Back to Journals » Journal of Pain Research » Volume 16
Education and Experience in Intrathecal Drug Delivery Systems (IDDS) During Pain Medicine Fellowships
Authors Mahmoud A, Aman MM , Trumbo JL, Paracha U, Langell A, Petersen E
Received 22 August 2023
Accepted for publication 18 November 2023
Published 27 December 2023 Volume 2023:16 Pages 4367—4377
DOI https://doi.org/10.2147/JPR.S428851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Andrea Tinnirello
Ammar Mahmoud,1 Mansoor M Aman,2 Jennifer L Trumbo,3 Umera Paracha,4 Ashley Langell,5 Erika Petersen6
1Department of Pain Medicine, Northern Light Eastern Maine Medical Center, Bangor, ME, USA; 2Department of Pain Medicine, Advocate Aurora Health, Oshkosh, WI, USA; 3Clinical Research Center, Northern Light Eastern Maine Medical Center, Bangor, ME, USA; 4Department of Neurology, Northern Light Eastern Maine Medical Center, Bangor, ME, USA; 5Department of Pain Medicine, Cleveland Clinic Foundation, Cleveland, OH, USA; 6Department of Neurosurgery, University of Arkansas for Medical Sciences, Little Rock, AR, USA
Correspondence: Ammar Mahmoud, Interventional Pain Management Clinic, Northern Light Eastern Maine Medical Center, 925 Union Street, Suite #2, Bangor, ME, USA, Tel +1 207-973-5918, Fax +1 207-973-8300, Email [email protected]
Objective: Targeted drug delivery (TDD) via intrathecal drug delivery systems (IDDS) exposure and clinical adoption remains low despite multiple well-designed trials that demonstrate safety, efficacy, reliability, and cost-saving benefits. This study aims to understand the possible contributing factors starting with Pain Medicine fellowship training.
Materials and Methods: An internet-based, anonymous pilot survey was distributed to pain medicine fellows enrolled in an Accreditation Council for Graduate Medical Education (ACGME) accredited pain medicine training program during the 2021– 2022 academic year. Fellowship programs were identified using published online ACGME accreditation data. The survey was distributed via email to fellowship program directors and coordinators and was made available through pain medicine societies.
Results: Seventy-one of four hundred and twenty-three pain medicine fellows (17% response rate) completed the survey. Nine percent of respondents evidence-informed opinion coincided with the most recent Polyanalgesic Consensus Conference (PACC) guidelines recommendations for IDDS treatment indications. Fifty-one percent of respondents felt there was an unmet need for IDDS training. About one-third of respondents felt that lack of curriculum, faculty, and cases were barriers to IDDS use, respectively. Thirty-one percent of fellows reported sufficient training for IDDS in their fellowship programs. The majority (70%) of respondents somewhat or strongly support direct training by IDDS manufacturers.
Conclusion: A wide variability exists surrounding IDDS training during ACGME accredited pain medicine fellowship. Insufficient case exposure and lack of a standardized curriculum may play a role in future therapy adoption. The results from this study call for a more standardized training approach with an emphasis on adequate clinical exposure, utilization of peer reviewed educational curriculum and supplemental material to aid pain medicine fellows’ education.
