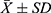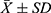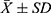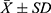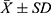Back to Journals » Journal of Pain Research » Volume 17
Effect of Sympathetic Blockade on Spontaneous Discharge and the H-Reflex at Myofascial Trigger Points in Rats
Authors Liu S, Liu L, Lu X, Yao T
Received 18 November 2023
Accepted for publication 20 March 2024
Published 27 March 2024 Volume 2024:17 Pages 1299—1311
DOI https://doi.org/10.2147/JPR.S449750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wendy Imlach
Shixuan Liu,* Lin Liu,* Xinyue Lu, Tingfeng Yao
Department of Rehabilitation, School of Sport Health, Nanjing Sport Institute, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lin Liu, Department of Rehabilitation, School of Sport Health, Nanjing Sport Institute, Nanjing, People’s Republic of China, Email [email protected]
Purpose: Myofascial trigger points (MTrPs) are the main cause of myofascial pain syndrome (MPS), and patients with MPS also have symptoms of sympathetic abnormalities. Consequently, this study aimed to investigate the potential relationship between MTrPs and sympathetic nerves.
Materials and Methods: Twenty-four seven-week-old male rats were randomly divided into four groups (six rats every group). Groups I and II were kept in normal condition (n=12), and groups III and IV underwent MTrPs modelling (n=12). After successful MTrPs modelling, differences in sympathetic outcomes between the MTrPs groups (III and IV) and non-MTrPs groups (I and II) were observed. Sympathetic blockade was then applied to groups III and I (n=12). Data were collected on peak inversion spontaneous potentials (PISPs) and the H-reflex-evoked electromyography during spontaneous discharge at the MTrPs before and after sympathetic blockade.
Results: Systolic blood pressure, diastolic blood pressure, mean arterial pressure, and heart rate were significantly higher in the MTrPs group than in the non-MTrPs group (P< 0.05). Compared with group I, group III had the PISPs potential lower wave amplitude, shorter duration and amplitude-to-duration ratio, and lower H latency and latency difference H-M (P< 0.05). Compared with group IV, group III had the PISPs potential lower wave amplitude, duration, amplitude-to-duration ratio, M-wave latency, H maximum wave amplitude, and maximal wave amplitude ratio H/M (P< 0.05). The changes before and after sympathetic blockade in the MTrPs group were significant, and the amplitude, duration, and amplitude-to-duration ratio of the PISPs potentials were lower after the blockade (P< 0.05).
Conclusion: MTrPs and sympathetic nerves interact with each other forming a specific relationship. MTrPs sensitize sympathetic nerves, and sympathetic nerve abnormalities affect local muscle myoelectric hyperactivity, leading to MTrPs. This finding is instructive for the clinical management of sympathetic disorders.
Keywords: myofascial trigger point, sympathetic blockade, electromyography, H-reflex, myofascial pain syndrome
Introduction
Myofascial trigger points (MTrPs) are discrete, palpable, hypertonic, and abnormally sensitive nodules in the skeletal muscle that often cause localized chronic pain or even distal pain, leading to myofascial pain syndrome (MPS), a typical non-organic disorder of the neuromuscular system.1,2 Previous studies have shown that the spontaneous electromyographic signals of MTrPs may originate from the muscle spindles of skeletal muscle, and that the muscle spindle, a proprioceptive organ, is neuro-physiologically affected by MTrPs to varying degrees.3 In addition, sympathetic nerves, as part of the peripheral central nervous system, can be functionally affected by both the afferent and efferent nerves where the myotomes are located.4
The sympathetic nervous system is implicated in both acute and chronic pain and may have analgesic or nociceptive effects under different circumstances.5 Studies in recent years have shown that the sympathetic nervous system can be involved in the pathogenesis of a wide range of diseases, such as ventricular arrhythmias, diseases associated with autonomic dysfunction (hyperhidrosis, complex regional pain syndrome, atherosclerotic occlusive disease of the lower limbs, and Raynaud’s disease, etc), bone and joint diseases (metabolic bone diseases, osteoporosis, degenerative spinal diseases, etc), vascular diseases (hypertension, atherosclerosis, pulmonary hypertension, etc), abnormalities in the regulation of energy metabolism (obesity, diabetes, etc), gastric cancer, and are even associated with the phenomenon of acupoint sensitization in Chinese medicine.6–14 Therefore, the sympathetic nervous system plays a key role in the human body and studying its related pathological mechanisms is of great importance in guiding various common clinical diseases.
