Back to Journals » Clinical Epidemiology » Volume 10
Early treatment with talk therapy or antidepressants in severely bereaved people and risk of suicidal behavior and psychiatric illness: an instrumental variable analysis
Authors Fenger-Grøn M, Kjaersgaard MIS, Parner ET , Guldin MB, Vedsted P , Vestergaard M
Received 24 November 2017
Accepted for publication 13 May 2018
Published 24 August 2018 Volume 2018:10 Pages 1013—1026
DOI https://doi.org/10.2147/CLEP.S157996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Vera Ehrenstein
Morten Fenger-Grøn,1,* Maiken Ina Siegismund Kjaersgaard,1,2,* Erik Thorlund Parner,2 Mai-Britt Guldin,1 Peter Vedsted,1 Mogens Vestergaard1
1Research Unit for General Practice and Section for General Medical Practice, Department of Public Health, Aarhus University, Aarhus, Denmark; 2Section for Biostatistics, Department of Public Health, Aarhus University, Aarhus, Denmark
*These authors contributed equally to the work
Purpose: Losing a loved one to death is a common and natural life-course experience. Still, bereavement has been associated with an increased risk of suicidal behavior and psychiatric hospitalization and little is known of how to counter these adverse events. We aimed to study the effect of early treatment in primary care with talk therapy (TT) or antidepressants (AD) in severely bereaved people.
Methods: We conducted a population-based cohort study including 207,435 adult Danes who experienced a severe loss in 1996–2013. We compared treatment and no treatment with either of the two treatment regimens within 6 months after the loss. The main outcome was a serious mental health condition (defined as suicide, deliberate self-harm, or psychiatric hospitalization) occurring >6 months after bereavement. Adjusted risk differences (RDs) 2 years after bereavement were calculated using both standard regression analysis and instrumental variable analysis (IVA) in which estimated physician preferences for treatment served as instruments.
Results: The standard adjusted regression analysis showed a higher risk of developing a serious mental health condition associated with both TT (RD, 7.1; 95% CI, 5.0 to 9.1 per 1000 people) and AD (RD, 30.1; 95% CI, 25.7 to 34.6 per 1000 people). The IVA, which was used to control for unmeasured confounding, showed that TT was associated with a lower risk of a serious mental health condition (RD, -17.1; 95% CI, -30.7 to -3.5 per 1000 people), whereas the results were inconclusive for AD (RD, -8.6; 95% CI, -62.6 to 45.4 per 1000 people).
Conclusion: This study suggests that early treatment with TT is associated with reduced long-term risk of serious mental health conditions in severely bereaved people. No clear benefit or harm of treatment with AD after bereavement was ascertained since the statistical precision was low.
Keywords: bereavement, loss, suicide, self-harm, practice variation
Plain language summary
Why was the study done? Losing a loved one to death is a common and often very emotionally painful experience. Bereaved people have an increased risk of suicidal behavior and psychiatric illness, and little is known about how to reduce this risk.
What did the researchers do and find? We studied the effect of early treatment in primary care with talk therapy (TT) or antidepressants (AD) on suicidal behavior and psychiatric hospitalization in bereaved people. A conventional regression analysis showed that both treatments were associated with a higher risk of serious mental health conditions. In contrast, a more advanced and partly novel analysis approach, which used physician prescription preferences as statistical instruments, showed that TT was associated with a lower risk of developing a serious mental health condition after the loss; no clear conclusion could be given for the effect of AD.
What do these results mean? This study suggests that early treatment with TT might reduce the long-term risk of developing a serious mental health condition after bereavement. Furthermore, the findings suggest that the performed analysis of physician treatment preferences might provide an informative supplement to more conventional approaches.