Keywords: intrathecal drug delivery system, targeted drug delivery, Accreditation Council for Graduate Medical Education, neuromodulation
Introduction
Targeted drug delivery (TDD) via intrathecal drug delivery systems (IDDS) is utilized in the treatment of refractory spasticity and intractable pain of cancer and non-cancer origin.1–7 IDDS consist of an intrathecal spinal catheter connected to a pump reservoir delivering prescribed medication into the subarachnoid space (Figure 1). The two current generation intrathecal pumps that are commonly utilized are the Medtronic SynchroMed TM II and the Flowonix Prometra II (Figure 2). Since their introduction, IDDS have been through several evolutionary changes allowing for more complex and eloquent medication delivery options. In addition, intrathecal medication guidelines and best practices continue to evolve based on basic and clinical research, clinical studies, and expert opinion.8–13 The Polyanalgesic Consensus Conference (PACC) guidelines help provide updated consensus statements and treatment algorithms to promote safe and efficacious evidence-based care.14
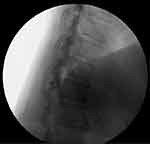 |
Figure 1 Fluoroscopic image intrathecal spinal catheter in the subarachnoid space. |
 |
Figure 2 Medtronic Syndromed II (Left) and Flowonix Prometera II (Right) IDDS. |
To keep up with these advances, surgical implantation of IDDS and intrathecal medication management are suggested training components during pain fellowship as stated by the Accreditation Council for Graduate Medical Education (ACGME).15 However, set guidelines defining the recommended extent or degree of exposure do not exist. Additionally, the current ACGME pain fellowship competency milestones do not account for the variability of practice style between those who perform intrathecal trials, permanent IDDS implant, and medically manage their patients to other practitioners who are only involved with one of these aspects of care. There are no current studies examining IDDS exposure, education, proficiencies and deficiencies at the post-graduate fellowship level.
We aimed to evaluate the current IDDS fellowship training landscape while exploring any unmet training needs using an online pilot survey sent to all post-graduate pain medicine fellows enrolled in an ACGME pain medicine training program during the 2021–2022 academic year. The goal of this study is to explore current fellow education and experience surrounding IDDS during pain medicine fellowship and its future impact on therapy adoption.
Materials and Methods
Study Design and Distribution
After Institutional Review Board exempt determination (Northern Light Eastern Maine Medical Center IRB #: 2021-082), researchers created a survey hosted by REDCap (redcap.vanderbilt.edu; supported by grant funding: UL1 TR000445 from NCATS/NIH). This 38-question questionnaire (Appendix 1) was modelled after the Pak et al survey of unmet training needs and factors to impact future practice in spinal cord stimulation.16 We utilized the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting statement checklist for this study. All post-graduate physicians in ACGME-accredited 2021–2022 pain fellowships were eligible to participate. This survey was made available through three venues in an attempt to reach the greatest proportion of fellows.
- The survey link was distributed to all ACGME pain medicine fellowship directors/coordinators, who then forwarded the email to their fellows.
- The survey was available through the virtual 2022 American Society of Pain and Neuroscience Innovation Summit. They host the largest fellow cadaver course that targets only ACGME pain medicine fellows.
- The survey was available at the virtual 2022 North American Neuromodulation Society Meeting.
Fellows were identified through program directors or coordinators who forwarded the survey via email and through a slide containing a QR code to the survey displayed at fellows-only events during the conferences listed above. In the latter case, fellows self-identified by attending those sessions.
Whether arriving to the survey page through survey link or QR code, fellows first viewed the research information sheet before choosing to continue to the survey or exit. To lessen the chance for duplicate survey responses, the researchers included a statement on the research information sheet and email to directors and coordinators that instructed fellows to complete the survey only once. The survey was offered via conferences beginning on January 8th and emailed to directors and coordinators on January 12th, 2022. One reminder email was sent to fellowship directors/coordinators two weeks after the initial send out date and the survey closed one month after initial email invitation.
The survey included questions about residency and pre-fellowship experiences, choice of fellowship program, current fellowship experience, future practice, industry involvement, and standardization of training. The survey was designed to be completely anonymous as all fellows used a universal invitation link. Participants were not linked to their responses and researchers did not collect any identifying information. Moreover, research staff did not have access to fellows’ contact information as recruitment was facilitated by directors and conference organizers as described above. As no questions were required, results presented are based on non-missing responses and the number of non-missing responses is specified for each question in tables and figures. No financial or other incentives were provided to survey respondents.
Statistical Analysis
Descriptive statistical analyses were performed on data collected following the end of the survey. Power analysis was not performed as our goal was to recruit the largest proportion of fellows possible out of the total population. Advanced statistical analyses were not warranted given the low response rate and lack of demographic data. Results are expressed as percentages out of the total number of non-missing responses per question. With a completely anonymous survey and no information available on non-respondents, we were not able to quantify potential biases in respondents. Descriptive analyses, tables, and figures were completed using SPSS V.25 (IBM, New York).
