhibited a leftward shift of the concentration responsiveness curve of RL compared with the NVD and HVD control groups, while there were no significant differences in the percentage change from baseline RL at different concentrations (p > 0.05, Figure 2A). After OVA sensitization/challenge, animals in the three different diet groups all exhibited a leftward shift in the concentration responsiveness curve of RL, suggesting an increased airway responsiveness compared with their control mice on the same diets (p < 0.05, Figure 2A). Vitamin D-deficient OVA-mediated mice showed stronger airway responsiveness and significantly increased RL at 8 mg/mL, 16 mg/mL, 64 mg/mL, 128 mg/mL, and 256 mg/mL concentrations of ACh compared to all three control groups (p < 0.05, Figure 2A). The -log PC100 declined dramatically after OVA sensitization/challenge in the LVD and NVD groups, and their levels in these two groups were lower than in the HVD+OVA group or all the three control groups, indicating AHR in these two groups (p < 0.05, Figure 2B). The -log PC100 was lower in the LVD+OVA group than in the NVD+OVA group, but there was no significant difference (p > 0.05, Figure 2B).
The Effects of Different Vitamin D Diets on Airway Inflammation in OVA Sensitized/Challenged Mice
The total cell count, as well as numbers of macrophages, eosinophils, and neutrophils in BALF samples of OVA sensitized/challenged mice, was universally higher than those from corresponding controls (p < 0.05, Figure 2C and D). Among all three OVA-mediated groups, mice fed the LVD diet exhibited the highest value of total cell counts and four differential cells in BALF and they were higher than those in NVD and HVD groups (p < 0.05, Figure 2C and D). After OVA sensitization and challenge, the HVD group exhibited fewer total cells, eosinophils, and lymphocytes compared to the NVD and LVD groups (p < 0.05, Figure 2C and D).
Histologically, before OVA sensitization and challenge, the airways of mice in the NVD group exhibited normal parenchyma around the respiratory epithelium. The LVD mice exhibited more airway inflammation than the other two control groups (p < 0.05, Figure 2E and F). After OVA sensitization/challenge, there were more inflammatory cells and peribronchial or perivascular inflammation in the lungs of all three different diet groups than in the corresponding control group, and the inflammation in the HVD+OVA group was less than in the LVD+OVA group (p < 0.05, Figure 2E and F).
The Effects of OVA Sensitization/Challenge on Cytokines in BALF in Different Vitamin D Diet Groups
A significant increase was found in the levels of Th2-related cytokines, IL-4, and IL-5 (p < 0.05, Figure 2G) in the BALF of all OVA-sensitized and challenged groups compared to the corresponding control group. LVD mice had a higher level of IL-5 and lower level of IL-10 in the BALF compared to NVD and LVD groups (p < 0.05, Figure 2G). After OVA sensitization and challenge, levels of IL-5 and IL-4 were more pronounced in vitamin D-deficient mice, while the level of IL-10 was decreased (p < 0.05, Figure 2G). A significant increase in IL-10 in BALF was demonstrated in the vitamin D-supplemented OVA-mediated mice compared to the other two OVA-mediated groups (p < 0.05, Figure 2G).
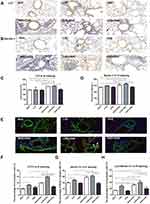
Immunohistochemical Analysis and Double Immunofluorescence Staining of LC3 and Beclin-1
The autophagy marker LC3 and Beclin-1 were detected by immunohistochemical analysis in the lung tissue. LC3 and Beclin-1 immunoreactivity was detected primarily in the cytoplasm of alveolar epithelial cells, macrophages, and the epithelial cells at the apical region of the airway in the mouse lung (Figure 3A and B). The H-score of LC3 and Beclin-1 was calculated. Accordingly, OVA sensitization and challenge could increase LC3 and Beclin-1 expression in the LVD and NVD groups, whereas in the vitamin D-deficiency group, the expression of LC3 and Beclin-1 was further increased (p < 0.05, Figure 3C and D). LC3 and Beclin-1 expression in LVD+OVA group was higher compared with the HVD+OVA group (p < 0.05, Figure 3C and D).
