Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Effect of Concomitant Benzodiazepine Use on Efficacy and Safety of Esketamine Nasal Spray in Patients with Major Depressive Disorder and Acute Suicidal Ideation or Behavior: Pooled Randomized, Controlled Trials
Authors Diekamp B, Borentain S , Fu DJ , Murray R, Heerlein K, Zhang Q, Schüle C, Mathews M
Received 15 April 2021
Accepted for publication 18 June 2021
Published 15 July 2021 Volume 2021:17 Pages 2347—2357
DOI https://doi.org/10.2147/NDT.S314874
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Bettina Diekamp,1 Stephane Borentain,2 Dong-Jing Fu,3 Robert Murray,4 Kristin Heerlein,1 Qiaoyi Zhang,5 Cornelius Schüle,6 Maju Mathews2
1Department of Medical and Scientific Affairs, Janssen-Cilag GmbH, Neuss, Germany; 2Department of Global Medical Affairs, Janssen Research & Development LLC, Titusville, NJ, USA; 3Department of Neuroscience Clinical Development, Janssen Research & Development LLC, Titusville, NJ, USA; 4Neuroscience Clinical Biostatistics, Janssen Research & Development LLC, Titusville, NJ, USA; 5Global Market Access, Neuroscience, Janssen Global Services, LLC, Titusville, NJ, USA; 6Ludwig-Maximilians-University Munich, Clinic for Psychiatry and Psychotherapy, Munich, Germany
Correspondence: Bettina Diekamp
Department of Medical and Scientific Affairs, Janssen-Cilag GmbH, Johnson & Johnson Platz 1, Neuss, 41470, Germany
Tel +49-21379556179
Email [email protected]
Purpose: The impact of benzodiazepines on the efficacy and safety of esketamine as a rapid-acting antidepressant remains unclear.
Materials and Methods: Data from two identically designed, randomized double-blind studies were pooled and analyzed on a post-hoc basis. In both studies, adults with major depressive disorder with acute suicidal ideation or behavior were randomized to placebo or esketamine 84 mg nasal spray twice-weekly for 4 weeks, each with comprehensive standard-of-care (initial hospitalization and newly initiated or optimized oral antidepressant[s]). Efficacy and safety were analyzed in two groups based on whether patients used concomitant benzodiazepines, which were prohibited within 8 hours before and 4 hours after the first dose of esketamine and within 8 hours of the primary efficacy assessment at 24 hours. The primary efficacy endpoint – change from baseline to 24 hours post-first dose in Montgomery-Asberg Depression Rating Scale (MADRS) total score – was analyzed using ANCOVA.
Results: Most patients (309/451, 68.5%) used concomitant benzodiazepines. Greater decrease in MADRS total score was observed with esketamine (mean [SD]: − 16.1 [11.73]) versus placebo (− 12.6 [10.56]) at 24 hours (least-squares mean difference: − 3.7, 95% CI: − 5.76, − 1.59). The differences between the esketamine and placebo groups were clinically meaningful, irrespective of benzodiazepine use (benzodiazepine: − 4.3 [− 6.63, − 1.89]; no benzodiazepine: − 3.1 [− 6.62, 0.45]). Among patients taking esketamine, change in MADRS total score was not significantly different between patients taking benzodiazepines (− 15.8 [11.27]) versus those not taking benzodiazepines (− 16.8 [12.82]) (least-squares mean difference: 1.1, [− 2.24, 4.45]). Among esketamine-treated patients, the incidence of sedation was higher with benzodiazepine use, whereas dissociation was similar.
Conclusion: Benzodiazepines do not meaningfully affect the rapid-acting antidepressant effect of esketamine at 24 hours post-first dose among patients with MDD and acute suicidal ideation or behavior.