Results
Response Rate
Seventy-one fellows completed at least one question in the 38-question survey, yielding an approximate response rate of 17%. Sixty surveys were returned complete (84.5%), while eleven surveys were returned incomplete (15.5%). The total number of pain medicine fellows was four hundred and twenty-three and was obtained from ACGME pain medicine fellowship database.17 No questions were required, and incomplete surveys with individual responses were included. Only one survey was returned with a single question answered. Excluding multiple-response questions, on average, 52 fellows responded to any given question in the survey.
Pre-Fellowship Demographics and IDDS Training
Of 60 respondents, 65% had residencies in anesthesiology, while 26.7% completed residency in physical medicine and rehabilitation, and 8.3% in other fields (Table 1). The majority of respondents (64%) had hands-on, didactic, and/or industry-sponsored training in IDDS prior to the start of their fellowship. Of those who reported previous industry sponsored IDDS training, Medtronic was the predominant provider. More than 25% of respondents had experience in at least one aspect of IDDS care prior to their fellowship, including IDDS trials, surgical implant procedures, pump refills, catheter access, and anesthetic care. Forty-three percent of respondents reported that IDDS training influenced fellowship choice, while 83.3% of respondents reported considering alumni use of IDDS in current practice as an influence in fellowship choice.
 |
Table 1 Training Demographics: Pre-Fellowship |
IDDS Training During Fellowship
The majority of respondents reported participating in 5 or fewer IDDS trials, surgical implants and catheter or pump access studies (63.6%, 68.5%, 87.2%, respectively; Table 2). Notably, 59.9% of respondents reported participating in at least 6 IDDS pump refills. Out of the eight areas of proficiency addressed (Table 3), the majority of respondents felt least experienced in performing catheter access port and rotor studies of IDDS pumps independently and most proficient in performing IDDS pump refills independently (43.4% and 38.3%, respectively). Given seven potential pain conditions for which IDDS treatment is offered, only 9% of respondents selected the correct response, “all of the above” (Figure 3). Over half of respondents felt there was an unmet need for IDDS training (Figure 4). Thirty-one percent of fellows reported sufficient training for IDDS in their fellowship programs.
 |
Table 2 Training Demographics: Number of Procedures Participated in During Fellowship Training |
 |
Table 3 Training Demographics: Perceived Fellow Proficiency During Fellowship Training |
 |
Figure 3 Intrathecal therapy should be suggested for which of the following pain conditions?. |
 |
Figure 4 How do you feel about your exposure to Intrathecal Drug Delivery Systems (IDDS) during your fellowship training. |
IDDS in Future Practice
Nearly 50% of respondents plan to enter private practice (Table 4). Thirty-four percent plan to conduct IDDS trials and implantations while in practice. Forty-nine percent reported that IDDS training will have little or no influence on their future position. Twenty-six percent of respondents anticipated no IDDS implants at their future practice.
 |
Table 4 Training Demographics: Future Practice |
Standardization and Considerations for Future Training
About one-third of respondents felt that lack of curriculum, faculty, and cases were barriers to IDDS use, respectively (Figure 5). Forty-six percent of respondents reported that ACGME/fellowship directors, pain medicine societies, and other groups should be involved in standardizing IDDS training (Table 5). Twelve percent reported that they do not believe it should be standardized. The majority (70%) of respondents somewhat or strongly support direct training by IDDS manufacturers. Out of five areas of IDDS learning, tracking patient outcomes and medication refill dates was the most selected at 30%.
 |
Figure 5 Indicate whether you consider the following to have been possible barriers for Intrathecal Drug Delivery System (IDDS) therapy training during your fellowship. Select all that apply. |
 |
Table 5 Areas of Learning |
Discussion
This study represents the first attempt to understand the landscape of IDDS education and trainee experience among fellows participating in ACGME-accredited pain medicine programs. The study results demonstrate a wide variability in IDDS training practices, case exposures and competencies among fellows and highlight potential targets for curriculum development.