In immunofluorescence analysis, the percentage of LC3 and Beclin-1-positive cells in the LVD+OVA and NVD+OVA groups was higher than in their corresponding control groups, whereas the expression of LC3 and Beclin-1 in LVD+OVA group was further increased (p < 0.05, Figure 3E-G). LC3 and Beclin-1 expression in the LVD+OVA group was higher compared with the HVD+OVA group (p < 0.05, Figure 3F and G). The merged signals of LC3 and Beclin-1 in the immunofluorescence images were yellow and detected primarily in the cytoplasm of alveolar epithelial cells (Figure 3E). The percentage of co-expression positive cells was higher in the LVD+OVA group compared with the HVD+OVA and NVD+OVA groups (p < 0.05, Figure 3H). Collectively, these results demonstrated that vitamin D deficiency produces autophagy-related signals.
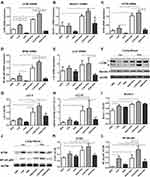
Expression of LC3B, Beclin-1, ATG5, NF-κB p65, and LL37 mRNA in the Lung Tissue of OVA-Sensitized and Challenged Mice
The mRNA of LC3B, the most studied autophagy family protein, was examined. LVD mice had a higher level of LC3B and NF-κB p65 mRNA in the lung compared to HVD mice (p < 0.05, Figure 4A and D). The level of LC3B mRNA in the LVD group was higher than in the NVD group, and that of NVD group was also higher than in the HVD group (p < 0.05, Figure 4A). After OVA sensitization and challenge, the expressions of LC3B, Beclin-1, ATG5, and NF-κB p65 mRNA in the LVD and NVD groups were all increased compared with their corresponding control group (p < 0.05, Figure 4A-D), while their expressions were higher in the HVD+OVA group than in the HVD group but differences were not significant (p > 0.05, Figure 4A-D). An increased expression of LC3B and NF-κB p65 mRNA was also observed in the LVD+OVA group compared to the NVD+OVA and HVD+OVA groups (p < 0.05, Figure 4A and D), and their expressions were also higher in the NVD+OVA group than in the HVD+OVA group (p < 0.05, Figure 4A and D). The expression of Beclin-1 and ATG5 mRNA was lower in HVD+OVA mice compared to LVD+OVA mice (p < 0.05, Figure 4B and C). LL37 mRNA in the NVD+OVA group and the LVD+OVA group was higher than in the HVD+OVA group (p < 0.05, Figure 4E), but there was no difference in all OVA-mediated groups compared to the corresponding group (p > 0.05, Figure 4E).
1,25(OH)2D3 Regulates the Expression of LC3B, Beclin-1, ATG5, and NF-κB p65 Protein in the Lung Tissue of OVA-Sensitized/Challenged Mice
In addition, the expressions of autophagy-related proteins, including LC3B, Beclin-1, and ATG5, and NF-κB p65 were investigated by Western blot analysis. LC3B is the most studied family protein, and exists in two forms, LC3-I and LC3-II. When autophagy is induced, a cytosolic form of LC3 (LC3-I) is converted to LC3-II for incorporation into membrane of autophagosomes. Therefore, LC3-II is a marker of autophagy. As expected, OVA sensitization and challenge could increase the level of LC3 II and ATG5 expression in LVD and NVD groups (p < 0.05, Figure 4F-K), while its expression had no significant change in the HVD and HVD+OVA groups (p > 0.05, Figure 4F-K). In addition, vitamin D deficiency dramatically upregulated LC3 II and ATG5 levels after OVA sensitization and challenge and these levels were higher than in the HVD+OVA group (p < 0.05, Figure 4F-K). After OVA sensitization and challenge, the level of Beclin-1 increased in the LVD and NVD groups (p < 0.05, Figure 4F and I), but there was no difference in all the three OVA-mediated groups (p > 0.05, Figure 4I). Collectively, these data suggest that autophagy proteins in the lung tissue were changed under differences in vitamin D status, especially after OVA sensitization and challenge. The level of NF-κB p65 expression in the LVD+OVA and NVD+OVA groups was increased compared with the corresponding group (p < 0.05, Figure 4J and L), while its expression was not greatly changed in the HVD and HVD+OVA groups (p > 0.05, Figure 4J and L). In addition, vitamin D deficiency dramatically upregulated the NF-κB p65 level after OVA sensitization and challenge, and its level was higher than that in the HVD+OVA and NVD+OVA groups (p < 0.05, Figure 4J and L).
