Back to Journals » Journal of Inflammation Research » Volume 16
Effect of Fipronil Exposure on Hematological Aspects of Rhesus Monkeys (Macaca mulatta): Risk and Toxicity Assessment in Agro-Workers
Authors Khan NH , Jiang E , Qureshi IZ
Received 3 September 2022
Accepted for publication 5 September 2023
Published 29 December 2023 Volume 2023:16 Pages 5755—5765
DOI https://doi.org/10.2147/JIR.S386145
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Nazeer Hussain Khan,1,2 Enshe Jiang,2 Irfan Zia Qureshi1
1Human and Animal Physiology Laboratory, Department of Animal Sciences, Quaid-I-Azam University, Islamabad, 44000, Pakistan; 2Institute of Nursing and Health, Henan University, Kaifeng, Henan, 475004, People’s Republic of China
Correspondence: Enshe Jiang; Irfan Zia Qureshi, Email [email protected]; [email protected]
Background: Fipronil (FPN) is a broad-spectrum phenylpyrazole insecticide, widely used in agriculture and veterinary medicine. Published research on FPN toxicity has established the fact that its inhalation or dermal exposure may lead to very serious clinical outcomes in non-target animals. In line to its exposure and toxicity related damage, FPN has been investigated in many invertebrates, however, its exposure-related noxiousness is less reported in higher animals.
Objective: To assess the FPN-induced effects to agro-workers in the field, in the present study, we used physiological human surrogates, adult rhesus monkeys as models.
Method: We exposed well habituated, chair restraint adult rhesus monkeys with a field spray concentration of FPN (0.3 mg/1 mL distilled water) through an inhalation route in the closed system. Animals were divided into control and treatment groups, each containing three animals. Inflammatory and hematological effects were determined by evaluating the kidney and liver biomarker enzymes; serum creatinine and alanine transaminase (ALT), aspartate transaminase (AST) levels respectively.
Results: Our findings reveal that FPN treated monkeys show significantly increased levels of ALT (p = 0.000461), AST (p = 0.0681) and creatinine (p = 0.00656) as compared to the control group. Furthermore, significant differences of red blood cells (RBCs) (p = 0.0139) and white blood cells (WBCs) (p = 0.00642) were also observed in the treated and control group monkeys which reflect strong toxic effects on the blood cells.
Conclusion: Our findings demonstrate that FPN exposure is very toxic to higher animals and causes severe damage to the liver and kidneys along with other clinical problems. The study highlights the effect and impact of passive inhalation of insecticides in intentionally carefree agro-workers and raises the concern of public awareness toward pesticides use.
Keywords: rhesus monkeys (Macaca mulatta), fipronil, hepatic damage, nephrotoxic, pesticides use in Pakistan
Introduction
To meet the increasing demand for food, human beings are in a constant struggle to increase per capita agricultural products by using different pesticides to control damaging crop organisms. These agrochemicals are used worldwide. According to a report, world pesticide usage at the producer level was worth nearly $56 billion annually in 2018.1 Corresponding to that increase in usage; there is mounting evidence of human illness and deaths with occupational exposure to these agrochemicals all over the world.2–4 Owing to improper regulations, monitoring systems, weak enforcement, improper training, low education, and poorly managed or non-existent personal protective equipment, high numbers of pesticide poisoning cases are reported in developing countries.5,6 The use of pesticides has become a worldwide trend which is now affecting an increasing number of species as more land is being used for intensive agriculture. There is a growing awareness that non-target species, including humans, often experience collateral damage from pesticides, often resulting in death or sub-lethal effects on behavior, physiology, or endocrinology.7–10 Therefore, more concerns have been expressed about safety measurements of pesticides toward human health.
