Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Effect of Silibinin on Dyslipidemia and Glycemic Alteration Associated with Polycystic Ovarian Syndrome: An Experimental Study on Rats
Authors Marouf BH
Received 15 June 2022
Accepted for publication 20 August 2022
Published 7 September 2022 Volume 2022:15 Pages 2771—2780
DOI https://doi.org/10.2147/DMSO.S377404
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Bushra Hassan Marouf
Department of Pharmacology and Toxicology, College of Pharmacy, University of Sulaimani, Sulaimani, Kurdistan Region, Iraq
Correspondence: Bushra Hassan Marouf, Tel +9647701562796, Email [email protected]
Purpose: Females with polycystic ovary syndrome (PCOS) are found to have hormonal and metabolic alterations. This study investigated the efficacy of the flavonolignan silibinin in restoring the metabolic alterations associated with letrozole-induced PCOS in rats.
Methods: The study allocated 42 albino rats into two groups. The first group was a normal control group (n=12) in which only the vehicle was given. The second group, the PCOS group (n=30), received letrozole (1 mg/kg/day) orally for 21 days. On day 21, six animals from the first group and six animals from the second group were sacrificed to confirm the development of PCOS, and the rest of the animals (n=24) in the second group were distributed equally into four groups: the PCOS group received vehicle, the metformin (MET) group received 300 mg/kg metformin orally, and the low-dose silibinin (LD-100) and high-dose silibinin (HD-200) groups received 100 and 200 mg/kg silibinin intraperitoneally, respectively. Blockade of the estrus cycle in the diestrus phase, hyperglycemia, and body weight elevation were related to a positive PCOS induction. An oral glucose tolerance test (OGTT) was also carried out for all animals on day 21 and on the last day of the experiment (day 40) to investigate the effect of silibinin on insulin resistance. The rats’ lipid profile, insulin level, estrus cycle patterns, body weight, and weights of the ovaries and uterus were also measured on day 40.
Results: A 19-day silibinin treatment led to the restoration of regular estrus cyclicity, reduced the glucose spike in OGTT analysis, and alleviated insulin resistance in PCOS rats. There was a statistically non-significant decrement in insulin level and lipid profile in the treatment groups.
Conclusion: Silibinin flavonolignan ameliorated some metabolic and reproductive alterations associated with PCOS. This could be related to the decreased insulin resistance, and antiandrogenic and phytoestrogenic activity of silibinin. Further study with longer term therapy is recommended to clarify more potential effects of silibinin and its mechanism of action in PCOS.
Keywords: metabolic alteration, impaired glucose tolerance, lipid profile, silibinin
Introduction
Polycystic ovary syndrome (PCOS) is described as an abnormal state of the female reproductive system with a multifaceted etiology. It manifests as menstrual irregularity, oligo-ovulation, and/or anovulation. Women with this disorder exhibit several metabolic changes and chronic diseases, including dyslipidemia, weight gain, hypertension, and metabolic syndrome.1 Although the pathogenesis of PCOS is not well defined, several theories have been suggested, such as impairment of androgen, lipid, glucose, and insulin metabolism. There are also metabolic alterations, such as insulin resistance, impaired glucose tolerance, and hyperlipidemia, which are considered as risk factors for type 2 diabetes mellitus (T2DM), cardiovascular disease, weight gain, infertility, and endometrial carcinogenesis.2 PCOS exhibits hyperglycemic spikes after an oral glucose tolerance test (OGTT), which contribute to insulin resistance, consequently resulting in hyperglycemia and metabolic syndrome.3 Insulin resistance, with reflex hyperinsulinemia, plays a part in metabolic changes associated with PCOS, and therefore PCOS and insulin resistance are often interrelated.4,5 In addition, androgen elevation and higher luteinizing hormone (LH) level are the principal biochemical