Excitation of the sympathetic nervous system has been shown to increase pain intensity, spontaneous potentiation of MTrPs, and cause an increase in the concentration of inflammatory substances around the myocytes of MTrPs, particularly norepinephrine.15–17 After sympathetic blockade in eight patients with fibromyalgia, six showed a significant reduction in pain and a decrease in the number of MTrPs.18 This abnormal sympathetic activity triggered by the noxious stimulation of recessive MTrPs may explain the autonomic symptoms in patients with chronic myalgia, such as vasoconstriction (whitening), coldness, sweating, and hairiness.19 Sympathetic neurons make close contact with neuromuscular junctions and form a network in skeletal muscle that may functionally couple different targets including blood vessels, motor neurons, and muscle fibers.20 The electrical activity recorded from animal myocytes indicates that electrical stimulation of cortical sympathetic dendrites may act on the reticular formation to influence myocyte sensitivity by affecting gamma motor nerve firing. This could also explain the sympathetically induced disruption of proprioceptive information as a potential mechanism for chronic muscle pain.21,22 Studies show that neurogenic inflammation of MTrPs may disrupt the balance between the sympathetic and parasympathetic nerves in the autonomic nervous system.23 These results suggest that sympathetic overactivity is associated with MTrPs formation. Increased sympathetic output in humans has been associated with increased sensitivity of the muscle spindle.24,25 Therefore, we also strongly suspect that the abnormal sensitivity of muscle spindle afferent nerves may be related to the pathophysiological mechanisms of MTrPs. Moreover, the intrinsic link between MTrPs and sympathetic nerves and the specific mechanisms involved still remain unclear. For these reasons, we suspect that increased sympathetic nervous system excitability further sensitizes the gamma motor nerve, which indirectly modulates the neurophysiological activity of the muscle, causing alterations in muscular discharge and ultimately affecting the proprioceptive function and pain level of the affected muscle. Therefore, this study aimed to explore the connection between MTrPs and sympathetic nerves through the construction of a MTrPs model and sympathetic blockade to provide clinical guidance for the diagnosis and treatment of sympathetic diseases and MPS due to MTrPs.
Materials and Methods
Experimental Animals
Male Sprague-Dawley (SD) rats (n=24; age, 7 weeks; weight, 270–350 g) were randomly divided into four groups. Groups I and II were used as controls without MTrPs modelling. Groups III and IV were used as the MTrPs modelling group. Sympathetic blockade was performed simultaneously in groups I and III. Finally, rats were divided into groups I (sympathetically blocked non-MTrPs group, n=6), II (unblocked non-MTrPs group, n=6), III (sympathetically blocked MTrPs group, n=6), and IV (unblocked MTrPs group, n=6).
All rats were housed in polypropylene cages with an 11 h/13 h light–dark cycle and kept in a temperature-controlled room (20–26°C) with a relative humidity of 40–60%. Food and water were provided ad libitum. The experimental procedures were performed in accordance with local guidelines for animal welfare and the National Research Council’s ‘Guide for the Care and Use of Laboratory Animals’ (National Academies Press, Washington DC, USA), and were approved by the Nanjing Sport Institute Science Research Ethics Committee (permission no. GZRDW-2020-02). All efforts were made to minimize the number of animals used and their suffering.
Establishment
Firstly, 24 SD rats were raised normally in order to acclimatize them to the environment. After two weeks, groups III and IV received the MTrPs modelling for eight weeks (n=12). After completing the MTrPs modelling, groups III and IV were allocated recovery times of 4 weeks. Finally, groups I and III received sympathetic blockade (n=12).
MTrPs Model
On the first day of each week, the rats were treated with a blow (fixed in the supine position under abdominal anesthesia and then hit once on the left lower limb in free fall from a height of 20 cm with a 1200 g weight percussion device, causing blunt contusions on the mid-superior part of the gastrocnemius muscle). On the second day, the rats were trained by centrifugal exercise on a running table (inclined at an angle of −16°, with the speed gradually increasing to 16 m/min for 90 min each time). The rats were fed normally for the remaining five days, and this intervention was repeated for eight weeks. The rats then entered a recovery period, during which they were fed normally for four weeks without any experimental intervention.