Fipronil (FPN) is a broad-spectrum phenylpyrazole insecticide. It has been widely used in agriculture and veterinary medicine to prevent aggressive insects from damaging agricultural crops and domesticated animals.11,12 Moreover, FPN has diverse uses in horticulture against a broad range of insects, such as termites, beetles, cockroaches, mosquitoes, locusts, ticks, weevils, and fleas.13 Chemically, it has a small molecular weight with low polarity and is soluble in low polar organic solvents like acetone and toluene. Under natural or biotic process, FPN is mainly dissipated by photolysis, hydrolysing into four degradation compounds, including fipronil-desulfinyl, fipronil-amide, fipronil-sulfide, and fipronil-sulfone.14 In water, the major degradation product is fipronil-sulfide or fipronil-desulfinyl while in many animals like in fishes, FPN is mainly metabolized to fipronil-sulfone through cytochrome P450 oxidation.15
Research on FPN toxicity shows that FPN is highly toxic to many aquatic species: it could deprive benthic macro invertebrate’s motor ability.15,16 Moreover, FPN exerts some sub-lethal effects, ranging from reproductive genotoxic and cytotoxic effects, to many vertebrate animals, such as fish, reptiles, amphibians, birds, mammals, and even humans.17 Studies have reported that FPN metabolites are even more toxic than their parent compound. For example, fipronil-sulfone is 20 times more active with mammals than in insect chloride channels.17,18 There are many studies about the FPN mode of action, pathway and its toxicity along with its pharmacodynamics on insects, aquatic reptiles, birds and lower mammals, especially the house mouse (Mus musculus).
In our previous study, we found that FPN combined with buprofezin is relatively more toxic to Cyprinus carpio in the combined state and induced physiologically relevant hematological, biochemical, molecular, and histopathological alterations.19 However, there's no literature about FPN's toxicity in higher animals like monkeys.
In the present study, to understand the mechanism, risk assessment, and toxicity associated with FPN exposure to agro-workers in the field, as an environmental toxin and bio-aerosol, we used physiological human surrogates, adult rhesus monkeys, as models. Monkeys are frequently used to understand the exposure–response relationships for allergens and pro-inflammatory agents to help in elucidating the disease mechanisms and quantitative data to assess risk.20–22 We exposed well habituated, chair restraint, adult rhesus monkeys to field spray concentrations of FPN (0.3 mg/1 mL distilled water) through the inhalation route in a closed system.
After the FPN-treatment, toxicity profiles of the treated animals were assessed in the form of nephrotoxic and hepatotoxic damage by measuring the kidney and liver enzymes; serum creatinine and alanine transaminase (ALT), aspartate transaminase (AST) level, respectively. Hematologic effects on the blood cell counts were also determined. Additionally, we also recorded the qualitative data of animals during and after the treatment. We anticipated that the present study would highlight the need to educate the agro-worker community and increase the awareness to consider all precautionary measures while doing field work.
Materials and Methods
Chemical - Materials
Chemicals, ALT, AST and creatinine activity assay kits were purchased from Jesan Company (Italy). Ketamine and heparin (Rotexmedica, Trittau, Germany), EDTA and gel tubes were purchased from the local supplier. Formulations of FPN (2.5% solution) were obtained from Planters (PVT) Limited, Islamabad, Pakistan under the trade name of Turmoil 50 SC and Anpon Co. Ltd. (Jiangsu, China), and Super Pesticides. A Teflon-cannula of Vasocan Branule, 0.8 mm/22 G O.D, (B. Braun Melsungen AG, Belgium) was used. In instruments, physiological parameters of animals, G3-monitor, BP apparatus, glucometer and video camera were used. For blood base analysis, spectrophotometer, URIT-2900Vit Plus Auto Hematology Analyzer (made in China) was used.
Method
Animal Habituation
Disease and parasite free, active and uniform size (8±0.5 kg) three adult nonhuman primates, male rhesus monkeys (Macaca mulatta) were chosen from those housed in an animal house. The monkeys were maintained at daily temperatures of 25 ± 2 °C, a relative humidity of 30–70%, with a sufficient air flow. Before the day of the experiment, all animals were habituated for restraining on a primate chair, with a cannula installation and to sit in a card-made large inhalation chamber for 40 days with friendly and empathetic handling. Before exposure to the FPN inhalation, the animals were relaxed with minimum handling stress with no estimated adverse effect level by the operating and handling approaches adopted as reported in the study.23
Venous Catheterization
A Teflon-cannula was inserted in the saphenous vein of habituated experimental animals. The free end of the cannula was linked to a syringe by a butterfly tube. This conduit was used for sequential blood sampling after FPN inhalation.