abnormalities in women with PCOS.6 An increase in body weight is associated with increased androgen levels in women with PCOS.7 Thus, a complex interrelationship exists between obesity, abdominal obesity, insulin resistance, androgen, and LH level in the etiology and pathogenesis of PCOS. The resulting hormonal imbalance, circulating hyperandrogenism and intraovarian androgen excess, lead to the appearance of a polycystic ovary, hyperglycemic condition, and metabolic disturbances. PCOS is frequently associated with various patterns of dyslipidemia, including a reduction of high-density lipoprotein cholesterol (HDL-C), and high levels of triglycerides (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C).8 Although the data from large series suggest that the mean values for circulating lipids in women with PCOS are within normal limits, up to 70% of patients have at least one abnormal lipid level according to the most widely used criteria of metabolic syndrome.9 Moreover, the conversion from normal to impaired glucose tolerance and from impaired glucose tolerance to T2DM is increased two- to five-fold in the PCOS population.10 Because of these increased metabolic risks, many organizations recommend screening for T2DM in PCOS women. Moreover, screening these women with an OGTT instead of fasting glucose is recommended because relying on fasting plasma glucose (FPG) alone is not adequate for the screening of disorders of glucose tolerance in women with PCOS; such diagnosis should rely on the results of an OGTT.11
Clomiphene citrate, exogenous gonadotropins, and insulin sensitizers, such as metformin, are used to reduce insulin resistance, which results in a reduction of ovarian androgen production and a consequent improvement in menstrual cyclicity. Because of the adverse effects that accompany conventional therapy, the use of alternative herbal medicines has been encouraged as a potential therapy for the treatment of PCOS. Silibinin phytoestrogen is a flavonolignan and it is a major active constituent of silymarin extract, derived from Silybum marianum. It has been documented to possess a wide variety of pharmacological activity, including anti-inflammatory, analgesic, antioxidant, antihyperglycemic, antihyperlipidemic, and antiobesity properties, and is also used in gynecological disorders.12,13 Silibinin possesses antihyperglycemic12,13 and antihyperlipidemic14 properties in diabetic and hypercholesterolemic rats, and also induces apoptosis in human prostate cancer cells through targeting Akt, NF-KB and androgen receptor signaling.15 In an in vivo diet-induced obesity study, silibinin restored glucose abnormalities in treated animals, and reversed hyperglycemia, hyperinsulinemia, and hypertriglyceridemia, through reduced adipose tissue inflammation, reversed obesity progression, and improved glucose homeostasis.12,14 In spite of these effects of silibinin, its action in PCOS disorder is unknown. On the basis of these actions of silibinin, we hypothesized that it may be beneficial in the management of PCOS induced by letrozole. Therefore, the aim of this study was to investigate the efficacy of silibinin on restoring the metabolic alterations associated with letrozole-induced PCOS in albino rats.
Materials and Methods
Experimental Animals
Forty-two female Wister albino rats aged 10–12 weeks (weighing 150–180 g) were used for the study. They were obtained from the animal house of the College of Pharmacy, University of Sulaimani. The rats were housed in plastic cages and acclimatized to the standardized environment with a 12-hour light–dark cycle, a temperature of 22±1°C, and a relative humidity of 50±5%, for 1 week. The experimental protocol was approved by the Ethical Committee of the College of Pharmacy, University of Sulaimani (registration number PH24-21, August 31, 2021). All the experimental procedures were conducted according to the European Communities Council Directives on Animal Care. All the animals were checked for three consecutive regular estrous cycles by vaginal smear examination. Rats with a normal estrus cycle were included in the study.