In OVA-Sensitized/Challenged Mice, the Level of LC3B mRNA Was Correlated with Inflammatory Markers in BALF
Through correlation analysis in OVA-sensitized/challenged mice, LC3B mRNA showed a positive correlation with the number of total cells, eosinophils, and neutrophils in BALF (p < 0.05, Figure 5A and B). The LC3B mRNA level was also positively correlated with the IL-4 level (p < 0.05, Figure 5C). In addition, the expression of LC3B mRNA was negatively correlated with IL-10, which has a protective role in airway inflammation (p < 0.05, Figure 5D).
1,25(OH)2D3 Regulates the Expression of LC3B, Beclin-1, ATG5, NF-κB p65 and LL37 mRNA in Macrophages in a Time- and Dose-Dependent Manner
To validate the role of 1,25(OH)2D3 in autophagy, AMs were collected from BALF in OVA-sensitized/challenged mice with sufficient levels of vitamin D in the diet and treated either with vehicle or 1,25(OH)2D3 (0.2, 2, 20, and 200 nM). The expression of LC3B, Beclin-1, ATG5, NF-κB p65, and LL37 mRNA were measured in cultured alveolar macrophages in the presence of different concentrations of 1,25(OH)2D3. As the concentration of 1,25(OH)2D3 increased, the expression of LC3B, ATG5, and NF-κB p65 mRNA decreased, while that of LL37 mRNA increased (p < 0.05, Figure 6A, C-E). These results confirm that vitamin D might suppress the expression of autophagy-related factors such as LC3B and ATG5 and at the same time reduce NF-κB p65 expression but increase LL37 expression. In addition, the expression of LC3B, ATG5, and NF-κB p65 mRNA decreased, while the concentration of 1,25(OH)2D3 increased in 12-h and 24-h co-culture (p < 0.05, Figure 6A, C and D). The expression of Beclin-1 mRNA decreased with 1,25(OH)2D3 in dose of 0.2, 20 and 200 nM in 12-h co-culture (p < 0.05, Figure 6B), while it tended to increase as the concentration of 1,25(OH)2D3 increased in 24h co-culture, but there was no statistical significance (p > 0.05, Figure 6B).
Discussion
Vitamin D deficiency is common in patients with asthma.3 A reason for the relationship between vitamin D deficiency and high incidence of asthma is that low 25(OH)D levels might be a cause of the disease.4,5 In a recent study,3 reduced molar ratios of 25(OH)D3 to vitamin D3 suggested that vitamin D metabolism is dysregulated in asthma patients. In our study, we found that the serum levels of 25(OH)D in all the three dietary treatment groups decreased after OVA sensitization and challenge. Thus, lower levels of 25(OH)D in OVA-mediated mice in our study may represent as a consequence of this condition, and further studies need to be conducted to determine the reasons. We also found the level of 25(OH)D in vitamin D-deficient OVA-mediated mice was extremely lower than in vitamin D-sufficient or supplemented OVA-mediated ones, suggesting that vitamin D deficiency might represent a reversible risk factor for allergic airway inflammation. In this article, we mainly focused on exploring the influence of different vitamin D concentrations on airway inflammation and its regulation of autophagy as a possible mechanism.
It has been reported that vitamin D affects the inflammatory pathways and cells (types, numbers, or functions) involved in the progression of allergic asthma.24,35,36 Our results are in agreement with the discoveries of Agrawal et al7 that vitamin D deficiency may contribute to a higher total cell number and BALF eosinophilia in an asthmatic murine model. In our study, the total cell number and eosinophil number in the BALF was significantly reduced in the vitamin D-supplemented OVA-mediated mice compared to that in the vitamin D-deficient and D-sufficient OVA-mediated ones. In addition, Brehm et al37 discovered that vitamin D levels were inversely related to peripheral blood eosinophil count in asthmatic children. These results suggested that vitamin D deficiency might be involved in the pathogenesis of asthma by modulating crucial processes in airway inflammation.