Introduction
Losing a loved one to death is a natural life-course experience, which can be very emotionally painful. For most people, reactions or symptoms will lessen with time without any professional intervention. However, some experience complicated grief (CG)1 with prolonged emotional suffering and difficulties in adjusting to the loss for a period of >6 months and bereavement has been associated with an increased risk of suicidal behavior and psychiatric hospitalization.2–10
Existing evidence provides little advice on how to reduce these adverse consequences of bereavement. Few studies have explored the effectiveness of pharmacotherapy on CG, and the results are ambiguous.11–13 Psychotherapy does not appear to prevent CG, but it may have a short-term effect on CG symptoms and suicidal ideation in people who already meet the criteria for CG.11,14 However, no similar evaluation of whether suicidal behavior and psychiatric illness in bereaved people can be prevented by treatment within the first 6 months after their loss, where they do not (by definition) fulfill the CG criteria, exists. Treatment within this period could prove important since bereaved people are at particularly high risk of suicidal behavior and psychiatric illness within the first 6–12 months after the loss.3
Indeed, studying the effect of early treatment on suicidal behavior and psychiatric illness among bereaved people also poses several challenges. Randomized controlled trials with sufficient power to study rare outcomes such as completed and attempted suicide are difficult to establish, and traditional observational studies are prone to bias, particularly from confounding by indication, ie, people receiving treatment are likely to have the most severe grief symptoms and hence probably also the greatest risk of adverse events.
Therefore, we performed a large population-based cohort study using an instrumental variable approach to estimate the influence of early treatment with TT or AD on the 2-year risk of suicide, deliberate self-harm, and psychiatric hospitalization in people who experienced a severe loss. As instruments, we applied primary care physicians’ preferences for treatment with AD and/or TT, which were estimated from adjusted treatment frequencies in the patients’ primary care populations. This approach yields results that are unaffected by confounding by indication since it does not attempt to assess the consequences of the actual treatment received by the patients but rather the consequences of their physician’s general intention to treat, which is not linked to the individual patient’s mental health status. As a reference of methodological interest, we estimated similar effects of AD and TT using the standard regression analysis.
Methods
Study design and setting
We conducted a population-based cohort study of 207,435 relatives who experienced a severe loss of a close relative between 1996 and 2012 by using Danish nationwide registers. The data were linked at the individual level by using the unique Civil Personal Registration (CPR) number assigned to all Danish citizens. Denmark has a tax-financed public health care system, and primary health care is provided by primary care physicians, who act as gatekeepers to specialized health care.15 Citizens are listed with a specific primary care practice, which they must first consult for medical advice.
Data sources
The following Danish administrative data sources were used in the study: 1) the Civil Registration System, which contains information on vital status and family relations,16 2) the Register of Causes of Death, which contains information on cause of death,17 3) the National Patient Register, which contains information on all somatic hospitalizations since 1977 and outpatient and emergency department contacts since 1995,18 4) the Psychiatric Central Research Register, which contains information on all psychiatric hospitalizations since 1969 and outpatient and emergency department contacts since 1995,19 5) the National Health Service Register, which contains information on all services provided by primary care physicians and psychologists since 1990,20 and 6) the National Prescription Register, which contains information on all redeemed prescriptions since 1995.21 The Danish registers cover the entire population with no loss to follow-up, and their validity is generally considered good.22
The study was approved by the Danish Data Protection Agency and the Danish Health Data Authority. According to Danish legislation, no further ethical approval or collection of informed consent is required for research projects entirely based on public administrative registers.
Cohort
We identified all Danes aged 18 years or older who experienced a severe loss of a child, spouse, registered partner, parent, or sibling in the study period. In agreement with relevant literature,2–10 we defined severe loss as any event in the hierarchy: 1) loss of a child, 2) loss of a spouse or registered partner younger than 50 years, 3) loss due to suicide, 4) multiple losses within 30 days, 5) loss due to homicide, 6) loss due to an accident, and 7) unexpected loss defined by a score of 0 or 1 in the age-adjusted Charlson Comorbidity Index.23 People who had experienced more than one of these types of losses or losses that fell into more than one of the categories were assigned to the category of their highest-listed event in this hierarchy, and they were included on the day of their first event of this category. Cohort composition according to the type of loss is found in Table 1.