Animal Grouping and PCOS Induction
The study protocol is illustrated in Figure 1. Rats showing a normal estrus cycle were selected and randomly divided into two groups. The control group (n=12) received distilled water. The PCOS group (n=30) received letrozole (Denk Pharma, Germany; 1 mg/kg/day dissolved in distilled water) orally by gavage tube for 21 days to induce PCOS, as previously described.16 On day 21, six rats from the first group and six rats from the second group were randomly selected and sacrificed for PCOS confirmation, and the rest (n=24) were distributed into four groups. The PCOS group received vehicle; the metformin (MET) group received 300 mg/kg body weight metformin (Merck Sante, Germany) orally; the low-dose silibinin (LD-100) and high-dose silibinin (HD-200) groups received 100 mg/kg and 200 mg/kg silibinin 98% (Glentham Life Sciences, UK) intraperitoneally, respectively. Animals with an irregular estrus cycle were excluded from the study. PCOS was confirmed by increasing weight and irregularity of the estrus cycle. Therefore, blockade of the estrus cycle in the diestrus phase, hyperglycemia, and body weight elevation were related to positive PCOS induction.17,18 The body weight of the animals was recorded on day 1 ie before administration of letrozole, on day 21 of administration of letrozole, and at the end of the experiment on day 40. At the end of the research period, the rats were euthanized and blood samples were collected from the heart of all the animals for measurement of the lipid profile and insulin level. The sexual organs (ovaries and uterus) and the abdominal fat around the ovaries (periovarian fat) and the uterine horns within the broad ligaments were dissected out and weighed separately for each uterine side.
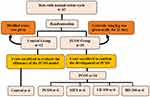 |
Figure 1 Flowchart of the study design. Abbreviations: PCOS, polycystic ovary syndrome-induced group; MET, metformin; LD-100, low-dose silibinin; HD-200, high-dose silibinin. |
Vaginal Smears
For determination of the estrus stage, vaginal smears were collected daily and evaluated microscopically using Giemsa stain to confirm the induction of PCOS. This examination was carried out daily starting from day 15 until day 21, as previously described.19 The estrus stage was determined by microscopic analysis of the predominant cell type in the vaginal smear. Proestrus and estrus stages consisted of a predominance of nucleated epithelial cells and anucleated cornified cells, respectively. The metestrus stage consisted of the same proportion among leukocytes, cornified and nucleated epithelial cells, while the diestrus stage primarily consisted of a predominance of leukocytes (Figure 2). Changes in vaginal cytology were used to interpret the changes in hormonal levels and modifications in the estrous cycle.20
Oral Glucose Tolerance Test (OGTT)
The OGTT was conducted on day 21, and after treatments with metformin and the two doses of silibinin. Rats were fasted for 16 hours and the glucose level was determined using tail blood samples by a glucometer (Accucheck-active, Roche Diagnostics) before a single oral administration of glucose (2.5 g/kg), which was considered as zero time, and at 30, 60, 90, and 120 min after administration, as previously described.18 The total area under the curve of the glucose response (AUC) was calculated using GraphPad Prism 9.3.1 (GraphPad Software, California, USA).
Biochemical Parameters
Serum insulin, lipid profile, TC, TG, HDL-C, and LDL-C, for all groups were measured on day 40 using an enzyme-linked immunosorbent assay (ELISA); the kits were purchased from BT-LAB Bioassay Technology Laboratory Jiaxing Korain Biotech Co. (Zhejiang, China).
Statistical Analysis
The results are expressed as mean ± SEM. The statistical significance of the data was determined by a one-way ANOVA followed by Tukey’s post-hoc tests, which were used to evaluate the lipid profile, insulin, and relative weight differences among the groups. A two-way ANOVA with repeated measures for time, followed by Tukey’s post-hoc test, was also used to test significant differences in OGTT and body weight between different time-points and different groups on days 21 and 40. The statistical analysis of the data was performed using GraphPad Prism 9.3.1 software (GraphPad Software). Statistical significance was set at p<0.05.