A few observational studies suggested that lower 25(OH)D levels were associated with worse lung function and substantially more severe asthma symptoms.38–40 However, Kang et al41 studied 96 children with asthma and found that vitamin D levels were not associated with FEV1 (forced expiratory volume in one second), FVC (forced vital capacity), and FEV1/FVC levels (p > 0.05). In animal experiments, Fischer et al42 found that vitamin D-supplementation alleviated AHR and airway inflammation in BALF in an asthma model of HRA-sensitized and HRA-challenged mice. In our study, animals in the three different diet groups all exhibited a leftward shift of the concentration responsiveness curve of RL after OVA sensitization and challenge, suggesting an increased airway responsiveness compared with the control mice. Vitamin D-deficient allergic mice showed stronger airway responsiveness and significantly increased RL, and -log PC100 in this group declined dramatically, indicating AHR in this group. The -log PC100 in vitamin D-sufficient or -deficient OVA-mediated groups was lower than in the vitamin D-supplemented OVA-mediated group, suggesting vitamin D supplementation may improve airway resistance. Liu et al's43 latest meta-analysis of vitamin D and lung function in patients with asthma showed that high blood vitamin D levels may be positively correlated with lung function. The -log PC100 in the HVD+OVA decreased than HVD group, but there was no significant difference. This could be caused by the reduced airway inflammation in the vitamin D-supplemented asthmatic group, suggesting vitamin D supplementation may help to decrease airway hyper-responsiveness.
In our study, vitamin D deficiency was associated with an exaggerated response to OVA antigen and was associated with more severe airway inflammation in histological comparisons with the vitamin D-sufficient group. Gorman et al8 also reported that vitamin D deficiency markedly enhanced the capacity of airway cells to proliferate and secrete cytokines in response to OVA sensitization and challenge, indicating that the ability of airways to respond to allergens was influenced by vitamin D deficiency. However, we emphasized that a high vitamin D level may help to alleviate airway inflammation and reduce the hallmarks of asthma rather than completely reverse the pathogenetic process of asthma. In another study, Wittke et al44 reported that oral supplementation of 1,25(OH)2D3 failed to modify lung inflammation in vitamin D3-deficient mice. The experimental protocols of Wittke et al44 were markedly different than ours, including the strain of mice and type of vitamin D used for the supplementation, as well as the treatment of vitamin D diets. Such differences in experimental conditions, strains of mice, and spatial and temporal contexts might explain the discrepant results.
Suppressing the levels of Th2 cytokines (IL-4, IL-5) has been shown to be a possible therapeutic option to alleviate airway inflammation.45,46 In the current study, sensitization with OVA antigen in vitamin D-deficient mice induced a substantial rise in IL-4 and IL-5 in BALF. We also discovered that vitamin D supplementation, to some extent, could reverse the levels of these cytokines in mice who had undergone OVA sensitization and challenge. However, the influence of vitamin D on the release and activity of IL-4 is still controversial. Some studies showed an increase in IL-4 level after vitamin D treatment,47,48 while some reports demonstrated that in human cord blood T cells, vitamin D suppressed IL-4 production and expression of IL-13 induced by IL-4.45 In a murine model of asthma, the administration of 1,25-dihydroxyvitamin D was shown to inhibit the IL-4 level in BALF, suppress T-cell migration, and restrain the inflammatory response.46 We observed no statistically significant alteration of IL-4 level in BALF from mice with supplemented vitamin D compared to that in the vitamin D-sufficient group after OVA sensitization and challenge. The underlying reason for the discrepancy between our results and others is not clear, but it could be related to the timing, dose, and administration of vitamin D supplementation. Additional studies are needed to further clarify this issue.