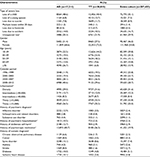  | Table 1 Baseline characteristics of full cohort and treatment groups Notes: aAge-adjusted Charlson Comorbidity Index23 score of 0 or 1. bCategories according to density and largest city coded as in Table S1B. cFive-year history of diagnosis coded as in Table S1C. dFive-year history of psychiatric inpatient hospitalization coded as in Table S1B. eFive-year history of deliberate self-harm coded as in Table S1B. fOne-year history of redeemed psychotropic medication coded as in Table S1C. gHistory of diagnosis any time before bereavement coded as in Table S1C. Abbreviations: AD, antidepressants; TT, talk therapy. |
Treatments
We categorized people as treated early with TT if a primary care physician or psychologist provided one or more TT services within 6 months of the loss. We defined people as treated early with AD if one or more prescriptions for AD had been redeemed from a pharmacy within 6 months after the loss; prescriptions for tricyclic AD, trazodone, and bupropion were excluded because of their frequent use for pain, insomnia, or smoking cessation. The two treatments were studied under mutual adjustment. The reference groups consisted of those who did not receive the respective treatments in the first 6 months. Coding definitions for treatments are found in Table S1A.
Outcome
A serious mental health condition was defined as the composite endpoint comprising suicide, deliberate self-harm, and psychiatric hospitalization. To identify deliberate self-harm in the Danish registers, we used a previously applied algorithm.24 The outcome coding definition is found in Table S1B.
Covariates
In accordance with previous literature in the field,3,25 the following covariates were a priori chosen for adjustment: gender, age (18–39, 40–49, 50–59, 60–69, and ≥70 years), calendar period (96–99, 00–03, 04–07, and 08–12), urbanization (densely, intermediate with ≥40,000, intermediate with <40,000, thinly with ≥15,000, and thinly with <15,000), and type of loss as well as dichotomous variables coded for information on 5-year history of psychiatric inpatient hospitalization, deliberate self-harm, and selected mental conditions (affective disorder, schizophrenia and related disorders, and substance abuse disorder), 1-year history of psychotropic medication redemption, and any history of selected physical conditions (chronic obstructive pulmonary disease, cancer, spine disorder, asthma, stroke, diabetes, and ischemic heart disease). Diagnostic criteria and classifications of medication and urbanization are included in Table S1C. Analyses were also adjusted for interactions between gender and age and between history of psychotropic medication redemption and selected mental conditions.
Statistical analyses
We assessed adjusted risk differences (RDs) for serious mental health conditions at 2 years after the loss using the pseudo-observation approach.26 Thus, pseudo-observations from the cumulative incidence function for the competing risks’ model with death from other causes than suicide as competing risk were computed and analyzed as outcome variable. Time since the loss was used as the underlying time scale, and individuals entered the study 6 months after the loss. People were censored by emigration or end of study on December 31, 2013, whichever came first. We excluded 1880 (0.9% of 216,759) people who died or were censored during the first 6 months after the loss. Adjustment was performed both using a standard generalized linear model (GLM) regression analysis with robust variance estimation and the more technical method of instrumental variable analysis (IVA).27
In the IVA approach, patients are not directly compared with respect to the actual treatments received as this might be heavily confounded by indication or severity in a way that cannot be accounted for by standard analytical approaches, rather the IVA compares groups of patients who differ in their likelihood of receiving the treatments. Therefore, at its best, this method holds the potential of reducing the impact from unmeasured confounding similar to randomization.28 The IVA approach requires the existence of variables (instruments), which can predict the receipt of AD or TT but have no direct effect on patient outcome and no indirect effect other than through the treatments. As our instruments, we applied measures of physician treatment preference (defined below). Consequently, the IVA estimates a treatment effect on a “marginal” population that is defined as people whose likelihood of receiving treatment depends on their physician’s preference for the treatment.29
For both AD and TT, we defined the corresponding instruments as how much more or less than expected the treatments were provided in the bereaved person’s general practice averagely in the 3 months before the date of loss. Thus, for each practice for each month, we calculated the ratios between the observed number of patients treated with AD and TT, respectively, and the corresponding expected numbers as predicted from a Poisson model including gender, age, calendar time, and mental and physical morbidity of the entire Danish population. This definition ensured that the instruments were not associated with unmeasured cohort characteristics through practice composition. We linked patients and practices through the application of a validated algorithm (98.6% overall agreement with the gold standard).30 To assess IVA assumptions, we reported baseline characteristics according to quintiles of instruments and estimated correlations between the treatments and the corresponding instrument. Details on estimation of the instrument and considerations concerning requirements for the validity of the application on pseudo-values are found in the Supplementary materials.