Inhalation Chamber
A card-made inhalation chamber of 5.1 feet in height and 3.2 feet in width was used to cover a chair restrained monkey inside. A sprinkling inlet as the source of FPN spray was made on the right side of the chamber (Figure 1). A glass window, facing the front of monkey, was fixed in the chamber that allowed the investigator to observe the animal's behavior during the experiment. At the right top of the chamber, small holes were made as air inlets.
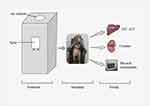 |
Figure 1 Sketch of experimental setup. |
Experimental Operation
The experiment began after positioning the habituated-restrained monkey on the primate chair followed by taking pre-inhalation measurements of physiological parameters: heart rate (HR), respiration rate (RR), rectal temperature (RT), partial pressure of oxygen (PO2), and blood glucose of the animals. Afterwards, the monkey was transferred into a closed-inhalation chamber where they were exposed to FPN spray for 10 min. Each animal, after exposure, underwent post-inhalation measurements of physiological parameters and sequential blood sampling for the next four hours with intervals of an hour (T0, T1, T2, T3, and T4).
Blood was collected in EDTA and gel tubes for complete blood count (CBC) and serum separation respectively. Hematological parameters were analyzed by a blood analyzer machine while assays for measuring the toxicity of the liver and kidneys were run in a spectrophotometer. Furthermore, during the experiment's duration, all possible qualitative data were measured.
Ethical Approval
The current study was conducted on the Rhesus monkey (Macaca mulatta) in the laboratory of Animal and Human Physiology, Department of Animal Sciences, Quaid-i-Azam University, Islamabad. Handling of animals was done according to the guidelines and regulations provided by ARRIVE (Animal Research: Reporting in vivo Experiments) and BEC-FBS-QAU-94 (Bioethical Ethical Committee of Faculty of Biological Sciences Quaid–i-Azam University). This study was approved by the Bioethical Committee of the Faculty of Biological Sciences, Quaid–i-Azam University.
Statistical Analysis
Data are presented as mean ± standard error of mean (SEM). Statistical analysis of the data was performed with SPSS, version 20 and graphs were drawn with the help of GraphPad Prism, version 5 software (San Diego, California, USA). Data were analyzed statistically by repeat measures one-way ANOVA followed by Dunnett’s post-hoc test. Results were considered statistically significant at p < 0.05.
Results
Measurement of Physiological Parameters
A significant difference in physiological parameters like PO2 (P = 0011), RR (p = 0.0019), HR (p = 0.0069) and RT (p = 0.0005) of treated monkeys was observed as compared to the control.
Figure 2 presents the paired T-test comparison of the pre- and post-treatment values of these parameters in FPN exposed monkeys which indicated the considerable decrease in physiological indicators in treated animals. These preliminary physiological findings demonstrate that FPN exposure may exert considerable restlessness and other clinical effects on the physiology of the target animals.
Measurement of Serum ALT and AST Level
Our findings on clinical serum chemistry parameters indicate that there was a significant increase in serum ALT and AST levels in FPN treated rhesus monkeys as compared to the control group over the time duration of 240 min (4 h). There was a significant rise in AST level at the final sample (p = 0.0.0681) as compared to the first reading, while ALT was p = 0.0.000461 after 3 hours of treatment (Figure 3). These findings strongly suggest that FPN exposure may damage the functioning of the liver of the target animals and determines the risk for unprotected agro-workers while doing FPN spraying in the field.
Measurement of Serum Creatinine Level
Following the FPN exposure, serum creatinine level of treated rhesus monkeys showed a significant upsurge compared to the control group over the time duration of 240 min (4h). Sequential sampling showed the significant rise in creatinine level at final sample (p= 0.00656) as compared to first reading (Figure 4). Likewise damage to liver, these findings highlight that FPN exposure may damage the functioning of kidneys of target animals and determine the risk for agro-workers while doing FPN spray in the field.