Vitamin D promotes the stimulation of CD4+CD25+ regulatory T cells (Tregs) by dendritic cells.49 IL-10 is one of the crucial factors for Treg induction,50 resulting in significant decreases in the levels of proinflammatory cytokines that contribute to the pathogenesis of airway inflammation, which means it plays a protective and regulatory role in the process of inflammation. Our results showed that OVA sensitization and challenge may cause reduction in IL-10 levels, and the reduction of IL-10 was more pronounced in vitamin D-deficient mice. Conversely, vitamin D supplementation resulted in a significant increase in IL-10 levels, suggesting that vitamin D might help to alleviate airway inflammation through elevating the level of IL-10.
Vitamin D has an inhibitory effect on NF-κB activation.20–22,51 Our data showed vitamin D supplementation reduced the expression of NF-κB p65 mRNA and thus resulted in decreased secretion of pro-inflammatory cytokines in vivo as in a previous study.51 We now observed increased expression of NF-κB p65 mRNA and protein in the lung tissue of OVA-sensitized and -challenged mice in all vitamin D groups. The expression of NF-κB p65 mRNA and protein was higher in vitamin D-deficient OVA-mediated mice compared to vitamin D-sufficient or -supplemented OVA-mediated ones. In vitro, the expression of NF-κB p65 mRNA decreased, while the concentration of 1,25(OH)2D3 increased. LL37, as an antimicrobial peptide, its mRNA level was also increased after OVA sensitization and challenge, but the difference was not significant. The LL37 mRNA level in the vitamin D-supplemented OVA-mediated group was lower than in the vitamin D-sufficient or -deficient OVA-mediated group, while in vitro, the LL37 mRNA level was increased as the concentration of 1,25(OH)2D3 increased; the inconsistency between in vivo and in vitro experiments may be due only to analyses of mRNA levels of LL37, which are less important than the measures of its protein levels. Additional studies are needed to verify these findings.
The elevated mRNA and protein expression of LC3B, Beclin-1, and ATG5 observed in vitamin D-sufficient or -deficient groups after OVA sensitization and challenge demonstrated that autophagy is involved in allergic airway inflammation. LC3 is one of the indicators of autophagosome formation and was shown to be significantly elevated in an OVA-induced mice model.26 Genetic variants of the autophagy gene ATG5 were related to acceleration of airway remodeling and impairment of lung function in childhood asthma.28 It has also been reported that ATG5 knockdown could attenuate AHR and airway inflammation.26 Beclin-1 expression was significantly higher in asthmatic epithelium and ciliated cells, suggesting a potential role of ciliophagy in asthma.52 Ban et al26 also demonstrated increased autophagy levels, including LC3 and Beclin-1, in the peripheral blood cells, peripheral blood eosinophils, and sputum granulocytes in patients with severe asthma compared with patients with mild asthma patients and healthy controls.
In our study, we also found that vitamin D deficiency was related to the highest expression of LC3, Beclin-1, and ATG5 in vivo in OVA-mediated mice. The vitamin D-supplemented OVA-mediated group had significantly reduced expression of LC3 and ATG5 in lung tissue compared with the vitamin D-sufficient or -deficient OVA-mediated groups, which further emphasizes the participation of autophagy proteins in allergic airway inflammation as serum vitamin D concentration varies. In vitro, the increased expression of LC3B and ATG5 mRNA was modified by 1,25(OH)2D3 in dose- and time-dependent manner, which further emphasizes the modulation of 1,25(OH)2D3 in autophagy in allergic inflammation. However, the expression of Beclin-1 protein in all three OVA-mediated groups showed no significant difference, and the level of Beclin-1 mRNA had no significant change as 1,25(OH)2D3 concentration increased at 24-h co-culture; this may be because Beclin-1 and LC3 are active in different phases of autophagy. Beclin-1 is involved in the very early stage of autophagosome formation (nucleation phase) and is regarded as an essential component for the initiation of autophagy, ATG5 and LC3 are involved in later phases, when autophagy is induced by various stresses for incorporation into the membrane of autophagosomes. Thus, the modulation of autophagy by vitamin D may mainly be focused on the LC3 phase.