Keywords: esketamine, benzodiazepine, depression, suicidal ideation, rapid-acting
Introduction
More than 1 in every 5 adults experience major depressive disorder (MDD) episodes over their lifetime.1,2 Almost a third of patients with MDD attempt suicide (lifetime prevalence 31% [95% confidence interval {CI} 27–34%]),3 most experiencing suicidal ideation beforehand.4 Despite the need for prompt intervention, oral antidepressants can take a month or more for optimal effect.5,6 Until recently, there was no approved pharmacotherapy for the rapid reduction of depressive symptoms in patients with MDD and acute suicidal ideation or behavior.6,7
Benzodiazepines are often prescribed by clinicians, in addition to antidepressant, for relieving acute agitation, anxiety, or insomnia in patients with depression,8 notably during an acute suicidal crisis9 and are advocated by treatment guidelines in some countries, primarily for acute treatment of these patients.6,10 However, the role of benzodiazepines in the setting of psychiatric emergency is a matter of debate,11 and concerns around abuse, addiction, and tolerance/rebound are well known.12
Esketamine (the S-enantiomer of ketamine racemate), a first-in-class glutamatergic N-methyl-D-aspartate receptor antagonist, was approved in 2019 for treatment-resistant depression (TRD). More recently, esketamine was approved in the United States for the treatment of depressive symptoms in adults with MDD with acute suicidal ideation or behavior,13 and in the European Union, co-administered with oral antidepressant therapy, in adults with a moderate-to-severe episode of MDD, as acute short-term treatment, for the rapid reduction of depressive symptoms, which according to clinical judgement constitute a psychiatric emergency.14 These most recent approvals for esketamine nasal spray were primarily based on efficacy and safety findings from two identically designed, Phase 3 global studies (ASPIRE I and ASPIRE II).15,16 In these studies, the esketamine plus standard-of-care group demonstrated rapid improvement of depressive symptoms over the placebo plus standard-of-care group as early as 4 hours and 24 hours after the first dose.
Benzodiazepines have been reported to attenuate ketamine’s antidepressant response in a case report17 and small, single-center clinical studies of patients with TRD.18,19 Although consensus on the mechanism for the interaction is lacking, Albott et al19 suggested that benzodiazepine agonism of the γ-aminobutyric acid (GABA) A receptor decreases ketamine’s excitatory glutaminergic signal transduction, thereby attenuating its therapeutic effect. We conducted a post hoc analysis of pooled data from the ASPIRE I and ASPIRE II studies in patients with MDD and acute suicidal ideation or behavior15,16 to examine the potential effects of benzodiazepines on the safety and rapid efficacy of esketamine nasal spray following first-dose treatment in these patients who were experiencing a psychiatric emergency and in urgent need of symptom control. Specifically, the aims of the analyses were to: compare the efficacy of esketamine to that of placebo in patients with or without concomitant benzodiazepines; evaluate the impact of benzodiazepines on the rapid antidepressant effect of esketamine; and, determine the effects of benzodiazepines on the safety profile of esketamine.
Materials and Methods
Institutional review boards/ethics committees (names provided in Table S1) approved the ASPIRE I and ASPIRE II protocols and their amendments, written consent was obtained from all patients before they participated in the studies, both studies were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, and both studies are registered at clinicaltrials.gov (identifiers: NCT03039192 [registered 31 January 2017] and NCT03097133 [registered 27 March 2017]). The study methods pertaining to the work reported here are summarized below.