Hematological Effects
Statistically significant differences in CBC parameters were observed in FPN spray exposed monkeys. A considerable decrease in RBC, haemoglobin, and haematocrit were observed in treated animals as compared to the control. Pre- and post-comparison of RBCs count revealed a significant difference of RBCs count (p = 0.0139) in FPN exposed animals while no difference in RBC count was shown in all control group animals. Intergroup comparison shows a gradual decrease in cell count from first sampling to last one. Similarly, a significant decrease in Hb content in FPN treated animals (p = 0.0139) has been observed, however, there was no difference in all control group animals (Table 1 and Table 2).
 |
Table 1 Effect of Fipronil Exposure on the Serum RBC in Treated Monkeys |
 |
Table 2 Effect of Fipronil Exposure on the Serum RBC in Treated Monkeys (Mean±SEM) |
White Blood Cell (WBC) Count
Contrast to RBCs, WBCs count was high in all treated animals. A comparison of the treated and control group animals shows a significant difference (p = 0.00642). Furthermore, an intergroup comparison shows a gradual increase in cell counts from first sampling to the last one (Figure 5). These findings show that FPN exposure may lead to activation of inflammatory and immune system mechanisms in the body which indicates the presence of an internal infection.
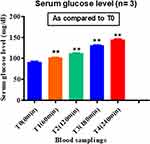
Serum Glucose Level
There was a significant increase in serum glucose level in the FPN treated monkeys (p = 0.00134), with itgoing up to 160 mg/dL. However, we observed no change in serum glucose level of the control group animals. An intergroup comparison shows a gradual increase in glucose level from first sampling to last (Figure 6). These readings clearly show the stress level of treated animals and provide insight to the mechanism of FPN action in inducing toxicity in target animals.
Evaluation of Qualitative Data
In the physical observations, no mortality was observed in the experimental animals exposed to the whole body FPN field spray in the inhalation chamber. However, the toxicological and clinical signs in the FPN treated group include urination and defecation within 5 minutes of removal from the inhalation chamber, drowsiness and restlessness were observed. Treated animals showed severe agitation and seizures, standing and falling with head downward in the cage. We also observed a change in activity and an abnormal walk among the experimental animals on the second day of the experiment.
Discussion
Through data mining, it has been well evaluated that FPN exposure may cause sub-lethal damage to non-target animals. The toxicological profile of FPN is well established in invertebrates and lower vertebrates, however, its mode of action and damage to the living body in higher animals has not been reported. To the best of our knowledge the present study is the first time that the toxicological damage associated with FPN exposure amongst higher animals has been explored.
The liver is an important organ in toxicological studies. It has the role to accumulate, biotransform and detoxify toxins, so hepatocytes are the first targets of toxic compounds in the human body.22,23 The clinical outcome of the present study indicates that field spray concentrations of FPN can cause liver damage. There is a significant increase in serum liver biomarkers, AST and ALT enzymes, along with the increased serum creatinine. These enzymes are responsible for metabolism, biosynthesis of macromolecules for different essential functions, and, most importantly, these enzymes detoxify many different toxins.23–25 So, they are used as specific indicators for liver damage.26 Research suggests that an increase in the concentration of these enzymes may be due to liver dysfunction and disturbance in the biosynthesis of these enzymes with noticeable changes in the permeability of the liver membrane.27
An elevated creatinine level in the serum of treated monkeys was also noted that shows an impaired kidney function.28,29 Our results confirmed the findings of previous studies on chronic FPN exposure in rats, which affected the thyroid, liver and kidney.30,31 Serum biochemical and other hematological parameters are used as biomarkers to evaluate the toxic stress, internal homeostasis, and functional status of the living organisms.32,33 In fishes, exposure to pesticides leads to a decrease in hematological indices, which is an indication that anemia might be due to chemosynthesis, osmoregulatory dysfunction or an increase in erythrocyte destruction in the hematopoietic organs.34,35 In the present study, there was a decrease in Hb and RBC count of adult monkeys upon exposure to the field spray concentration of FPN in an inhalation chamber. Such results indicate definitive anemia. These results show a similarity with already published literature on Nile tilapia exposed to sub lethal concentrations of FPN.36 We observed the significant increase in the WBC count in all FPN treated monkeys. The increase in WBC count in treated monkeys, reflects the hypersensitivity of WBC to FPN exposure.37 An increase in WBC count was also reported in Cyprinus carpio fry.38 A leukocyte increase might be due to the immunological response by the tissues.