To assess the relevance and potential impact of IDDS education during fellowship, respondents were surveyed regarding future practice plans and impact of IDDS training. With 43% of fellows reporting plans to participate in IDDS management in some capacity in their future practices and an additional 22% giving it continued consideration at the time of this survey, it is reasonable to infer that IDDS training is a valuable and pertinent aspect of fellow education. Results from this study demonstrate highly variable mastery of IDDS management among fellowship level trainees. We aimed to assess experience across the continuum of care including identification of factors influencing a patient’s IDDS candidacy, procedural experience, as well as medication and complication management. Nine percent of respondents evidence-informed opinion coincided with the PACC guideline recommendations for IDDS treatment indications. This is a discouraging result, as the PACC guidelines represent a comprehensive and evidence-based recommendations for intrathecal therapy. The remaining experience measures were self-reported, and results revealed wide variation in fellow comfort level with IDDS procedures and management, with the highest rated experience reported to be independent performance of pump refills and the lowest reports reflecting aspects of device interrogation, medication management and complication management. These identified areas of decreased experience represent potential targets for educational development in the future.
Half of respondents felt there was an unmet need for IDDS training. Lack of curriculum, lack of faculty preceptors with adequate IDDS subject expertise, and insufficient case exposure were each identified as potential barriers to IDDS use. Reported case exposures were relatively low overall, with the vast majority of respondents reporting participation in five or fewer trials, implants, and catheter access port and rotor studies. Notably, a third of respondents reported having had no participation in IDDS trials during fellowship, 37% reported no participation in implants, and more than half reported no participation in catheter access studies. This further supports insufficient case exposure as a possible barrier to therapy adoption and raises educational concerns regarding inconsistent exposure to IDDS during training.
The ACGME program requirements for graduate medical education in pain medicine outline core competencies and describe the required domains for fellows to transition into autonomous practice. Among them is a requirement for demonstrated competence in medical knowledge of intrathecal drug administration systems and intrathecal medication management. However, minimum required IDDS case numbers have not been established for pain medicine fellows. Interestingly, in the field of neurosurgery, graduating residents of ACGME-accredited programs are required to participate in a minimum of ten supervised and five independent cases that involve either spinal pumps, stimulators, or lesions. Recommendations for minimum case numbers could be a reasonable step toward standardizing pain medicine trainees’ experiences with IDDS, however it is unclear what constitutes an adequate threshold of exposure and whether implementation of such a process is feasible in the current training construct.
A recent publication by Ambardekar et al highlighted the steps taken to revise total case minimums for ACGME pediatric anesthesiology fellowship. This was a joint effort by the Pediatric Anesthesiology Program Directors Association and Society of Pediatric Anesthesia Task Force for Pediatric Anesthesiology Graduate Medical Education. Thirty-seven faculty participated in a Delphi process using iterative rounds of surveys until a final revision of categories and minimums was achieved. The threshold for inclusion was two-thirds agreement.18 A similar society and program director collaboration could be undertaken for pain fellowships for proposing changes to the ACGME minimums as determined by key stakeholders.
Whether implementation of a more standardized approach to IDDS education would improve fellow experience and comfort with this therapy remains to be seen. Nevertheless, 88% of respondents favored greater standardization of training and the majority supported an approach to standardization that involves collaboration between the ACGME/fellowship directors and pain medicine societies. The Zwisch scale has been used as an assessment of intraoperative faculty guidance and resident autonomy in general surgery residency.19 It is a graduated approach that includes four categories – show and tell, active help, passive help, and supervision only. Pittelkow et al conducted a pilot study evaluating neuromodulation surgical skills in pain medicine fellows where a predetermined set of criteria were established, and faculty were instructed to use the Zwisch scale as a guide to evaluate fellow surgical performance during a neuromodulation case. Both faculty and fellows reported improved satisfaction, consistency and efficiency with feedback provided.20 Another method of perioperative instruction that can be considered is the briefing, intraoperative teaching, debriefing (BID model). A short interaction takes place pre-operatively to assess the trainee knowledge prior to starting a case and setting objectives, intraoperative teaching with direct feedback and a debriefing at the end of the case.21,22
To complement ACGME training, fellows often gain additional exposure through medical societies and industry sponsored training workshops offering didactic and hands-on cadaver training. The North American Neuromodulation Society (NANS) has recently published an educational curriculum for IDDS implantation and management that is available online to fellows at a discounted membership fee.23 The American Society of Pain Medicine and Regional Anesthesia (ASRA) also offers supplemental materials on a range of topics including perioperative management, medication selection, billing guidance, and troubleshooting. Supplemental training approaches like these are readily accessible to fellows and offer a potential means of augmenting existing educational deficiencies.