Criteria for the successful establishment of the animal model (using internationally accepted characteristics for the presence of MTrPs): After the rats were restrained in the supine position under abdominal anesthesia, muscles were dissected from the medial ankle to the thigh, and the gastrocnemius and hamstring muscles were bluntly dissected to detect the presence of contracture nodes, local twitch responses, high-frequency spontaneous potentials at the nodes, and other outcomes of successful modelling.3
Sympathetic Blockade
After successful establishment of the MTrPs model, group III (MTrPs) and group I (non-MTrPs) were selected and injected intraperitoneally at 9:00 am each day with 0.2 mL 6-hydroxydopamine blocked (6-OHDA, Sigma, injection standard: 100 mg/kg) dissolved in sterilized saline containing 0.01% ascorbic acid; the other two groups of rats were injected intraperitoneally at 9:00 am each day with 0.2 mL of 0.01% ascorbic acid containing sterilized saline, which was injected intraperitoneally for five consecutive days. Mean arterial pressure (MAP), heart rate (HR), and urinary norepinephrine (NE) levels in each group were monitored before each injection.
Sympathetic Excitability
After the rats in the MTrPs groups were successfully modeled, the Panlab NIBP System noninvasive blood pressure measurement was used to measure the SBP, DBP, MAP and HR in the tail artery of each rat five times at an interval of 3 min, and the mean values were taken as the blood pressure and HR levels.26
Subsequently, urine from each group of rats was collected using the metabolic cage method; 1 mL of 1 mol/L hydrochloric acid was added to avoid the degradation of NE, and urine was collected after 6 h. The urine was centrifuged at 4°C 3000 r/min for 5 min, the supernatant was aspirated for 1.5 mL, and the urinary NE excretion was measured by high-performance liquid chromatography to reflect the sympathetic activity.
Electromyography (EMG)
After the successful sympathetic blockade, the peak inversion spontaneous potentials (PISPs) were recorded in the resting state of MTrPs and non-MTrPs in each group. Two fine needle electrodes (Ф0.3 mm) were connected to, and examined with, an EMG device (Z2J-NB-NCC08, NuoCheng, Shanghai) to record myoelectrical signals at confirmed MTrPs. The positive needle of the instrument was pricked into the muscle belly, while the negative needle was pricked at the MTrPs (muscle twitching occurred after the needle pole was pricked). The EMG data (wave amplitude, duration, and amplitude-to-duration ratio) of the PISPs were assessed and analyzed by stabbing the rat gastrocnemius muscle belly by a needle electrode. Amplitude indicates the strength of muscle contraction, reflecting the number of muscle fibers involved in mixing neuromuscular action potentials; wavelength indicates the duration of muscle contraction, reflecting the time it takes for a muscle action potential to deviate from baseline and return to baseline again, indirectly reflecting the speed of nerve conduction; and amplitude-to-wavelength ratio reflects the relationship between the force and duration of muscle contraction, and at the same time reflects the specific PISP The wave amplitude to wavelength ratio can reflect the relationship between the force and duration of muscle contraction, and the specific shape of PISP.
H-Reflex
Based on the success of the modelling of MTrPs and sympathetic blockade, the position of each electrode was kept constant, and a 2–3 cm longitudinal incision was made in the popliteal fossae of rats in both groups. The biceps femoris muscle was dissected to expose the sciatic nerve and its branches. The tibial nerve was dissected inferiorly from the sciatic nerve and its branches. A pair of bipolar hook silver electrodes were placed on the tibial nerve and served as stimulus electrodes. The recording electrodes of rats in the control group were placed at non-MTrPs, which were in the same position as the MTrPs in the gastrocnemius muscle of the rats in the experimental group. The stimulus parameters were set as follows: current, constant square; stimulus frequency, 0.5 Hz; stimulus pulse width, 0.05 ms; current increment, 0.1 mA; magnification, 50 times; scan speed, 1 ms/D; sensitivity, 1 mV/D; high cut, 3000 Hz; and low cut, 20 Hz. When the stimulus was initiated, the current was gradually increased from zero to obtain continuous records of needle EMG of the H-reflex evoked by the rats. After the stimulus, we analyzed the available needle EMG of the H-reflex and evaluated the H-reflex outcomes (M latency, H latency, M maximum wave amplitude, H maximum wave amplitude, H-M latency difference, and H/M maximum wave amplitude ratio).3
Statistical Analysis
All statistical analyses of sympathetic outcomes (SBP, DBP, MAP, and HR) and EMG outcomes (EMG wave amplitude, duration, amplitude-to-duration ratio, and H-reflex) between the model groups and normal controls and between each of the model groups were performed using SPSS 22.0. Data were expressed as mean ± standard deviation (ieq), and the same index between two groups of rats was tested by the t-test for unpaired samples, while the same index between multiple groups of rats was analyzed by one-factor analysis of variance followed by post hoc Tukey’s test, with P<0.01 as highly significant and P<0.05 as significant.