Furthermore, in this pesticide exposure-response, we observed a significant increase in blood glucose level in FPN treated monkeys. Glucose is one of the most susceptible indicators of stress. A high blood glucose level is an indication that the animal is in a stressed state and using its storage glycogen in liver and muscles.39 Stress hormones, like corticosterone, trigger the glycogenolysis and gluconeogenesis that cause an increase in glucose level of an animal under stress.40 Similar to our study, an increase in serum glucose level has already documented in fish under stress conditions.41 The increase in glucose level in the rhesus monkeys is an indication of induced toxicity to the animals and stress.
In our experiment we observed physiological parameters like, PO2, RR, HB, Hb, BP, and RT. There was a significant difference in the pre- and post-exposure measurements. PO2 measurement reflects the Hb content and oxygen supply to the tissues of the body. Our findings on the difference in pre- and post-values of RR, PO2, and HB support the decrease in both RBC and Hb, indicating the anemic condition of the treated monkeys. There was a noticeable decrease in rectal temperature of the treated animals.
Limitations of the Study
The present study has assessed the toxicological effect of FPN in higher animals, in this case rhesus monkeys, and provided an insight for the risk evaluation of FPN exposure to care-free agro-workers while they are spraying pesticides in the field. However, these experiments provide only an initial evaluation toward FPN toxicity. The study does not include tissue and cell level validation. Therefore, it is important to look further, with in-depth molecular exploration of FPN at the tissue level and its action on other bodily systems, eg, the central nervous system.
Conclusion
In conclusion, the present study demonstrates that inhalation of field spray concentration of FPN is very toxic and imposed serious clinical problems on the physiology of the treated animals (rhesus monkeys). It damaged the liver and kidney of the rhesus monkeys. As monkeys are beings used as physiological human surrogates, so the findings highlight the risk of FPN toxicity in intentionally unprotected agro-workers during spraying in the field. The study has raised special concerns to promote community education about the right and safe use of these pesticides. We encourage further studies to investigate the harmful effects of FPN on the environment and its collateral damage to living organisms.
Abbreviations
FPN, fipronil; EDTAA, ethylenediamine tetraacetic acid; ATL, alanine transaminase; AST, aspartate aminotransferase; PO2, partial pressure of oxygen; RR, respiration rate; HB, heartbeat; Hb, hemoglobin; BP, blood pressure; RT, rectal temperature; RBC, red blood cell; WBC, white blood cells; CBC, complete blood count.
Acknowledgments
The authors are thankful to the National Veterinary Laboratory, Peshawar for their valued cooperation and Ponch University Azad, Kashmir and Henan University, China for the collaboration and funding in this project. The authors would like to pay countless thanks to Abdul Aziz, Abdul Basit, Syed Aftab Hussain Shah and Bilal Hussain, and Mr. Manzoor (Attendant Animal House) for their help in animal habituation and project administration.
Funding
This work was supported by the mutual funding grants from the Higher Education Commission of Pakistan and Quaid-I-Azam University, Islamabad and the Postgraduate Education Reform and Quality Improvement of Henan Province, China (No. YJS2022KC30, to E.J.).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zaller JG, Zaller JG. What is the Problem? Pesticides in Our Everyday Life. Daily Poison: Pesticides-an Underestimated Danger; 2020:1–125.
2. Horrigan L, Lawrence RS, Walker P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ Health Perspect. 2002;110(5):445–456. doi:10.1289/ehp.02110445
3. García-García CR, Parrón T, Requena M, et al. Occupational pesticide exposure and adverse health effects at the clinical, hematological and biochemical level. Life Sci. 2016;145:274–283. doi:10.1016/j.lfs.2015.10.013
4. Rohlman DS, Ismail A, Bonner MR, et al. Occupational pesticide exposure and symptoms of attention deficit hyperactivity disorder in adolescent pesticide applicators in Egypt. Neurotoxicology. 2019;74:1–6. doi:10.1016/j.neuro.2019.05.002
5. Roberts DM, Aaron CK. Management of acute organophosphorus pesticide poisoning. BMJ. 2007;334(7594):629–634. doi:10.1136/bmj.39134.566979.BE
6. Thundiyil JG, Stober J, Besbelli N, Pronczuk J. Acute pesticide poisoning: a proposed classification tool. Bull World Health Organ. 2008;86:205–209. doi:10.2471/BLT.08.041814
7. Weis JS, Smith G, Zhou T, Santiago-Bass C, Weis P. Effects of contaminants on behavior: biochemical mechanisms and ecological consequences: killifish from a contaminated site are slow to capture prey and escape predators; altered neurotransmitters and thyroid may be responsible for this behavior, which may produce population changes in the fish and their major prey, the grass shrimp. Bioscience. 2001;51(3):209–217.