Results
Body Weight Changes
The body weight of the animals prior to PCOS induction was similar in all groups. The body weights of the animals after induction of PCOS by letrozole on day 21 were significantly higher than in the control group (p<0.05). There was a statistically non-significant change in the mean body weight of all the groups between day 21 and day 40, except in the LD-100 group, where a significant reduction of body weight was observed on day 40 (Table 1).
 |
Table 1 Body Weight Changes of the Rats Throughout the Treatment Period |
Relative Ovarian and Uterine Weights, and Abdominal Fat Weight at the End of the Experiment
The changes in the relative weight of the ovaries and uteri at the end of the experiment were not significant in any of the treated groups compared to the control, the PCOS group, or within treatment groups. The weight of abdominal fat decreased in the silibinin groups, and the reduction was statistically significant in the HD-200 group (p<0.05) (Table 2).
 |
Table 2 Relative Ovarian and Uterine Weights, and Abdominal Fat Weight, on Day 40 |
Oral Glucose Tolerance
An OGTT was performed on day 21 and day 40 for all rats in the experiments.
On day 21, OGTT analysis showed that the glucose level at 60 min after glucose loading was significantly increased in the PCOS groups (PCOS, MET, LD-100, HD-200) compared to the control group (Figure 3A). In general, in normal conditions after glucose loading, the serum glucose level increases to the peak level, and within 120 min it comes down into the normal range again, but this was not observed in the PCOS groups (PCOS, MET, LD-100, HD-200) on day 21. This indicates the induction of PCOS and development of insulin resistance status.
On day 40, OGTT analysis in animals treated with low and high doses of silibinin, as well as MET, showed no significant spike in glucose level after 60 min compared to the control group, suggesting an amelioration in the impaired glucose tolerance status that developed as a result of letrozole. However, at 120 min, all groups exhibited a decrement in glucose level in a significant manner, nearly comparable to the control group (Figure 3B). Furthermore, no differences in the 0 to 120-min AUC value for glucose existed among the groups (Table 3).
 |
Table 3 Area Under the Curve for Oral Glucose Tolerance Test on Days 21 and 40 |
Insulin Level
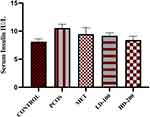
The serum level of insulin on day 21 was elevated, which indicated hyperinsulinemia in letrozole-treated rats. On day 40, the serum insulin level remained high in the PCOS group. There was a statistically non-significant decrement in insulin level in the treatment groups of metformin, LD-100, and HD-200 (Figure 4).
Lipid Profile
Administration of letrozole to the animals in all the groups for 21 days resulted in considerable elevations in TC, TG, and LDL-C, and a reduction in HDL-C level. At the end of the experiment, the study data showed that the treatment interventions with metformin and LD-100 did not have any effect on the return to normal values of the elevated level of these parameters owing to letrozole treatment, while HD-200 noticeably reverted the elevation of TG and LDL-C levels nearly to the control values, in a non-significant manner (p>0.05). None of the treatments used in the experiment reverted the reduction in HDL-C to the normal value (Figure 5A–D).
Discussion
It is well known that PCOS is accompanied by reproductive complications (hyperandrogenism, menstrual irregularity, anovulation, and infertility) and metabolic dysfunction (dyslipidemia, glucose intolerance state, T2DM, and cardiovascular complications).21 Insulin resistance with compensatory hyperinsulinemia is proposed as a key pathophysiological feature of PCOS, contributing to both reproductive and metabolic disturbances.22 Not all women with PCOS are insulin resistant or develop compensatory hyperinsulinemia, implying that these features are not essential for the development of PCOS. However, androgen excess and higher LH level, with metabolic dysfunction, are the principal biochemical abnormalities in women with PCOS. To validate the effect of the silibinin on metabolic alterations associated with PCOS, the present study investigated the glucose intolerance state, lipid profile, and some weight parameters in a PCOS model developed by oral administration of letrozole in albino rats.