ASPIRE I and ASPIRE II were identically designed, double-blind, randomized, placebo-controlled phase 3 studies conducted between June 2017 and April 2019 in North America, Europe (both studies), Asia (ASPIRE I only), and South America (ASPIRE II only). The ASPIRE studies enrolled adults (18–64 years) with a diagnosis of MDD without psychotic features, according to Diagnostic and Statistical Manual of Mental Disorders 5th edition20 (DSM-5) and confirmed by the Mini-International Neuropsychiatric Interview (MINI).21 Potential study participants were screened soon after arriving at an emergency department or being admitted to an inpatient psychiatric ward, where they were to stay for 5 days (14 days in several European Union countries) after entering the studies. To be eligible, patients must have had a current suicidal ideation with intent, confirmed by affirmative responses to MINI questions B3 (Think about suicide [killing yourself]?) and B10 (Intend to act on thoughts of killing yourself in the past 24 hours?) within 24 hours of randomization, been in clinical need of acute psychiatric hospitalization due to imminent suicide risk, and had a Montgomery-Asberg Depression Rating Scale22 (MADRS) total score >28 prior to the first dose of the study drug on day 1. Patients must have voluntarily agreed to comprehensive standard-of-care treatment, including the initial hospitalization. Key exclusion criteria were current or prior DSM-5 diagnosis of a psychotic disorder or MDD with psychotic features, current DSM-5 diagnosis of bipolar disorder, obsessive-compulsive disorder, antisocial personality disorder, or borderline personality disorder, and DSM-5 diagnosis of moderate-to-severe substance or alcohol use disorder in the recent 6–12 months prior to screening. Full lists of the inclusion and exclusion criteria for each study are published.15,16
Patients meeting eligibility criteria were randomized to 84 mg esketamine nasal spray or matching placebo nasal spray. Patients self-administered the study drug twice-weekly for 4 weeks, beginning on day 1, with dosing supervised by medical staff at the study centers. In addition, patients received standard-of-care oral antidepressant(s) – either monotherapy or antidepressant plus augmenting agents (ie, second antidepressant, an atypical antipsychotic, or a mood stabilizer) – determined by the investigator and initiated or optimized at the time they were randomized.
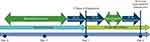
Benzodiazepines (dosage equivalent to lorazepam ≤6 mg/day) were allowed during the study, as long as they were not taken during the 8 hours preceding each intranasal study drug administration, the 4 hours following the first intranasal study drug dosing, and within 8 hours of day 2 assessments (Figure 1). Prohibition of benzodiazepines during these hours before and after dosing and before day 2 assessments was driven by potential confounding due to benzodiazepine-related sedation on efficacy and safety assessments of esketamine.
Investigators assessed depressive symptom severity using MADRS, before and 4 and 24 hours after the first dose of intranasal esketamine or intranasal placebo (days 1–2), pre-dose at all successive visits during double-blind treatment, and 4 hours post-dose on day 25. A difference between treatment groups of 2 points or greater for change in total MADRS score was considered clinically meaningful.23,24
Adverse events were monitored throughout the study. Dissociative symptoms were assessed pre-dose and 40 minutes and 1.5 hours post-dose on dosing days using the Clinician Administered Dissociative States Scale (CADSS).25 Sedation/alertness was assessed every 15 minutes from predose to 1.5 hours postdose on dosing days using Modified Observer’s Assessment of Alertness/Sedation scale (MOAA/S).26
Statistical Analyses
Efficacy and safety were each analyzed by treatment arm and by benzodiazepine use (yes/no) in pooled data from the ASPIRE I and ASPIRE II studies. Point estimates of the treatment differences (either difference in means or proportions) and 95% CIs are reported. Of note, 95% CIs that do not include zero for differences in means and proportions correspond to a two-sided P-value that is less than 0.05 (ie, statistical significance). CIs were presented rather than P-values as they provide information on the direction and magnitude of the effect. As this was a post-hoc analysis, no adjustments for multiple comparisons were made.
For efficacy assessments, which were performed on day 2 (24 hours post-first dose), benzodiazepine users included patients who took a benzodiazepine(s) (standing or as-needed dosing) between day −2 and day 2. For safety assessments, including MOAA/S and CADSS that were assessed up to 1.5 hours post-dose, benzodiazepine users included patients who took a benzodiazepine(s) on day −2 and/or day −1 (before dosing of the study drug began).
Demographic and baseline characteristics were summarized by benzodiazepine use.
The primary efficacy endpoint – change in MADRS total score from baseline (day 1, predose) to 24 hours post-first dose (day 2) – was analyzed on last observation carried forward (LOCF) data using analysis of covariance (ANCOVA) with study number, treatment, analysis center within study number, standard-of-care antidepressant (as randomized), benzodiazepine group, and treatment-by-benzodiazepine group as factors, and baseline MADRS total score as a continuous covariate. A sensitivity analysis on the primary efficacy endpoint, based on the timing of benzodiazepine use (ie, between day −2 and day −1 and between day 1 and day 2) was conducted using ANCOVA.