Both the standard GLM regression analysis and the IVA were adjusted for the measured potential confounders that are mentioned in the “Covariates” section. In both approaches, we excluded 7444 (3.4% of 216,759) people who could not be allocated to a practice at the time of the loss.
In a sub-analysis, we restricted the cohort to people who received no treatment with AD or TT in the 3 months before the loss. In sensitivity analyses, the treatment window of 6 months was extended to 9 months and the people treated with both AD and TT were pooled with either the AD only group or the TT only group. All analyses were conducted with Stata 14 (StataCorp LP, College Station, TX, USA).
Results
Among 207,435 bereaved adults, 17,211 (8%) adults were treated with AD and 30,818 (15%) adults were treated with TT during the first 6 months after the loss; 5298 (3%) of them received both treatments. Of the 30,818 people treated with TT, 13,754 (45%) people received therapy exclusively from a psychologist, 12,137 (39%) people received therapy exclusively from a primary care physician, and 4927 (16%) people received therapy from both a primary care physician and a psychologist. Among people treated with AD in the 6 months after the loss, 7091 (41%) people received no AD treatment in the 3 months before. People receiving these treatments were more often women and more often had a history of mental illness compared to the entire cohort. Additionally, people treated with AD were generally older, whereas people treated with TT were younger than the remaining cohort (Table 1). People treated with AD more often suffered from both mental ill-health and physical ill-health compared to both the entire cohort and people receiving TT. A flow diagram of cohort selection is found in Figure 1.
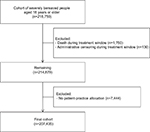  | Figure 1 Flow diagram of cohort selection. |
The baseline characteristics of the study population were reasonably balanced between the quintiles of the instruments (ie, when people were categorized into five groups according to how often their practice provided treatment during the 3 months before the loss while taking the composition of the patient population into account) and the likelihood of treatment increased with increasing quintiles (Table 2). This may support the assumption of validity of our instruments. However, the correlation between the treatment and the corresponding instrument was markedly lower for AD than for TT (Table S2).
  | Table 2 Baseline characteristics of cohort across quintiles of instruments Notes: aUpper range is the mean of the five largest values (to prevent the identification of personal information). bFive-year history of diagnosis coded as in Table S1C. cFive-year history of psychiatric inpatient hospitalization coded as in Table S1B. dFive-year history of deliberate self-harm coded as in Table S1B. eOne-year history of redeemed psychotropic medication coded as in Table S1C. Data presented as percentages unless otherwise stated. Abbreviations: AD, antidepressants; TT, talk therapy. |
The crude risk of serious mental health conditions between 6 months and 2 years was 31.9 (95% CI, 29.9 to 33.9) per 1000 people treated with TT and 91.2 (95% CI, 86.9 to 95.5) per 1000 people treated with AD (Table 3), whereas the corresponding figure was 15.7 (95% CI, 15.1 to 16.3) among people receiving neither AD nor TT. Deliberate self-harm events accounted for the majority of events, whereas completed suicides were rare.
  | Table 3 Crude risk of serious mental health conditions from 6 months to 2 years after bereavement in the entire cohort and treatment groups Notes: aRisk per 1000 people as estimated by the Nelson–Aalen approach accounting for censuring due to emigration or end of follow-up and for competing the risk of death from other causes than suicide. Only the first outcome in the period was considered, and for the three components, the risk was estimated from their proportional contribution to the composite outcome. bComposite outcome and the three underlying components defined as outlined in Table S1B. Abbreviations: AD, antidepressants; TT, talk therapy. |
In the standard GLM regression analysis, the adjusted risk was 30.1 (95% CI, 25.7 to 34.6) per 1000 people higher among bereaved people treated with AD and 7.1 (95% CI, 5.0 to 9.1) per 1000 people higher among bereaved people treated with TT compared with people who did not receive the respective treatments (Table 4).