In this study, the PCOS-induction model was achieved by a 3-week administration of letrozole,16 a non-steroidal aromatase inhibitor, which suppresses aromatization, paracrine signaling, folliculogenesis, and ovarian function.23 It has been used to produce endogenous hyperandrogenism.24 In female rats, letrozole disrupts estrus cyclicity and increases the weight of the ovaries, with multiple cysts, thin granulosa cell layers, thickening of the theca cell layers, and atretic follicles. It can also increase gonadotropin and testosterone levels and decrease estrogen levels.16,25 In the present study, administration of letrozole for 21 days successfully enabled the induction of PCOS in all allocated animals. This was evidenced by irregularity in the estrus cycle, particularly blockade of the estrus cycle in the diestrous phase for a longer time (data not shown), and increased body weight, hyperglycemia, and disruption of lipid profile parameters, as previously reported.17,26 Vaginal smear histology analysis is a key indicator of ovarian physiology, and in the present study it was used as a diagnostic tool to assess the normality of the estrus cycle. Obesity and abdominal obesity worsen the clinical features of menstrual irregularity and infertility,27 and are related to the metabolic dysfunction accompanying PCOS development.28
Most polyphenolic compounds with pleiotropic activities exert their anti-PCOS action through a reduction of body weight and the restoration of hormonal and estrus cycle irregularities.29 Herbal medicines with phytoestrogenic activity have a potential binding affinity for estrogen receptors;30,31 this feature provides the possibility for silibinin agent to be used as a therapeutic approach in the treatment of PCOS. Although the 19-day treatment with metformin 300 mg/kg and the two doses of silibinin had a tremendous effect on restoring the estrus cycle (data not shown), the effects of the treatments on body weight were not significant at the end of the experiment. This may be related to the duration of the treatments, which was too short to prevent fat accumulation or act on adipocyte parameters. Comparable results were reported with respect to metformin in an in vivo study conducted by Ndeingang et al, in which a high dose of metformin (500 mg/kg/day) was unable to reduce body weight; however, the use of clomiphene as a first line treatment for PCOS was superior in the amelioration of body weight.32 In another in vivo study on PCOS, in which metformin was used as a standard control in assessing the effect of rutin on metabolic and biochemical aspects of PCOS, the results showed no significant changes in weight gain, abdominal circumference, or thoracic circumference among rats in different groups.26
In an in vivo study of diet-induced obesity, silibinin reversed the progression of obesity, improved glucose homeostasis, and ameliorated inflammation of adipose tissue.33 Attenuation of inflammation by silibinin treatment has also been described in several disease models, and the effects were attributed mainly to silibinin’s ability to suppress the nuclear factor kappa B (NF-κB) pathway, and its downstream expression of pro-inflammatory cytokines, particularly tumor necrosis factor-ɑ and interleukin-1β.34 In addition, silibinin can activate transcriptional factors involved in cellular defense against inflammatory and oxidative challenges, such as nuclear factor-erythroid-2 (NF-E2)-related factor 2 (Nrf2).35 These effects are coupled with the potent antioxidant effects of silibinin, by which further attenuation of inflammatory events is achieved. In agreement with these theories, the well-established inhibitory effects of silibinin on NF-κB could explain the antiobesity effects of silibinin.
In the PCOS condition, the ovarian and uterine weights of the animals did not increase significantly; these non-significant findings may be contributed to the rapid metabolic response of the animals, or a longer duration of exposure of letrozole may have been required to obtain significant changes in ovarian pathology.26
A previous investigation on obese women with PCOS reported that the women had significantly increased glucose levels during an OGTT.5 In the present study, letrozole treatment led to dysglycemia, including impairment on the OGTT. The effect on the overall glucose intolerance status was not superior following silibinin treatment, at the end of the experiment. This could be explained by the short duration of the treatment, which was sufficient for restoration of the estrous cyclicity to the normal phases, but was not long enough to revert and improve the glucose intolerance status and insulin resistance.