8. Hayes T, Falso P, Gallipeau S, et al. The cause of global amphibian declines: a developmental endocrinologist’s perspective. J Exp Biol. 2010;213(6):921–933. doi:10.1242/jeb.040865
9. Tuomainen U, Candolin U. Behavioural responses to human‐induced environmental change. Biol Rev. 2011;86(3):640–657. doi:10.1111/j.1469-185X.2010.00164.x
10. Jansen M, Stoks R, Coors A, et al. Collateral damage: rapid exposure‐induced evolution of pesticide resistance leads to increased susceptibility to parasites. Evolution. 2011;65(9):2681–2691. doi:10.1111/j.1558-5646.2011.01331.x
11. Khan M, Damalas CA. Factors preventing the adoption of alternatives to chemical pest control among Pakistani cotton farmers. Int J Pest Manag. 2015;61(1):9–16. doi:10.1080/09670874.2014.984257
12. Zhao X, Yeh JZ, Salgado VL, et al. Sulfone metabolite of fipronil blocks γ-aminobutyric acid-and glutamate-activated chloride channels in mammalian and insect neurons. J Pharmacol Exp Ther. 2005;314(1):363–373. doi:10.1124/jpet.104.077891
13. Wright I. Fipronil: a microcosm of flea control? UK Vet Companion Anim. 2013;18(4):139–141. doi:10.12968/coan.2013.18.4.139
14. Gunasekara AS, Truong T, Goh KS, et al. Environmental fate and toxicology of fipronil. J Pestic Sci. 2007;32(3):189–199. doi:10.1584/jpestics.R07-02
15. Brennan AA, Harwood AD, You J, et al. Degradation of fipronil in anaerobic sediments and the effect on porewater concentrations. Chemosphere. 2009;77(1):22–28. doi:10.1016/j.chemosphere.2009.06.019
16. Tian Y, Zhang X, Huang Y, et al. Amphiphilic prodrug nano-micelles of fipronil coupled with natural carboxylic acids for improving physicochemical properties and reducing the toxicities to aquatic organisms. Chem Eng J. 2022;439:135717. doi:10.1016/j.cej.2022.135717
17. Gibbons D, Morrissey C, Mineau P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ Sci Pollut Res. 2015;22(1):103–118. doi:10.1007/s11356-014-3180-5
18. Qu H, Ma R-X, Liu D-H, et al. The toxicity, bioaccumulation, elimination, conversion of the enantiomers of fipronil in Anodonta woodiana. J Hazard Mater. 2016;312:169–174. doi:10.1016/j.jhazmat.2016.03.063
19. Qureshi IZ, Bibi A, Shahid S, et al. Exposure to sub-acute doses of fipronil and buprofezin in combination or alone induces biochemical, hematological, histopathological and genotoxic damage in common carp (Cyprinus carpio L.). Aquat Toxicol. 2016;179:103–114. doi:10.1016/j.aquatox.2016.08.012
20. Martonen T, Katz I, Musante C. A nonhuman primate aerosol deposition model for toxicological and pharmaceutical studies. Inhal Toxicol. 2001;13(4):307–356. doi:10.1080/089583701750127412
21. Jennings M, Prescott MJ, Refinement JWGO. Refinements in husbandry, care and common procedures for non-human primates: ninth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab Anim. 2009;43(1_suppl):1–47. doi:10.1258/la.2008.007143
22. Yang Y, Shi Y, Chen D, et al. Bisphenol A and its analogues in paired urine and house dust from South China and implications for children’s exposure. Chemosphere. 2022;294:133701. doi:10.1016/j.chemosphere.2022.133701
23. Gad MF, Mossa A-TH, Refaie AA, et al. Benchmark dose and the adverse effects of exposure to pendimethalin at low dose in female rats. Basic Clin Pharmacol Toxicol. 2022;130(2):301–319. doi:10.1111/bcpt.13683
24. Akter S, Shekhar HU, Akhteruzzaman S. Application of biochemical tests and machine learning techniques to diagnose and evaluate liver disease. Adv Biosci Biotechnol. 2021;12(6):154–172. doi:10.4236/abb.2021.126011
25. Kamada Y, Hashimoto R, Yamamori H, et al. Impact of plasma transaminase levels on the peripheral blood glutamate levels and memory functions in healthy subjects. BBA Clin. 2016;5:101–107. doi:10.1016/j.bbacli.2016.02.004