Results
Effect of MTrPs on Sympathetic Nerves
As shown in Table 1, SBP, DBP, MAP, HR, and urinary NE levels were significantly higher in the MTrPs group than in the non-MTrPs group (P<0.05). This suggests that the sympathetic nerves of the rats in the MTrPs model were more active.
 |
Table 1 Comparison of Data for Each Indicator Related to Sympathetic Nervous System Before Blockade ( |
Effects of Sympathetic Blockade
Blood Pressure and HR
When comparing the data before the blockade, the sympathetic outcomes of rats in groups I and III after the blockade significantly decreased, while the outcomes of rats in groups II and IV were relatively stable. The differences in each sympathetic nerve index between groups I and III and groups II and IV were significant after the blockade (P<0.01) (Table 2).
 |
Table 2 Comparison of Data on Various Sympathetic-Related Indicators Before and After Sympathetic Blockade in Each Group( |
When comparing the sympathetic outcomes in each group (Table 2, after blockade), there was a certain decrease in both groups I and III compared to groups II and IV. Group I showed the most significant decrease (P<0.05). In contrast to group I, group III showed an increase in all outcomes (P<0.05) (Figure 1).
Urinary NE Excretion
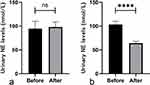
According to the ELISA test results, there was no significant difference between NE emissions in the urine of unblocked groups II and IV before and after sympathetic blockade. There was a significant difference in NE emission in the urine of the blocked groups I and III before and after sympathetic blockade, and NE emission in the urine was lower after blockade (P<0.05) (Figure 2).
Therefore, as the changes in sympathetic nerve parameters and urinary NE excretion before and after the blockade were significant, the protocol of blocking the sympathetic nerve of rats by continuous intraperitoneal injection of 0.2 mL of 6-hydroxydopamine dissolved in sterile saline containing 0.01% ascorbic acid for five days to model sympathetic nerve-blocked rats was successful, and it ensured the validity of the follow-up experiments.
EMG
Changes in PISP
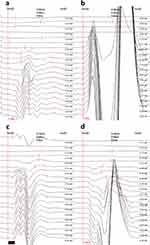
Figures 3 and 4 show the characteristics of the PISP in the EMG between the groups of rats. Compared with group II, group I after sympathetic blockade showed partial PISP, while the wave amplitude of PISP was lower in group III than in IV (P<0.05).
 |
Figure 4 Comparison of outcomes of EMG in groups ( Abbreviation: EMG, Electromyography. |
Compared with group II, group I showed a significant increase in wave amplitude and amplitude-to-duration ratio (P<0.05). The duration and amplitude-to-duration ratios of rats in group III were significantly lower (P<0.05), whereas the wave amplitude, duration, and amplitude-to-duration ratios of rats in group IV were significantly higher (P<0.05). Groups IV and I showed significantly higher wave amplitude, duration, and amplitude-to-duration ratios than group III (P<0.05). The duration in group I was lower and the amplitude-to-duration ratio was higher than in group IV (P<0.05) (Figure 4).
Effect of Sympathetic Blockade on the Changes in the PISPs of Rats in the MTrPs Group
The outcomes of PISP in EMG before and after sympathetic blockade in the MTrPs group changed significantly, and the EMG wave amplitude, duration, and wave amplitude amplitude-to-duration ratio were significantly higher (P<0.05) after the blockade (Figure 5).