Dyslipidemia is another characteristic feature associated with PCOS.36 Although many women with PCOS have normal levels of lipids, up to 70% of patients have at least one abnormal lipid level.37 In our study, elevation of several lipid profile contents, ie cholesterol, TG, and LDL-C, and a noticeable reduction in HDL-C, were observed in the letrozole-treated group. There were no ameliorative effects of metformin and silibinin on these metabolic changes associated with PCOS, except that HD-200 slightly reduced TG and LDL-C. This finding is inconsistent with the effects of Silybum marianum in decreasing LDL-C, TC, and plasma glucose levels, reported by Tóth et al in a meta-analysis.38
The restoration of the estrus cycle and amelioration of some metabolic parameters in the silibinin-treated groups could be associated with the antiandrogenic and phytoestrogenic activity of silibinin. The antiandrogenic effect of silibinin has been documented in in vitro studies of prostate cancer cells, which investigated anticancer effects in human prostate cancer cells through androgen receptor degradation,39 or inhibition of lipid metabolism in prostate cancer cells. It is well established that letrozole-induced PCOS alters the normal physiology and endocrine secretion owing to the increased androgen levels. Moreover, an abnormal increase in androgens leads to the induction of insulin resistance, thus reducing glucose tolerance.40 Many preclinical and clinical studies have revealed the antihyperglycemic action of silibinin, which reduces the fasting blood glucose level and glycosylated hemoglobin level, as well as reducing the glycemic level in patients with T2DM.12,41 Based on the findings of Chen et al, silibinin protects β-cells from glucotoxicity through the regulation and improvement of the insulin-induced gene 1/sterol regulatory element binding protein-1c (Insig-1/SREBP-1c) pathway,42 and it takes part in potentiating the insulin secretion by the β-cells, restores glucose homeostasis, and reverses hyperglycemia, hyperinsulinemia, and hypertriglyceridemia.33 Furthermore, it has been suggested that silibinin may be a potential agent for glycemic control and metabolic disorders through farnesyl X receptor (FXR) activation and NF-κB inhibition.43
The findings of this study revealed that silibinin has a role in the treatment of PCOS induced by the aromatase inhibitor letrozole. It can be seen that this pleiotropic agent exerts many pharmacological effects, which improve the glucose intolerance status and lipid profile. However, the current study found less significant beneficial effects on PCOS compared to other studies.
Conclusion
Silibinin flavonolignan ameliorated some of the abnormal metabolic and reproductive alterations associated with PCOS. This could be related to the decreased insulin resistance, and antiandrogenic and phytoestrogenic activity of silibinin. Further study with longer term therapy is recommended to clarify more potential effects of silibinin and its mechanism of action in PCOS.
Acknowledgments
The author would like to thank the College of Pharmacy and the College of Education, Biology Department, University of Sulaimani, for their support in conducting this research.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Orio F, Muscogiuri G, Nese C, et al. Obesity, type 2 diabetes mellitus and cardiovascular disease risk: an uptodate in the management of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;207:214–219. doi:10.1016/j.ejogrb.2016.08.026
2. Mu N, Zhu Y, Wang Y, Zhang H, Xue F. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol. 2012;125(3):751–757. doi:10.1016/j.ygyno.2012.03.032