We discovered that LC3B mRNA showed a positive correlation with total cell number, eosinophil number, and IL-4 in BALF and a negative correlation with IL-10 in BALF in OVA-induced airway inflammation. As mentioned above, IL-10 plays a protective role in airway inflammation. Therefore, these results may suggest that in allergic airway inflammation, autophagy may be correlated with the degree of inflammation. McAlinden et al52 found that an autophagy inhibitor could reduce airway inflammation in HDM-induced asthmatic mice, which is in accord with our results that vitamin D might function as an inhibitor to regulate autophagy and suppress airway inflammation. However, the mechanisms that anti-inflammatory effect of vitamin D may be through the suppression of autophagy need to be further studied.
Conclusion
In conclusion, OVA sensitization and challenge induced dramatically allergic airway inflammation and higher RL in vitamin D-deficient mice as compared with vitamin D-sufficient or - supplemented groups. The expression of ATGs, including LC3B, Beclin-1, and ATG5, and NF-κB p65 in lung tissue in the vitamin D-deficient asthmatic group was increased compared with the vitamin D-supplemented OVA sensitized/challenged group. In vitro, 1,25(OH)2D3 also regulates the expression of LC3B, Beclin-1, ATG5, and NF-κB mRNA in AMs in a time- and dose-dependent manner. Thus, vitamin D deficiency may increase airway inflammation and enhance the expression of autophagy proteins. Supplementation of vitamin D could alleviate airway inflammation and reduce the expression of autophagy proteins. The study of vitamin D and autophagy in allergic airway inflammation is still at an initial stage. We will continue to investigate whether vitamin D supplementation would help alleviate allergic airway inflammation in patients and its possible role in regulating autophagy proteins and cell autophagy.
Abbreviations
ACh, acetylcholine chloride; AHR, airway hyper-responsiveness; AMs, alveolar macrophages; cDNA, complementary DNA; ELISA, enzyme-linked immunosorbent assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; H&E, hematoxylin and eosin; HVD, vitamin D-supplemented; LC3, light chain 3; LVD, vitamin D-deficient; NVD, vitamin D-sufficient; OVA, ovalbumin; RL, pulmonary airway resistance.
Data Sharing Statement
The data that support the findings of this study are available from Min Zhang upon reasonable request.
Ethics Approval
The study was approved by the Ethics Committee for Animal Studies at Shanghai General Hospital, China (IACUC: 2019-A011-01) and carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011).
Consent for Publication
Written informed consent for publication of this study was obtained from all participants.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (Grant No.81900016, No. 81873402 and No.81970022) and the Shanghai Municipal Health Committee (Grant No. 201740039). The sponsors had no role in the design or interpretation of the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
All authors have reported no conflicts of interest for this work and that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
References
1. Holgate ST, Holloway J, Wilson S, et al. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J Allergy Clin Immunol. 2006;117:496–506.
2. Morales E, Duffy D. Genetics and gene-environment interactions in childhood and adult onset asthma. Front Pediatr. 2019;7:499.
3. Jolliffe DA, Stefanidis C, Wang Z, et al. Vitamin D metabolism is dysregulated in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;202(3):371–382.
4. Mohammadzadeh I, Darvish S, Qujeq D, Hajiahmadi M, Vaghari-Tabari M. Association of serum 25-OH vitamin D3 with serum IgE and the Pediatric Asthma Severity Score in patients with pediatric asthma. Allergy Asthma Proc. 2020;41:126–133.
5. von Mutius E, Martinez FD. Vitamin D supplementation during pregnancy and the prevention of childhood asthma. N Engl J Med. 2020;382:574–575.
6. Litonjua AA, Lange NE, Carey VJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials. 2014;38:37–50.
7. Agrawal T, Gupta GK, Agrawal DK. Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clin Exp Allergy. 2013;43:672–683.
8. Gorman S, Tan DH, Lambert MJ, Scott NM, Judge MA, Hart PH. Vitamin D (3) deficiency enhances allergen-induced lymphocyte responses in a mouse model of allergic airway disease. Ped Allergy Immunol. 2012;23:83–87.
9. Kazemian E, Akbari ME, Moradi N, et al. Effect of vitamin D receptor polymorphisms on plasma oxidative stress and apoptotic biomarkers among breast cancer survivors supplemented vitamin D3. Eur J Cancer Prev. 2020;29(5):433–444.