Contrarily, in the IVA, the estimated adjusted risk of developing a serious mental health condition between 6 months and 2 years after the loss was lower in bereaved people treated with TT (RD, -17.1; 95% CI, -30.7 to -3.5 per 1000 people) or AD (RD, -8.6; 95% CI, -62.6 to 45.4 per 1000 people), but the statistical precision on the latter estimate was very low (Table 4). When we restricted the cohort to people not treated with AD or TT during the 3 months before the loss, the association for TT tended to be stronger (RD, -22.3; 95% CI, -36.5 to -8.2 per 1000 people). In a sensitivity analysis, we excluded people with extreme instrument values (n=923), but this did not change the IVA estimates essentially (Table S3).
The sensitivity analyses showed that the estimates did not change substantially if we pooled people treated with both AD and TT with either the AD only group or the TT only group, or if we extended the treatment window to 9 months (Tables S4–S6).
Discussion
Main findings
This large population-based cohort study suggests that people exposed to a severe loss had significantly lower risk of developing serious mental health conditions if they were treated with TT during the first 6 months after the loss. A slightly stronger association was observed when we restricted our analysis to people who had received no recent treatment. No relevant benefit or harm of AD treatment was revealed since, for this treatment, the statistical precision was low.
Comparison with existing studies
To our knowledge, our study is the first to examine the association between early treatment with TT and AD and development of serious mental health conditions in severely bereaved people. However, a meta-analysis of randomized controlled trials on CG suggested short-term benefits from a range of individual or group-based psychotherapeutic interventions.14 In a recent randomized controlled trial on the treatment of CG, Shear et al11 found a reduction in CG symptoms and self-reported suicidal ideation after 20 weeks of “complicated grief therapy”, both alone and when added to citalopram treatment. Furthermore, the authors found that citalopram treatment reduced the depressive symptoms when added to the therapy, whereas citalopram alone had no effect on CG symptoms and suicidal ideation. Previous nonrandomized studies have indicated that AD can be helpful in reducing grief symptoms among patients with CG or bereavement-related depression.12,13
Strengths and limitations
We used a population-based cohort of severely bereaved people for whom bereavement was reliably recorded in Danish registers. Loss to follow-up was virtually nonexistent in these data, which made selection bias unlikely. However, to study the most severe losses, we selected our cohort by including the first loss in the highest category of our hierarchy within the study period. This involved an element of conditioning on the future, which is unwarranted from a theoretical mathematical perspective, but this has limited practical influence.
Primary health care in Denmark is based on a principle of free and equal access for all. Patients receive public subsidies to cover part of the costs of prescription drugs and, when referred by their primary physician, of consultations with privately practicing psychologists. However, some of the people who were categorized as not treated with TT in our study might have received TT elsewhere as we had no information on bereavement care provided by privately paid psychologists, in palliative care, or by various free support services (including grief support groups or pastoral care). Furthermore, we did not include treatment provided by privately practicing psychiatrists, but only few people received such treatment within 6 months after the loss.
Suicides may be underreported as some may be erroneously categorized as other causes of death,31 and deliberate self-harm that did not lead to hospitalization cannot be identified in our data. Conversely, earlier studies applying the same algorithm for the identification of deliberate self-harm conclude that some over-reporting may be taking place as well.32 Both types of misclassification will cause an underestimation of the studied associations if it is not associated with the exposures, but over-reporting may be most frequent in people receiving treatment for mental health conditions, and this could entail over-estimation of the risk associated with treatment. However, it does not seem plausible that misclassification would bias the standard regression results upward and the IVA results downward.
Two categories of confounding seem important to consider in our study. The first is confounding by indication, which may arise because people with the most severe grief reactions are more likely to be identified and treated. The second is residual confounding, which may arise because we had no information on sociodemographic factors besides urbanization and because our data on comorbid conditions did not hold information on the severity of the conditions.
In our standard regression analysis, both TT and AD were deemed harmful even after extensive adjustments. However, as no detailed information was available on the study participants’ coping with their loss, these findings are likely to suffer from confounding by indication.
IVA has the potential to control for such unmeasured confounding, but at least three important prerequisites must be fulfilled.