3. Lim SS, Kakoly NS, Tan JWJ, et al. Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression: metabolic syndrome in PCOS. Obes Rev. 2019;20(2):339–352. doi:10.1111/obr.12762
4. Cassar S, Misso ML, Hopkins WG, Shaw CS, Teede HJ, Stepto NK. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic–hyperinsulinaemic clamp studies. Hum Reprod. 2016;31(11):2619–2631. doi:10.1093/humrep/dew243
5. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi:10.1210/er.2011-1034
6. Nisenblat V, Norman RJ. Androgens and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2009;16(3):224–231. doi:10.1097/MED.0b013e32832afd4d
7. Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes. 2002;26(7):883–896. doi:10.1038/sj.ijo.0801994
8. Kim JJ, Choi YM. Dyslipidemia in women with polycystic ovary syndrome. Obstet Gynecol Sci. 2013;56(3):137. doi:10.5468/ogs.2013.56.3.137
9. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. doi:10.1161/circ.106.25.3143
10. Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90(6):3236–3242. doi:10.1210/jc.2004-1843
11. Ortiz-Flores AE, Luque-Ramírez M, Fernández-Durán E, Alvarez-Blasco F, Escobar-Morreale HF. Diagnosis of disorders of glucose tolerance in women with polycystic ovary syndrome (PCOS) at a tertiary care center: fasting plasma glucose or oral glucose tolerance test? Metabolism. 2019;93:86–92. doi:10.1016/j.metabol.2019.01.015
12. Chu C, Li D, Zhang S, et al. Role of silibinin in the management of diabetes mellitus and its complications. Arch Pharm Res. 2018;41(8):785–796. doi:10.1007/s12272-018-1047-x
13. Xu F, Yang J, Negishi H, et al. Silibinin decreases hepatic glucose production through the activation of gut–brain–liver axis in diabetic rats. Food Funct. 2018;9(9):4926–4935. doi:10.1039/C8FO00565F
14. Gobalakrishnan S, Asirvatham SS, Janarthanam V. Effect of silybin on lipid profile in hypercholesterolaemic rats. J Clin Diagn Res. 2016;10(4):FF01–05. doi:10.7860/JCDR/2016/16393.7566
15. Deep G, Gangar SC, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin A induces apoptosis in human prostate cancer cells via targeting Akt, NF-κB, and androgen receptor signaling. Mol Carcinog. 2010;49(10):902–912. doi:10.1002/mc.20670
16. Kafali H, Iriadam M, Ozardalı I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35(2):103–108. doi:10.1016/j.arcmed.2003.10.005
17. Mvondo MA, Mzemdem Tsoplfack FI, Awounfack CF, Njamen D. The leaf aqueous extract of Myrianthus arboreus P. Beauv. (Cecropiaceae) improved letrozole-induced polycystic ovarian syndrome associated conditions and infertility in female Wistar rats. BMC Complement Med Ther. 2020;20(1):275. doi:10.1186/s12906-020-03070-8
18. Kakadia N, Patel P, Deshpande S, Shah G. Effect of Vitex negundo L. seeds in letrozole induced polycystic ovarian syndrome. J Tradit Complement Med. 2019;9(4):336–345. doi:10.1016/j.jtcme.2018.03.001
19. Kim EJ, Jang M, Choi JH, Park KS, Cho IH. An improved dehydroepiandrosterone-induced rat model of polycystic ovary syndrome (PCOS): post-pubertal improve PCOS’s features. Front Endocrinol. 2018;9:735. doi:10.3389/fendo.2018.00735
20. Nallathambi A, Bhargavan R. Regulation of estrous cycle by Cynodon dactylon in letrozole induced polycystic ovarian syndrome in Wistars albino rats. Anat Cell Biol. 2019;52(4):511. doi:10.5115/acb.19.114
21. Gambineri A, Patton L, Altieri P, et al. Polycystic ovary syndrome is a risk factor for type 2 diabetes. Diabetes. 2012;61(9):2369–2374. doi:10.2337/db11-1360
22. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women 1. J Clin Endocrinol Metab. 1999;84(1):165–169. doi:10.1210/jcem.84.1.5393
23. Maliqueo M, Sun M, Johansson J, et al. Continuous administration of a P450 aromatase inhibitor induces polycystic ovary syndrome with a metabolic and endocrine phenotype in female rats at adult age. Endocrinology. 2013;154(1):434–445. doi:10.1210/en.2012-1693