In other analyses, frequency distributions were provided by the treatment and benzodiazepine (yes/no) group for responders to treatment (defined as ≥50% decrease from the baseline MADRS total score) and patients in remission of depressive symptoms (defined as MADRS ≤12) at day 2, for adverse events with onset within 24 hours of treatment, and for CADSS total score and MOAA/S total score at day 1.
Results
In the ASPIRE studies, 456 patients were randomized to study drug, 229 to esketamine plus standard-of-care and 227 to placebo plus standard-of-care. Four patients (2 in each treatment group) were excluded from analyses of efficacy and safety because they did not receive a dose of intranasal study drug. One additional patient (in the esketamine group) was excluded from analyses of efficacy due to the absence of post-baseline MADRS assessments.
Benzodiazepine use was common during the period evaluated (ie, day −2 to day 2) (309/451, 68.5% of patients). Patients who did and those who did not receive benzodiazepines were generally similar with respect to demographic and baseline clinical characteristics, including depression severity (MADRS score) (Table 1).
 |
Table 1 Demographic and Baseline Characteristics by Benzodiazepine Use in ASPIRE Studies (Safety Analysis Set) |
Mean MADRS total score decreased from baseline to 24 hours post-first dose, with greater improvement in depressive symptoms among those treated with esketamine plus standard-of-care as compared to placebo plus standard-of-care in the overall population (mean [SD]: esketamine −16.1 [11.73]) versus placebo −12.6 [10.56]) at 24 hours; least-squares [LS] mean difference: −3.7, 95% CI: −5.76, −1.59). Clinically meaningful improvement in depressive symptoms was observed with esketamine versus placebo in the subgroups of patients with or without benzodiazepine use (Figure 2). The difference of LS means (95% CI) was −4.3 (−6.63, −1.89) for the patients taking benzodiazepines and −3.1 (−6.62, 0.45) for the patients who did not take benzodiazepines. Among esketamine-treated patients, no statistically significant difference was observed in the reduction of MADRS total score between patients taking benzodiazepines (mean [SD]: −15.8 [11.27]) versus those not taking benzodiazepines (−16.8 [12.82]) (difference of LS means [95% CI], 1.1 [−2.24, 4.45]). Sensitivity analyses on change in MADRS score from baseline to 24 hours post-first dose revealed similar results when timing of benzodiazepine use was defined by the period only before esketamine dose, and also by the period only after esketamine dose (Table S2).
On day 2, the proportion of patients who were responders and the proportion of patients in remission were numerically higher among those treated with esketamine plus standard-of-care as compared to placebo plus standard-of-care, with little influence by concomitant benzodiazepine use (Figure 3).
In terms of safety, dissociation was reported as a treatment-emergent (ie, within 24 hours of study drug administration) adverse event for 29.5% (67/227) of esketamine-treated patients and 3.6% (8/225) of placebo-treated patients, with incidence similar between those who did and did not take concomitant benzodiazepines within each treatment group (Table 2). In line with these findings, mean total CADSS score at 40 minutes following the first dose was increased from predose, substantially more so among the esketamine- versus the placebo-treated patients, but, within the esketamine group, similarly among those taking a benzodiazepine compared to those not taking a concomitant benzodiazepine. Mean total CADSS total score returned toward the predose level by 1.5 hours post-dose in all analysis groups.
 |
Table 2 Treatment-Emergent Dissociation and Sedation by Benzodiazepine Use in ASPIRE Studies (Safety Analysis Set) |
More patients in the esketamine plus standard-of-care group (17/227 [7.5%]) had a MOAA/S score ≤3 (indicating moderate or greater sedation) on day 1, versus patients in the placebo plus standard-of-care group (2/225 [0.9%]). None of these patients required medical intervention. The incidence of sedation, based on adverse event reporting, was higher among esketamine-treated patients who had taken concomitant benzodiazepines compared to those who had not, and compared to placebo-treated patients, regardless of concomitant benzodiazepine usage (Table 2).
Most adverse events of dissociation were mild or moderate (esketamine 92.0%, placebo 100%) and had onset within one hour of study drug administration (94.3% and 90.9% of events in the respective treatment groups). All events of sedation were mild or moderate and had onset within one hour of study drug administration.