First, the chosen instruments must be associated with the exposures of interest. In our data, this condition was satisfied. However, the observed associations were weak after covariate adjustment, particularly for AD treatment. This is most likely because the practice variation for the prescription of ADs was small, with only limited capability to predict whether AD treatment was used in people with severe loss.
Second, the instrument must not be associated with any unmeasured confounders. For logical reasons, this requirement cannot be directly verified in the data and we had no good proxy for severity of the grief reaction, which could have been used to justify an assumption of independence between the overall practice treatment patterns and the mental health among the people under study. Yet, it appears plausible that experiences related to the studied types of loss may cause serious mental reactions in the bereaved, regardless of their practice population profile. Additionally, it is reassuring that the measured confounders were rather balanced between the quintiles of the instruments (Table 2), although some differences still had to be compensated by adjustments in the final analysis model.
Third, the association between the instrument and the outcome must not be mediated by causal pathways that do not involve the studied exposures. In our study, this assumption must be carefully considered as we cannot rule out that the practices in which patients have a high probability of receiving TT also do other things of benefit to the patients than offering registered TT sessions. Still, our analyses were corrected for the concomitant prescription of ADs.
Summing up, we tend to consider our findings from the IVA approach more reliable than the results from the standard regression analyses. Hence, we believe that a high probability of receiving TT in primary care in the first 6 months after a severe loss is associated with a lower risk of suicide, deliberate self-harm, and psychiatric hospitalization. However, while a TT session in the present study constitutes a well-defined entity from an administrative point of view, it is not well specified in terms of content, duration, theoretical framework, and so on. Although the observed association suggests that physicians with a high preference for TT treatment do something right, our findings provide no precise information on what this is. It cannot be ruled out that physicians’ use of TT is merely a marker of other more decisive aspects of their daily practice, for instance their general focus on a biopsychosocial approach or the density of their time schedules.
Conclusion
This population-based cohort study suggests that severely bereaved people benefit from TT early after bereavement. However, requirements for the therapy to have effect cannot be specified and the suggested benefit may even be driven by other characteristics of the general practices that frequently offer TT. Standard regression approaches to estimate treatment effects in observational studies may be seriously impaired by confounding by indication, which could lead to erroneous clinical decisions. Analyses with physician treatment preferences as instrumental variables might provide useful supplementary information.
Acknowledgments
The authors would like to thank Lone Niedziella for language editing and Claus Høstrup Vestergaard for technical support, both at the Research Unit for General Practice at Aarhus University. This study was supported by an unrestricted grant (R155-2012-11280) from the Lundbeck Foundation (MISK, MF-G, and MV), a grant from the Novo Nordisk Foundation and the Danish Cancer Society (MISK and PV), a grant from the Danish foundation TrygFonden (M-BG), the Program for Clinical Research Infrastructure, which was established by the Lundbeck Foundation and the Novo Nordisk Foundation and administered by Danish Regions (MISK and MV), and the Danish Research Foundation for General Practice (MISK). The funders had no role in the design and conduct of the study; acquisition, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153–160. | ||
Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370(9603):1960–1973. | ||
Guldin MB, Kjaersgaard MIS, Fenger-Grøn M, et al. Risk of suicide, deliberate self-harm and psychiatric illness after loss of a close relative: a nationwide cohort study. World Psychiatry. 2017;16(2):193–199. | ||
Christakis NA, Allison PD. Mortality after the hospitalization of a spouse. N Engl J Med. 2006;354(7):719–730. | ||
Elwert F, Christakis NA. The effect of widowhood on mortality by the causes of death of both spouses. Am J Public Health. 2008;98(11):2092–2098. | ||