24. Dellapasqua S, Colleoni M. Letrozole. Expert Opin Drug Metab Toxicol. 2010;6(2):251–259. doi:10.1517/17425250903540246
25. Mannerås L, Cajander S, Holmäng A, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148(8):3781–3791. doi:10.1210/en.2007-0168
26. Jahan S, Munir F, Razak S, et al. Ameliorative effects of rutin against metabolic, biochemical and hormonal disturbances in polycystic ovary syndrome in rats. J Ovarian Res. 2016;9(1):86. doi:10.1186/s13048-016-0295-y
27. Ozcan Dag Z, Dilbaz B. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. 2015;16(2):111–117. doi:10.5152/jtgga.2015.15232
28. Rojas J, Chávez M, Olivar L, et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med. 2014;2014:1–17. doi:10.1155/2014/719050
29. Jahan S, Abid A, Khalid S, et al. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: a histological and a biochemical study. J Ovarian Res. 2018;11(1):26. doi:10.1186/s13048-018-0400-5
30. Zhao L, Mao Z, Brinton RD, Select A. Combination of clinically relevant phytoestrogens enhances estrogen receptor β-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. 2009;150(2):770–783. doi:10.1210/en.2008-0715
31. Jiang Y, Gong P, Madak‐Erdogan Z, et al. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013;27(11):4406–4418. doi:10.1096/fj.13-234617
32. Ndeingang EC, Defo Deeh PB, Watcho P, Kamanyi A. Phyllanthus muellerianus (Euphorbiaceae) restores ovarian functions in letrozole-induced polycystic ovarian syndrome in rats. Evid Based Complement Alternat Med. 2019;2019:1–16. doi:10.1155/2019/2965821
33. Alsaggar M, Bdour S, Ababneh Q, El-Elimat T, Qinna N, Alzoubi KH. Silibinin attenuates adipose tissue inflammation and reverses obesity and its complications in diet-induced obesity model in mice. BMC Pharmacol Toxicol. 2020;21(1):8. doi:10.1186/s40360-020-0385-8
34. Kim BR, Seo HS, Ku JM, et al. Silibinin inhibits the production of pro-inflammatory cytokines through inhibition of NF-κB signaling pathway in HMC-1 human mast cells. Inflamm Res. 2013;62(11):941–950. doi:10.1007/s00011-013-0640-1
35. Podder B, Kim YS, Zerin T, Song HY. Antioxidant effect of silymarin on paraquat-induced human lung adenocarcinoma A549 cell line. Food Chem Toxicol. 2012;50(9):3206–3214. doi:10.1016/j.fct.2012.06.007
36. Xiang SK, Hua F, Tang Y, Jiang XH, Zhuang Q, Qian FJ. Relationship between serum lipoprotein ratios and insulin resistance in polycystic ovary syndrome. Int J Endocrinol. 2012;2012:1–4. doi:10.1155/2012/173281
37. Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111(8):607–613. doi:10.1016/S0002-9343(01)00948-2
38. Tóth B, Németh D, Soós A, et al. The effects of a fixed combination of berberis aristata and Silybum marianum on dyslipidaemia – a meta-analysis and systematic review. Planta Med. 2020;86(02):132–143. doi:10.1055/a-1063-1649
39. Deep G, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin B causes androgen receptor degradation in human prostate carcinoma cells via PI3K-Akt-Mdm2-mediated pathway. Oncogene. 2008;27(28):3986–3998. doi:10.1038/onc.2008.45
40. Liao EP. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med. 2012;125(10):S1–S2. doi:10.1016/j.amjmed.2012.05.001
41. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res. 2006;20(12):1036–1039. doi:10.1002/ptr.1988
42. Chen K, Zhao L, He H, Wan X, Wang F, Mo Z. Silibinin protects β cells from glucotoxicity through regulation of the Insig-1/SREBP-1c pathway. Int J Mol Med. 2014;34(4):1073–1080. doi:10.3892/ijmm.2014.1883
43. Gu M, Zhao P, Huang J, et al. Silymarin ameliorates metabolic dysfunction associated with diet-induced obesity via activation of farnesyl X receptor. Front Pharmacol. 2016;7. doi:10.3389/fphar.2016.00345
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.