H-Reflex
Figures 6 and 7 show the differences in the H-reflex between the four groups of rats, with the sympathetic blockade groups being significantly inhibited and having a smaller reflex amplitude. H latency and latency difference in H-M were significantly lower in the rats in group III (P<0.05) than in group I; in rats in group IV, M latency and the maximal wave amplitude ratio H/M were significantly higher, while H latency, M maximal wave amplitude and the latency difference in H-M were significantly lower (P<0.05); M maximal wave amplitude was significantly lower in rats in group III (P<0.05) than in group II; the M maximum wave amplitude and the latency difference in H-M were significantly lower and the maximum wave amplitude ratio was significantly higher in group IV rats (P<0.05); the M latency, H maximum wave amplitude and the maximum wave amplitude ratio H/M were significantly higher in group IV rats than in group III (P<0.05).
 |
Figure 7 Comparison of H-reflex outcomes after blockade between groups ( |
Discussion
Recent studies have shown that treatment with MTrPs facilitates healing and recovery from sympathetic-related disorders.19,27,28 However, the intrinsic link between MTrPs and sympathetic nerves and the specific mechanisms involved remain unclear. This study showed that MTrPs can sensitize peripheral sympathetic nerves and affect their regulation, leading to an increase in HR and blood pressure, which may ultimately lead to related diseases. Simultaneously, a decrease in sympathetic modulation (eg, sympathetic blockade in this study) also results in a corresponding suppression of local EMG at the MTrPs and H-reflex, which evokes muscle spindle discharge. Thus, if we think backwards, the disease-causing sympathetic sensitization could be the cause of MTrPs formation and even worsening. Localized MTrPs sensitize the corresponding sympathetic nerves. This has adverse psychological and physiological effects on patients and should be noted in clinical practice.
Effects of MTrPs Formation on Sympathetic Nerves
This study showed that the formation of chronic MTrPs significantly increased HR, blood pressure, and urinary NE levels in rats, suggesting that chronic MTrPs formation may lead to central sympathetic hyperactivity, which is involved in the activation of cardiac and renal autonomic nerves in rats. Previous studies have shown that central sensitization of the spinal cord is the main neurophysiological mechanism involved in the painful symptoms of MTrPs.29 Pain induced by central sensitization in the spinal cord can directly affect cardiovascular activity, such as blood pressure, HR, and renal metabolic activity by enhancing sympathetic effusion and regulating the balance between the vagus and sympathetic nerves. In addition, several clinical studies have shown that symptoms such as the localized twitch response, abnormal sweating, abnormal skin temperature, and the sympathetic skin response in MPS patients point to possible localized sympathetic reorganization and abnormal innervation of MTrPs.30 Therefore, the inactivation of MTrPs may be necessary for the treatment of sympathetic symptoms.
Effects of the Changes in Sympathetic Function on MTrPs
Comparing the outcomes of the PISP between groups, significant changes were also demonstrated. However, unlike the more monotonous changes in sympathetic nerves, the various changes in the PISPs and H-reflex in EMG were simultaneously affected by both sympathetic blockade and the formation of MTrPs. The possible interactions between the two have not yet been determined; therefore, their effects on the PISPs and H-reflex are comprehensive. The formation of MTrPs led to an increase in local EMG and greater proprioceptive excitation, whereas sympathetic blockade inhibited the production of electromyographic signals, as discussed in the previous paragraph. Therefore, both sympathetic blockade and the effects of the formation of MTrPs have a more or less comprehensive effect on EMG, causing changes in the values of its parameters, and the PISP in the EMG itself is a myoelectric feature of MTrPs. Therefore, it is conceivable that the operation of sympathetic nerves may also directly or indirectly influence the formation of MTrPs and their development. In addition, the changes in the H-reflex suggest that sympathetic blockade can inhibit the H-reflex pathway, which is associated with proprioceptors in skeletal muscle and may have caused abnormal discharge of the sarcomere and generated the PISP, which ultimately induced or promoted the formation and development of MTrPs.