10. Asbaghi O, Sadeghian M, Mozaffari-Khosravi H, et al. The effect of vitamin d-calcium co-supplementation on inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Cytokine. 2020;129:155050.
11. Murdaca G, Tonacci A, Negrini S, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev. 2019;18:102350.
12. Abdel-Mohsen MA, El-Braky AA, Ghazal AAE, Shamseya MM. Autophagy, apoptosis, vitamin D, and vitamin D receptor in hepatocellular carcinoma associated with hepatitis C virus. Medicine (Baltimore). 2018;97(12):e0172.
13. Zhen C, Feng X, Li Z, et al. Suppression of murine experimental autoimmune encephalomyelitis development by 1,25-dihydroxyvitamin D3 with autophagy modulation. J Neuroimmunol. 2015;280:1–7.
14. Shen Q, Bi X, Ling L, Ding W. 1,25-Dihydroxyvitamin D3 attenuates angiotensin ii-induced renal injury by inhibiting mitochondrial dysfunction and autophagy. Cell Physiol Biochem. 2018;51(4):1751–1762.
15. Lu R, Zhang YG, Xia Y, Sun J. Imbalance of autophagy and apoptosis in intestinal epithelium lacking the vitamin D receptor. FASEB J. 2019;33:11845–11856.
16. Yang L, Fan Y, Zhang X, Liu J, Ma J. Effect of 1,25(OH)2D3 on high glucose-induced autophagy inhibition in peritoneum. Mol Med Rep. 2017;16(5):7080–7085.
17. Dai J, Liang Y, Li H, et al. Vitamin D enhances resistance to aspergillus fumigatus in mice via inhibition of excessive autophagy. Am J Transl Res. 2018;10(2):381–391.
18. Yurt M, Liu J, Sakurai R, et al. Vitamin D supplementation blocks pulmonary structural and functional changes in a rat model of perinatal vitamin D deficiency. Am J Physiol Lung Cell Mol Physiol. 2014;307(11):L859–67.
19. Foong RE, Shaw NC, Berry LJ, Hart PH, Gorman S, Zosky GR. Vitamin D deficiency causes airway hyperresponsiveness, increases airway smooth muscle mass, and reduces TGF‐β expression in the lungs of female BALB/c mice. Physiol Rep. 2014;2(3):e00276.
20. Lai G, Wu C, Hong J, Yingfang S. 1,25-Dihydroxyvitamin D(3) (1,25-(OH)(2)D(3)) attenuates airway remodeling in a murine model of chronic asthma. J Asthma. 2013;50(2):133–140.
21. Song Y, Hong J, Liu D, Lin Q, Lai G. 1,25-dihydroxyvitamin D3 inhibits nuclear factor kappa B activation by stabilizing inhibitor IκBα via mRNA stability and reduced phosphorylation in passively sensitized human airway smooth muscle cells. Scand J Immunol. 2013;77(2):109–116.
22. Zhang H, Yang N, Wang T, Dai B, Shang Y. Vitamin D reduces inflammatory response in asthmatic mice through HMGB1/TLR4/NF-κB signaling pathway. Mol Med Rep. 2018;17(2):2915–2920.
23. Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros. 2007;6(6):403–410.
24. Ramos-Martínez E, López-Vancell MR, Fernández de Córdova-aguirre JC, et al. Reduction of respiratory infections in asthma patients supplemented with vitamin D is related to increased serum IL-10 and IFNγ levels and cathelicidin expression. Cytokine. 2018;108:239–246.
25. Liu JN, Suh DH, Trinh HK, Chwae YJ, Park HS, Shin YS. The role of autophagy in allergic inflammation: a new target for severe asthma. Exp Mol Med. 2016;48(7):e243.
26. Poon A, Eidelman D, Laprise C, Hamid Q. ATG5, autophagy and lung function in asthma. Autophagy. 2012;8(4):694–695.
27. Groves NJ, Kesby JP, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav Brain Res. 2013;241:120–131.
28. Roggenbuck M, Anderson D, Barfod KK, et al. Vitamin D and allergic airway disease shape the murine lung microbiome in a sex-specific manner. Respir Res. 2016;17(1):116.
29. Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J. 2006;20:455–465.
30. Bao W, Zhang Y, Zhang M, et al. Effects of ozone repeated short exposures on the airway/lung inflammation, airway hyperresponsiveness and mucus production in a mouse model of ovalbumin-induced asthma. Biomed Pharmacother. 2018;101:293–303.
31. Xue Y, Bao W, Zhou Y, et al. Small-airway dysfunction is involved in the pathogenesis of asthma: evidence from two mouse models. J Asthma Allergy. 2021;14:883–896.
32. Maclean A, Bunni E, Makrydima S, et al. Fallopian tube epithelial cells express androgen receptor and have a distinct hormonal responsiveness when compared with endometrial epithelium. Human Reprod. 2020;9(9):2097–2106.
33. Dogan S, Vasudevaraja V, Xu B, et al. DNA methylation-based classification of sinonasal undifferentiated carcinoma. Mod Pathol. 2019;32(Suppl):1447.
34. Rao Z, Zhang N, Xu N, et al. 1,25-Dihydroxyvitamin D inhibits LPS-Induced High-Mobility Group Box 1 (HMGB1) Secretion via targeting the NF-E2-related factor 2-Hemeoxygenase-1-HMGB1 pathway in macrophages. Front Immunol. 2017;8:1308.
35. Cho SW, Kim JH, Choi JH, Lim DH. Preventive and therapeutic effects of vitamin D in a mouse model of allergic asthma. Asian Pac J Allergy Immunol. 2019;37(3):130–137.
36. Foong RE, Bosco A, Troy NM, et al. Identification of genes differentially regulated by vitamin D deficiency that alter lung pathophysiology and inflammation in allergic airways disease. Am J Physiol Lung Cell Mol Physiol. 2016;311(3):L653–63.
37. Brehm JM, Celedon JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765–771.
38. Wu AC, Tantisira K, Li L, Fuhlbrigge AL, Weiss ST, Litonjua A. Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med. 2012;186:508–513.
39. Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Serum vitamin D levels and severe asthma exacerbations in the childhood asthma management program study. J Allergy Clin Immunol. 2010;126:52–58.e55.
40. Ginde AA, Mansbach JM, Camargo CA
41. Kang Q, Zhang X, Liu S, Huang F. Correlation between the vitamin D levels and asthma attacks in children: evaluation of the effects of combination therapy of atomization inhalation of budesonide, albuterol and vitamin D supplementation on asthmatic patients. Exp Ther Med. 2018;15:727–732.
42. Fischer KD, Hall SC, Agrawal DK. Vitamin D supplementation reduces induction of epithelial-mesenchymal transition in allergen sensitized and challenged mice. PLoS One. 2016;11(2):e0149180.
43. Liu J, Dong YQ, Yin J, et al. Meta-analysis of vitamin D and lung function in patients with asthma. Respir Res. 2019;20(1):161.
44. Wittke A, Chang A, Froicu M, et al. Vitamin D receptor expression by the lung micro-environment is required for maximal induction of lung inflammation. Arch Biochem Biophys. 2007;460:306–313.
45. Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 alpha, 25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52:12–18.
46. Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur J Immunol. 2004;34:1068–1076.
47. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha, 25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980.
48. Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160:5314–5319.
49. Boissier MC, Assier E, Biton J, Denys A, Falgarone G, Bessis N. Regulatory T cells (Treg) in rheumatoid arthritis. Joint Bone Spine. 2009;76:10–14.
50. Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/ Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33.
51. Zhou Y, Wang GF, Yang L, et al. Treatment with 1,25(OH)2D3 induced HDAC2 expression and reduced NF-κB p65 expression in a rat model of OVA-induced asthma. Braz J Med Biol Res. 2015;48:654–664.
52. McAlinden KD, Deshpande DA, Ghavami S, et al. Autophagy activation in asthma airways remodeling. Am J Respir Cell Mol Biol. 2019;60(5):541–553.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.