26. Lala V, Goyal A, Minter DA. Liver function tests. In: StatPearls [Internet]. StatPearls Publishing; 2021.
27. McGill MR. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016;15:817. doi:10.17179/excli2016-800
28. Lameire N, Hoste E. Reflections on the definition, classification, and diagnostic evaluation of acute renal failure. Curr Opin Crit Care. 2004;10(6):468–475. doi:10.1097/01.ccx.0000144939.24897.71
29. Luan D, Liu A, Wang X, et al. Robust two-stage location allocation for emergency temporary blood supply in postdisaster. Discrete Dyn Nat Soc. 2022;2022:1–20. doi:10.1155/2022/6184170
30. Mossa A-TH, Swelam ES, Mohafrash SM. Sub-chronic exposure to fipronil induced oxidative stress, biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol Rep. 2015;2:775–784. doi:10.1016/j.toxrep.2015.02.009
31. Ardeshir RA, Zolgharnein H, Movahedinia A, et al. Comparison of waterborne and intraperitoneal exposure to fipronil in the Caspian white fish (Rutilus frisii) on acute toxicity and histopathology. Toxicol Rep. 2017;4:348–357. doi:10.1016/j.toxrep.2017.06.010
32. Fazio F. Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture. 2019;500:237–242. doi:10.1016/j.aquaculture.2018.10.030
33. SuiX Z. RSL3drivesferroptosisthrough GPX4inactivationandROSproductionincolorectalcancer. FrontPharmacol. 2018;9:1371.
34. Seth N, Saxena K. Hematological responses in a freshwater fish Channa punctatus due to fenvalerate. Bull Environ Contam Toxicol. 2003;71:1192–1199. doi:10.1007/s00128-003-8732-1
35. Saravanan M, Kumar KP, Ramesh M. Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: cypriniformes) during acute and chronic sublethal exposure to lindane. Pestic Biochem Physiol. 2011;100(3):206–211. doi:10.1016/j.pestbp.2011.04.002
36. El-Murr A, Hakim Y TSI, Ghonimi WA, et al. Histopathological, immunological, hematological and biochemical effects of fipronil on Nile tilapia (Oreochromis niloticus). J Vet Sci Technol. 2015;6(5):2–9. doi:10.4172/2157-7579.1000252
37. Ali F, Khan MQ, Anjum MZ, Khattak I. Toxic effect of atrazine herbicide on the hematological indices of snow carp (Schizothorax plagiostomus): an indigenous fish species of economic importance. Fresenius Environ Bull. 2018;27:3075–3080.
38. Gupta SK, Pal AK, Sahu NP, et al. Dietary microbial levan ameliorates stress and augments immunity in Cyprinus carpio fry (Linnaeus, 1758) exposed to sublethal toxicity of fipronil. Aquac Res. 2014;45(5):893–906. doi:10.1111/are.12030
39. Saravanan M, Devi KU, Malarvizhi A, et al. Effects of Ibuprofen on hematological, biochemical and enzymological parameters of blood in an Indian major carp, Cirrhinus mrigala. Environ Toxicol Pharmacol. 2012;34(1):14–22. doi:10.1016/j.etap.2012.02.005
40. Davis KB, McEntire M. Comparison of the cortisol and glucose stress response to acute confinement among white bass, Morone chrysops, striped bass, Morone saxatilis and sunshine bass, Morone chrysops x Morone saxatilis; 2009.
41. Fırat Ö, Cogun HY, Yüzereroğlu TA, et al. A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol Biochem. 2011;37(3):657–666. doi:10.1007/s10695-011-9466-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.