This study showed that sympathetic blockade significantly suppressed the PISP wave amplitude, wave frequency, and the induced H-reflex at MTrPs, suggesting that the PISP potentials observed at MTrPs may be related to sympathetic overactivity. Anatomical and electrophysiological evidence has confirmed that sympathetic innervation is not restricted to the blood vessels supplying the spindles and that the intrafusal fibers are directly innervated by the sympathetic nervous system.31–33 Some muscle spindles also receive autonomic axons that are in a nonselective neuro-effective association with intrafusal muscle fibers. The presence of one or two very small-diameter (less than 0.5 µm) unmyelinated nerve fibers in the human muscle spindle without any endings were observed and considered sympathetic.34,35 Based on this evidence, we suggest that sympathetic blockade inhibits the hypersensitive H reflex pathway at MTrPs, possibly by inhibiting MTrPs myosin Iα afferent nerves in order to achieve a lower H max. In addition, sympathetic hyperexcitability not only contributed to the increased sensitivity of the innervated musculus but also significantly increased the excitability of the entire H-reflex pathway, which also induced central sensitization and overactive α-motor neurons in the spinal cord, resulting in dysfunction of the skeletal muscle motor end plate and acceleration of MTrPs formation. In addition, it has been shown that sympathetic blockade significantly promotes the repair of MTrPs myocytes and reduces their local inflammation, providing a strong molecular biological basis for the extrapolation of this study.36 Therefore, sympathetic overactivity may be an important cause of chronic MTrPs formation.
Hypothesis Between the Association of Sympathetic Nerves and MTrPs
Taken together, this is sufficient to confirm the conjecture of the earlier part of this study that there may be structural or functional links between MTrPs and sympathetic nerves. Furthermore, the excitability of sympathetic nerves and the formation of MTrPs may be positively correlated; that is, relatively abnormally excited sympathetic nerves may promote or even induce the formation and development of MTrPs, whereas the deterioration of MTrPs may sensitize sympathetic nerves. This leads to a vicious cycle of “MTrPs latency to sympathetic sensitization and then MTrPs activation”. In clinical practice, physicians often have difficulty associating sympathetic nervous system disorders with MTrPs, and therefore may misdiagnose them as having other etiologies and miss the optimal time to treat the disease. In future clinics, there may be many sympathetic nervous system-related diseases that can be cured by inactivating MTrPs. In conclusion, there is a link between MTrPs and sympathetic nerves and the two interact, and their effects may be positively correlated.
Conclusion
In this study, the interaction between MTrPs and sympathetic nerves was demonstrated by combining an MTrPs model and sympathetic nerve block, and by monitoring blood pressure, HR, and EMG signals. MTrPs sensitize the sympathetic nerves, and sympathetic nerve abnormalities affect local muscle myoelectric hyperactivity, leading to MTrPs. This finding is instructive for the clinical management of sympathetic disorders and chronic muscle pain. However, evidence for a lack of correlation between MTrPs and sympathetic nerves remains to be investigated.
Abbreviations
MTrPs, myofascial trigger points; MPS, myofascial pain syndrome; PISPs, peak inversion spontaneous potentials; EMG, electromyography; NE, norepinephrine; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate.
Data Sharing Statement
Data openly available in a public repository.
Author Contributions
All authors contributed significantly to the reported work, including conception, study design, data acquisition, analysis, and interpretation. Shixuan Liu, Xinyue Lu and Tingfeng Yao took part in drafting, revising or critically reviewing the article. Lin Liu gave final approval of the version to be published. We have agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Funding
This study was funded by the National Natural Science Foundation of China, Grant No. 32000829 and the Jiangsu Province University “Qinglan project” Program 2022.
Disclosure
The authors reports no conflicts of interest in this work.
References
1. Huang QM, Lv JJ, Ruanshi QM, Liu L. Spontaneous electrical activities at myofascial trigger points at different stages of recovery from injury in a rat model. Acupunct Med. 2015;33(4):319–324. doi:10.1136/acupmed-2014-010666
2. Zhang H, Lü JJ, Huang QM, Liu L, Liu QG, Eric OA. Histopathological nature of myofascial trigger points at different stages of recovery from injury in a rat model. Acupunct Med. 2017;35(6):445–451. doi:10.1136/acupmed-2016-011212
3. Liu L, Huang QM, Liu QG, Nguyen TT, Yan JQ, Bo CZ. Relationship between muscle spindles and myofascial trigger spots according to Hoffmann reflex pathway and tissue morphology characteristics in a rat model. Acupunct Med. 2020;38(2):109–116. doi:10.1136/acupmed-2017-011626
4. Raja SN. Role of the sympathetic nervous system in acute pain and inflammation. Ann Med. 1995;27(2):241–246. doi:10.3109/07853899509031966
5. Doroshenko M, Turkot O, Horn DB. Sympathetic nerve block. In: StatPearls. StatPearls Publishing; 2023. Available from: http://www.ncbi.nlm.nih.gov/books/NBK557637/. Accessed November 7, 2023.