A weakness of this study is that the results of the survey are not widely generalizable because non-responders could not be characterized. Our findings are limited by the low response rate and the lack of demographic data on the respondents. This raises concerns about the representativeness of the sample and the generalizability of the findings to the broader population of pain medicine fellows. Although trainees were instructed not to complete duplicate surveys, the common-link, anonymous nature with which the survey was conducted cannot wholly exclude the potential for redundant and misinterpreted responses. It should be noted that the survey was conducted from January to February of 2022. Given the July–August start dates typical of fellowships, this would put trainees at approximately the midpoint of their training at the time the surveys were completed. Any educational opportunities that occurred in the latter half of the fellowship training period were therefore not captured here. Additionally, there is no way to know if the respondents were skewed towards certain program with higher case volumes or conscientious fellowship directors who encouraged participation. Finally, this survey was distributed immediately post the COVID19 pandemic and may reflect programs that were still recovering from a slump in cases. Nevertheless, the results suggest high variability in IDDS training and exposure among fellowship-level trainees within the first six months of training. These limitations affect the generalizability of the conclusions and suggest that further research is needed to confirm the findings and develop recommendations for improving IDDS training during pain medicine fellowships.
Conclusion
This survey-based study provides a glimpse into the landscape surrounding pain medicine fellows’ exposure and experience with targeted drug delivery via IDDS. A wide variability exists for IDDS training amongst graduating pain medicine fellows. The results from this study call for a more standardized approach with an emphasis on adequate clinical exposure, utilization of peer reviewed educational curriculum and supplemental material to aid pain medicine fellows’ exposure and training.
Acknowledgments
The authors thank the anonymous referees for their useful suggestions on our article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Dr Mahmoud reports personal fees from Boston Scientific. Dr Aman reports personal fees from Abbott, Medtronic, Vertos, SPR Therapeutics and Bioventus. Dr Erika Petersen reports personal fees from Abbott Neuromodulation, Biotronik, Medtronic Neuromodulation, Nalu, Neuros Medical, Nevro, Saluda Medical, MainStay, SPR, Presidio Medical, Versos and Nalu; board of directors and stock options from SynerFuse; stock options from neuro.42. The authors declare no other conflicts of interest in this work.