Discussion
Patients with MDD having acute suicidal ideation or behavior are in a psychiatric emergency. During this psychiatric emergency, clinicians prescribe benzodiazepines for rapid relief of acute agitation, anxiety, or insomnia, which accompanies depression in many patients.6,10,27,28 We observed high usage of benzodiazepines (69% of study patients) at the onset of the ASPIRE studies (day −2 to day 2), within the constraints of the protocol-specified time frame.
Among esketamine-treated patients, improvement in depressive symptoms was similar, regardless of benzodiazepine use. Concomitant treatment with benzodiazepines (ie, taken between day −2 and day 2) did not augment or diminish the rapid, robust antidepressant effect observed after the first dose of esketamine in these patients (primary efficacy endpoint), which is specifically relevant in the setting of psychiatric emergencies.
The findings from the ASPIRE studies do not support prior studies in which benzodiazepines were shown to attenuate the acute antidepressant effect of ketamine (Ford et al17 [case study, n=1]; Frye et al18 [post-hoc analysis, n=10]; Albott et al19 [post-hoc analysis, n=14]; Andrashko et al29 [n=47, excluded patients with suicidal risk assessed by clinical examination]). The disparity may be explained, at least in part, by the small sample size and uncontrolled methods of the ketamine studies and, perhaps, that the ketamine studies included patients who had TRD but were not in acute crisis. In a post-hoc analysis of 10 patients with TRD given ketamine infusions twice weekly for up to 2 weeks, Frye et al18 reported that non-responders received significantly higher mean daily doses of benzodiazepines than responders (≥50% decrease from baseline MADRS total score). Likewise, in a study of 47 patients with MDD and without suicidal risk who received a single ketamine infusion as an add-on to ongoing antidepressant treatment, Andrashko et al29 reported a significantly lower response rate among those taking high-dose benzodiazepines (defined as >8 mg diazepam equivalent, n=13). And, in a post hoc analysis of 13 patients with TRD who received a ketamine infusion 3 times weekly for 2 weeks, Albott et al19 reported no significant differences between benzodiazepine and non-benzodiazepine users in response or remission rates, or depression relapse rate over 4-week follow-up, but those taking benzodiazepine took significantly longer time to reach these outcomes and experienced significantly shorter therapeutic effect.18
The incidences of esketamine-associated dissociation and sedation are consistent with expectations from TRD studies.13,14 Most events of dissociation and sedation were mild or moderate and began within one hour of study drug administration. Dissociation, based on adverse event reporting and post-dose mean total CADSS score, was more common with esketamine compared to placebo, and was not influenced by benzodiazepine use prior to esketamine dosing. Sedation, based on adverse event reporting and MOAA/S score ≤3, was also more common with esketamine compared to placebo, especially so among those who had taken benzodiazepines prior to esketamine dosing. This result was expected given the well-known sedative effects of benzodiazepines.
The most important observation of the study is that the rapid antidepressant effect of esketamine nasal spray is not attenuated or augmented by the concomitant administration of benzodiazepines in patients with MDD and acute suicidal ideation or behavior. While clinicians often prescribe benzodiazepines in combination with conventional antidepressants for the initial treatment of anxiety, due to latency of efficacy of these antidepressants, this may not be necessary even in the initial phase of treatment with esketamine because of its rapid effect. In addition, the use of benzodiazepines during treatment with esketamine may lead to an increase in adverse events, particularly sedation, and may not reduce the incidence of dissociative effects at the beginning of esketamine treatment.
Limitations
Our results are limited by analyses being conducted on a post hoc basis. Furthermore, the ASPIRE studies did not allow concomitant benzodiazepine use within 8 hours of esketamine dosing and within 8 hours of primary efficacy (day 2) assessment; thus, we could not examine the potential effect of benzodiazepines on the efficacy or tolerability of esketamine if they were used together. And, the analyses reported herein are confined to effects following the first dose of esketamine, informing on the effects of benzodiazepine treatment in the context of the need for urgent control of symptoms; the impact of chronic benzodiazepine use on the long-term efficacy/safety of esketamine was not explored.