6. Jia H, Yu B. Sympathetic neuromodulation for ventricular arrhythmias. Chin J Cardiac Pacing Electrophysiol. 2022;36(01):4–7. doi:10.13333/j.cnki.cjcpe.2022.01.002
7. Zhu JJ, Tao JC, Ni HD, Huang B, Yao M. New advances in the clinical application of sympathetic modulation. Chin J Pain Med. 2021;27(9):688–692.
8. Huang C, Cao P, Hu B, Dou YB. Role and mechanism of sympathetic neuromodulation in bone and joint diseases. J Guangzhou Med Univ. 2021;49(5):165–167.
9. Chi HJ, Chen LX, Liu SF, Zhao XY. Sympathetic influence on osteoporotic bone metabolism and its clinical application. Chin J Osteoporosis. 2020;26(4):590–594.
10. Zhou Y, Hu YH, Chai RN, Zhang XS, Du YH, Zhu YG. CiteSpace-based visualization of the study of the relationship between sympathetic nerves and hypertension. China Med Herald. 2021;18(26):17–23.
11. Chen C, Lu ZB, Wang XY. Progress in the study of sympathetic nervous system and pulmonary hypertension. China Cardiovasc Dis Res. 2020;18(2):180–184.
12. Tang F, Zhang JX. Progress in the study of obesity and sympathetic nerve interaction. South China J Def Med. 2021;35(7):537–540. doi:10.13730/j.issn.1009-2595.2021.07.015
13. Qi YH Mechanisms of sympathetic infiltration promoting gastric adenocarcinoma progression. PhD. Shanxi Medical University.2020. doi:10.27288/d.cnki.gsxyu.2020.000186.
14. Li HC, Chen W, Yu QQ, et al. Sensory/sympathetic involvement in skin temperature, blood perfusion, and skin tissue inflammatory cytokine changes in sensitized areas of rats in a model of colitis. Chin Acupuncture. 2022;42(7):785–793. doi:10.13703/j.0255-2930.20210914-k0002
15. Ellaway PH, Taylor A, Durbaba R. Muscle spindle and fusimotor activity in locomotion. J Anat. 2015;227(2):157–166. doi:10.1111/joa.12299
16. Ge HY, Fernández-de-las-Peñas C, Arendt-Nielsen L. Sympathetic facilitation of hyperalgesia evoked from myofascial tender and trigger points in patients with unilateral shoulder pain. Clin Neurophysiol. 2006;117(7):1545–1550. doi:10.1016/j.clinph.2006.03.026
17. Hsieh YL, Yang SA, Yang CC, Chou LW. Dry needling at myofascial trigger spots of rabbit skeletal muscles modulates the biochemicals associated with pain, inflammation, and hypoxia. Evid Based Complement Alternat Med. 2012;2012:342165. doi:10.1155/2012/342165
18. Bäckman E, Bengtsson A, Bengtsson M, Lennmarken C, Henriksson KG. Skeletal muscle function in primary fibromyalgia. Effect of regional sympathetic blockade with guanethidine. Acta neurol Scand. 1988;77(3):187–191. doi:10.1111/j.1600-0404.1988.tb05893.x
19. Kimura Y, Ge HY, Zhang Y, Kimura M, Sumikura H, Arendt-Nielsen L. Evaluation of sympathetic vasoconstrictor response following nociceptive stimulation of latent myofascial trigger points in humans. Acta Physiol. 2009;196(4):411–417. doi:10.1111/j.1748-1716.2009.01960.x
20. Khan MM, Lustrino D, Silveira WA, et al. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc Natl Acad Sci U S A. 2016;113(3):746–750. doi:10.1073/pnas.1524272113
21. Hellström F, Roatta S, Thunberg J, Passatore M, Djupsjöbacka M. Responses of muscle spindles in feline dorsal neck muscles to electrical stimulation of the cervical sympathetic nerve. Exp Brain Res. 2005;165(3):328–342. doi:10.1007/s00221-005-2309-7