References
1. Jain S, Malinowski M, Chopra P, Varshney V, Deer TR. Intrathecal drug delivery for pain management: recent advances and future developments. Expert Opin Drug Deliv. 2019;16(8):815–822. doi:10.1080/17425247.2019.1642870
2. Smith TJ, Coyne PJ, Staats PS, et al. An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM). Ann Oncol. 2005;16(5):825–833. doi:10.1093/annonc/mdi156
3. Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20(19):4040–4049. doi:10.1200/JCO.2002.02.118
4. Stearns LJ, Narang S, Albright RE, et al. Assessment of health care utilization and cost of targeted drug delivery and conventional medical management vs conventional medical management alone for patients with cancer-related pain. JAMA Netw Open. 2019;2(4):e191549. doi:10.1001/jamanetworkopen.2019.1549
5. Stearns LM, Abd-Elsayed A, Perruchoud C, et al. Intrathecal drug delivery systems for cancer pain: an analysis of a Prospective, Multicenter Product Surveillance Registry. Anesth Analg. 2020;130(2):289–297. doi:10.1213/ANE.0000000000004425
6. Sindt JE, Odell DW, Dalley AP, Brogan SE. Initiation of intrathecal drug delivery dramatically reduces systemic opioid use in patients with advanced cancer. Neuromodulation. 2020;23(7):978–983. doi:10.1111/ner.13175
7. Sindt JE, Odell DW, Tariq R, Presson AP, Zhang C, Brogan SE. Initial intrathecal dose titration and predictors of early dose escalation in patients with cancer using a 100:1 oral to intrathecal morphine conversion ratio. Neuromodulation. 2021;24(7):1157–1166. doi:10.1111/ner.13517
8. Aman MM, Mahmoud A, Deer T, et al. The American Society of Pain and Neuroscience (ASPN) best practices and guidelines for the interventional management of cancer-associated pain. J Pain Res. 2021;14:2139–2164. doi:10.2147/JPR.S315585
9. Rizvi S, Kumar K. History and present state of targeted intrathecal drug delivery. Curr Pain Headache Rep. 2015;19(2):474. doi:10.1007/s11916-014-0474-8
10. Perruchoud C, Dupoiron D, Papi B, Calabrese A, Brogan SE. Management of cancer-related pain with intrathecal drug delivery: a systematic review and meta-analysis of clinical studies. Neuromodulation. 2022;26(6):1142–1152. doi:10.1016/j.neurom.2021.12.004
11. Rauck RL, Wallace MS, Leong MS, et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31(5):393–406. doi:10.1016/j.jpainsymman.2005.10.003
12. Abd-Elsayed A, Karri J, Michael A, et al. Intrathecal drug delivery for chronic pain syndromes: a review of considerations in practice management. Pain Physician. 2020;23(6):E591.
13. Prager J, Deer T, Levy R, et al. Best practices for intrathecal drug delivery for pain. Neuromodulation. 2014;17(4):354–372; discussion 372. doi:10.1111/ner.12146
14. Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20(2):96–132. doi:10.1111/ner.12538
15. Epstein RH, Dexter F, Pearson ACS. Pain medicine board certification status among physicians performing interventional pain procedures in the state of Florida between 2010 and 2016. Pain Physician. 2020;23(1):E7.
16. Pak DJ, Gruber J, Deer T, et al. Spinal cord stimulator education during pain fellowship: unmet training needs and factors that impact future practice. Reg Anesth Pain Med. 2019;44(3):407–414. doi:10.1136/rapm-2018-100065
17. Public ACfGMEA. Number of accredited programs; 2022. Available from: https://apps.acgme.org/ads/Public/Reports/Report/3.
18. Ambardekar AP, Furukawa L, Eriksen W, McNaull PP, Greeley WJ, Lockman JL. A consensus-driven revision of the accreditation council for graduate medical education case log system: pediatric anesthesiology fellowship education. Anesth Analg. 2022;136(3):446–454.
19. George BC, Teitelbaum EN, Meyerson SL, et al. Reliability, validity, and feasibility of the Zwisch scale for the assessment of intraoperative performance. J Surg Educ. 2014;71(6):e90–e96. doi:10.1016/j.jsurg.2014.06.018
20. Pittelkow TP, Hagedorn JM, Bendel MA, et al. Pain medicine fellow neuromodulation surgical skill assessment tool: a pilot. Reg Anesth Pain Med. 2019. doi:10.1136/rapm-2019-100761
21. Madeline B, Torres PMQ. Assessing resident autonomy: what tools are available? Bull Am Coll Surg. 2018;103(8):46–52.
22. George BC. The language of progressive autonomy: using the Zwisch Scale for more than just assessment; 2017.
23. Chaiban G, Abdallah RT, Abd-Elsayed A, et al. North American Neuromodulation Society Educational Curriculum for intrathecal drug delivery systems implantation and management. Neuromodulation. 2022;26(6):1208–1217. doi:10.1016/j.neurom.2021.11.012
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