Conclusions
The findings from this analysis support the efficacy and safety of esketamine nasal spray for MDD patients with acute suicidal ideation or behavior, irrespective of benzodiazepine use. Specifically, benzodiazepines had minimal impact on the rapid antidepressant efficacy of esketamine, but increased the occurrence of sedation. Therefore, the use of benzodiazepines for patients being treated with esketamine for psychiatric crisis may not be warranted. Further investigation is needed to confirm and expand upon these findings.
Abbreviations
ANCOVA, analysis of covariance; CADSS, Clinician Administered Dissociative States Scale; CGI-SS-r, Clinical Global Impression–Severity revised version; CI, confidence interval; DSM-5, Diagnostic and Statistical Manual of Mental Disorders 5th edition; LOCF, last observation carried forward; LS, least-squares; MADRS, Montgomery Åsberg Depression Rating Scale; MDD, major depressive disorder; MINI, Mini-International Neuropsychiatric Interview; MOAA/S, Modified Observer’s Assessment of Alertness/Sedation scale; SD, standard deviation; SE, standard error; TRD, treatment-resistant depression.
Acknowledgments
We acknowledge Sandra Norris, PharmD of the Norris Communications Group LLC for medical writing assistance and Ellen Baum, PhD (Janssen Global Services, LLC) for additional editorial support.
The abstract of this paper was presented at the European College of Neuropsychopharmacology (ECNP) Congress 2020 as a poster. The posters abstract was published in the Supplement 33rd ECNP Congress – Posters in European Neuropsychopharmacology (2020) 40, S420-421: https://www.sciencedirect.com/science/article/pii/S0924977X20308191/pdfft?md5=f447f43d1745ae592464b62756aa3fda&pid=1-s2.0-S0924977X20308191-main.pdf. It was also presented at the Psych Congress 2020: https://www.psychiatryadvisor.com/home/conference-highlights/psych-congress-2020-coverage/effect-of-benzodiazepines-on-esketamine-nasal-spray-in-patients-with-major-depressive-disorder-and-suicidal-ideation/) and at the German Association for Psychiatry, Psychotherapy and Psychosomatics Congress (DGPPN) Congress 2020, Berlin.
Author Contributions
Robert Murray conducted the statistical analyses. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors meet ICMJE criteria and all those who fulfilled those criteria are listed as authors.
Funding
The ASPIRE I and ASPIRE II studies were funded by Janssen Research & Development, LLC, Titusville, NJ, USA. Employees of the Sponsor, as noted in Author Contributions, were involved in data analysis or interpretation and/or other aspects pertinent to the studies. Authors had full access to all of the data in the study, were involved in writing and/or revising the manuscript, and had final responsibility for the decision to submit for publication.
Disclosure
Drs. Bettina Diekamp, Stephane Borentain, Dong-Jing Fu, Kristin Heerlein, Qiaoyi Zhang, Maju Mathews, and Mr. Robert Murray are employees of Janssen-Cilag GmbH or Janssen Research & Development, LLC and some hold company equity. Dr. med. Cornelius Schüle has served as a member of advisory boards for Janssen-Cilag GmbH and Janssen Pharmaceutica NV. The authors report no other conflicts of interest in this work.