22. Passatore M, Grassi C, Filippi GM. Sympathetically-induced development of tension in jaw muscles: the possible contraction of intrafusal muscle fibres. Pflugers Arch. 1985;405(4):297–304. doi:10.1007/BF00595681
23. Liu Q, Zhang W, Tian T, et al. Latent myofascial trigger points injection therapy for adult cough variant asthma: a randomized controlled trial. Front Med. 2023;10:937377. doi:10.3389/fmed.2023.937377
24. Ge HY, Serrao M, Andersen OK, Graven-Nielsen T, Arendt-Nielsen L. Increased H-reflex response induced by intramuscular electrical stimulation of latent myofascial trigger points. Acupunct Med. 2009;27(4):150–154. doi:10.1136/aim.2009.001099
25. Ribot-Ciscar E, Rossi-Durand C, Roll JP. Increased muscle spindle sensitivity to movement during reinforcement manoeuvres in relaxed human subjects. J Physiol. 2000;523 Pt 1(Pt 1):271–282. doi:10.1111/j.1469-7793.2000.t01-1-00271.x
26. Tan YY, Wang YY, Zhang Q. Effects of electroacupuncture at ”Quchi” point on abnormal sympathetic nerve activity in rats with central angiotensin II-induced elevation of arterial blood pressure. Acupuncture Res. 2016;41(2):144–149. doi:10.13702/j.1000-0607.2016.02.009
27. Cao L, Gao Y, Wu K, Li Y, Chen C, Yuan S. Sympathetic hyperinnervation in myofascial trigger points. Med Hypotheses. 2020;139:109633. doi:10.1016/j.mehy.2020.109633
28. Zhang Y, Ge HY, Yue SW, Kimura Y, Arendt-Nielsen L. Attenuated skin blood flow response to nociceptive stimulation of latent myofascial trigger points. Arch Phys Med Rehabil. 2009;90(2):325–332. doi:10.1016/j.apmr.2008.06.037
29. Fernández-de-las-peñas C, Ge HY, Arendt-Nielsen L, Cuadrado ML, Pareja JA. Referred pain from trapezius muscle trigger points shares similar characteristics with chronic tension type headache. Eur J Pain. 2007;11(4):475–482. doi:10.1016/j.ejpain.2006.07.005
30. Abbaszadeh-Amirdehi M, Ansari NN, Naghdi S, Olyaei G, Nourbakhsh MR. Neurophysiological and clinical effects of dry needling in patients with upper trapezius myofascial trigger points. J Bodyw Mov Ther. 2017;21(1):48–52. doi:10.1016/j.jbmt.2016.04.014
31. Radovanovic D, Peikert K, Lindström M, Domellöf FP. Sympathetic innervation of human muscle spindles. J Anat. 2015;226(6):542–548. doi:10.1111/joa.12309
32. Hjortskov N, Skotte J, Hye-Knudsen C, Fallentin N. Sympathetic outflow enhances the stretch reflex response in the relaxed soleus muscle in humans. J Appl Physiol. 2005;98(4):1366–1370. doi:10.1152/japplphysiol.00955.2004
33. Kamibayashi K, Nakazawa K, Ogata H, Obata H, Akai M, Shinohara M. Invariable H-reflex and sustained facilitation of stretch reflex with heightened sympathetic outflow. J Electromyogr Kinesiol. 2009;19(6):1053–1060. doi:10.1016/j.jelekin.2008.11.002
34. Barker D, Saito M. Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proc R Soc Lond B Biol Sci. 1981;212(1188):317–332. doi:10.1098/rspb.1981.0042
35. Passatore M, Filippi GM, Grassi C. Cervical sympathetic nerve stimulation can induce an intrafusal muscle fibre contraction in the rabbit. In: Boyd IA, Gladden MH, editors. The Muscle Spindle. UK: Palgrave Macmillan; 1985:221–226. doi:10.1007/978-1-349-07695-6_32
36. Yuan SG, Yan LM, Wu K, Xu MK, Li YK, Zhou YC. Effect of chemical sympathectomy on myofascial excitation point inflammation and myosatellite cell myogenic differentiation. J Pract Med. 2020;36(15):2059–2065.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.