References
1. Vandeleur CL, Fassassi S, Castelao E, et al. Prevalence and correlates of DSM-5 major depressive and related disorders in the community. Psychiatry Res. 2017;250:50–58. doi:10.1016/j.psychres.2017.01.060
2. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. doi:10.1001/jamapsychiatry.2017.4602
3. Dong M, Zeng LN, Lu L, et al. Prevalence of suicide attempt in individuals with major depressive disorder: a meta-analysis of observational surveys. Psychol Med. 2019;49(10):1691–1704. doi:10.1017/S0033291718002301
4. Sokero TP, Melartin TK, Rytsälä HJ, Leskelä US, Lestelä-Mielonen PS, Isometsä ET. Suicidal ideation and attempts among psychiatric patients with major depressive disorder. J Clin Psychiatry. 2003;64(9):1094–1100. doi:10.4088/JCP.v64n0916
5. Simon GE, Savarino J. Suicide attempts among patients starting depression treatment with medications or psychotherapy. Am J Psychiatry. 2007;164(7):1029–1034. doi:10.1176/ajp.2007.164.7.1029
6. Wasserman D, Rihmer Z, Rujescu D, et al. European Psychiatric Association. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur Psychiatry. 2012;27(2):129–141. doi:10.1016/j.eurpsy.2011.06.003
7. van der Feltz-cornelis CM, Sarchiapone M, Postuvan V, et al. Best practice elements of multilevel suicide prevention strategies: a review of systematic reviews. Crisis. 2011;32(6):319–333. doi:10.1027/0227-5910/a000109
8. Bushnell GA, Stürmer T, Gaynes BN, Pate V, Miller M. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001–2014. JAMA Psychiatry. 2017;74(7):747–755. doi:10.1001/jamapsychiatry.2017.1273
9. Winkler D, Kaltenboeck A, Frey R, Kasper S, Pjrek E. Changes over time of the diagnostic and therapeutic characteristics of patients of a psychiatric intensive care unit in Austria Compr Psychiatry. Comprehensive Psychiatry. 2019;93:20–26. doi:10.1016/j.comppsych.2019.06.004
10. Davidson JR. Major depressive disorder treatment guidelines in America and Europe. Clin Psychiatry. 2010;71(SupplE1):e04. doi:10.4088/JCP.9058se1c.04gry
11. Furukawa TA, Streiner DL, Young LT. Is antidepressant-benzodiazepine combination therapy clinically more useful? A meta-analytic study. J Affect Disord. 2001;65(2):173–177. doi:10.1016/S0165-0327(00)00254-8
12. Dell’osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry. 2013;28(1):7–20. doi:10.1016/j.eurpsy.2011.11.003
13. SpravatoTM (esketamine) nasal spray Prescribing Information. ©. Janssen Pharmaceutical Companies; 2020.
14. Spravato (esketamine) Summary of Product Characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/spravato-epar-product-information_en.pdf.
15. Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191. doi:10.4088/JCP.19m13191
16. Ionescu DF, Fu DJ, Qiu X, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. 2021;24(1):22–31. doi:10.1093/ijnp/pyaa068
17. Ford N, Ludbrook G, Galletly C. Benzodiazepines may reduce the effectiveness of ketamine in the treatment of depression. Aust N Z J Psychiatry. 2015;49(12):1227. doi:10.1177/0004867415590631
18. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334–336. doi:10.1097/JCP.0000000000000316
19. Albott CS, Shiroma PR, Cullen KR, et al. The antidepressant effect of repeat dose intravenous ketamine is delayed by concurrent benzodiazepine use. J Clin Psychiatry. 2017;78(3):e308–e309. doi:10.4088/JCP.16l11277
20. American Psychiatric Association: diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Arlington, TX: American Psychiatric Publishing; 2013.
21. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):
22. Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192:52–58. doi:10.1192/bjp.bp.106.032532
23. Melander H, Salmonson T, Abadie E, van Zwieten-boot B. A regulatory Apologia--a review of placebo-controlled studies in regulatory submissions of new-generation antidepressants. Eur Neuropsychopharmacol. 2008;18(9):623–627. doi:10.1016/j.euroneuro.2008.06.003
24. Montgomery SA, Möller HJ. Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol. 2009;24(3):111–118. doi:10.1097/YIC.0b013e32832a8eb2
25. Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress. 1988;11(1):125–136. doi:10.1023/A:1024465317902
26. Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244–251.
27. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–351. doi:10.1176/appi.ajp.2007.06111868
28. Lamers F, van Oppen P, Comijs HC, et al. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands study of depression and anxiety (NESDA). J Clin Psych. 2011;72(3):341–348. doi:10.4088/JCP.10m06176blu
29. Andrashko V, Novak T, Brunovsky M, Klirova M, Sos P, Horacek J. The antidepressant effect of ketamine is dampened by concomitant benzodiazepine medication. Front Psychiatry. 2020;11:844. doi:10.3389/fpsyt.2020.00844
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.