Li J, Precht DH, Mortensen PB, Olsen J. Mortality in parents after death of a child in Denmark: a nationwide follow-up study. Lancet. 2003;361(9355):363–367. | ||
Li J, Laursen TM, Precht DH, Olsen J, Mortensen PB. Hospitalization for mental illness among parents after the death of a child. N Engl J Med. 2005;352(12):1190–1196. | ||
Qin P, Agerbo E, Mortensen PB. Suicide risk in relation to family history of completed suicide and psychiatric disorders: a nested case-control study based on longitudinal registers. Lancet. 2002;360(9340):1126–1130. | ||
Qin P, Mortensen PB. The impact of parental status on the risk of completed suicide. Arch Gen Psychiatry. 2003;60(8):797–802. | ||
Agerbo E. Midlife suicide risk, partner’s psychiatric illness, spouse and child bereavement by suicide or other modes of death: a gender specific study. J Epidemiol Comm Health. 2005;59(5):407–412. | ||
Shear MK, Reynolds CF III, Simon NM, et al. Optimizing treatment of complicated grief: a randomized clinical trial. JAMA Psychiatry. 2016;73(7):685–694. | ||
Zisook S, Shuchter SR, Pedrelli P, Sable J, Deauciuc SC. Bupropion sustained release for bereavement: results of an open trial. J Clin Psychiatry. 2001;62(4):227–230. | ||
Simon NM. Treating complicated grief. JAMA. 2013;310(4):416–423. | ||
Wittouck C, Van Autreve S, De Jaegere E, Portzky G, van Heeringen K. The prevention and treatment of complicated grief: a meta-analysis. Clin Psychol Rev. 2011;31(1):69–78. | ||
Pedersen KM, Andersen JS, Søndergaard J. General practice and primary health care in Denmark. J Am Board Fam Med. 2012;25(suppl 1):S34–S38. | ||
Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 suppl):22–25. | ||
Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39(7 suppl):26–29. | ||
Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. | ||
Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39(7 suppl):54–57. | ||
Andersen JS, Olivarius Nde F, Krasnik A. The Danish national health service register. Scand J Public Health. 2011;39(7 suppl):34–37. | ||
Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7 suppl):38–41. | ||
Erlangsen A, Fedyszyn I. Danish nationwide registers for public health and health-related research. Scan J Public Health. 2015;43(4):333–339. | ||
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. | ||
Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry. 2011;68(10):1058–1064. | ||
Crump C, Sundquist K, Sundquist J, Winkleby MA. Sociodemographic, psychiatric and somatic risk factors for suicide: a Swedish national cohort study. Psychol Med. 2014;44(2):279–289. | ||
Klein JP, Andersen PK. Regression modeling of competing risks data based on pseudovalues of the cumulative incidence function. Biometrics. 2005;61(1):223–229. | ||
Kjaersgaard MIS, Parner ET. Instrumental variable method for time-to-event data using a pseudo-observation approach. Biometrics. 2016;72(2):463–472. | ||
Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33(13):2297–2340. | ||
Harris KM, Remler DK. Who is the marginal patient? Understanding instrumental variables estimates of treatment effects. Health Serv Res. 1998;33(5 pt 1):1337–1360. | ||
Kjaersgaard MIS, Vedsted P, Parner ET, et al. Algorithm linking patients and general practices in Denmark using the Danish national health service register. Clin Epidemiol. 2016;8:273–283. | ||
Kapusta ND, Tran US, Rockett IRH, et al. Declining autopsy rates and suicide misclassification: a cross-national analysis of 35 countries. Arch Gen Psychiatry. 2011;68(10):1050–1057. | ||
Schuerch M, Gasse C, Robinson NJ, et al. Impact of varying outcomes and definitions of suicidality on the associations of antiepileptic drugs and suicidality: comparisons from UK Clinical Practice Research Datalink (CPRD) and Danish national registries (DNR). Pharmacoepidemiol Drug Saf. 2016;25(suppl 1):142–155. |
Supplementary materials
Details on the definition of the instruments and evaluation of the analysis assumptions
For each practice for each month, we calculated the observed numbers of patients treated with antidepressants (AD) and talk therapy (TT) and the corresponding expected numbers as predicted from a Poisson model of the entire patient Danish population. This model allowed for any history of the 19 Charlson comorbidities;1 five-year history of affective disorder, schizophrenia and related disorders, and substance abuse disorder; and interactions between gender and restricted cubic splines for age with knots at 40, 50, 60, and 70 years as well as for calendar period with knots at year 2000, 2004, and 2008.2 For each person in our cohort, we defined the instruments for AD and TT as the practice-specific ratios of observed over predicted number of treated patients in the 3 months (lag period) before the loss.
With two treatments and two instruments, it is important that the IV model is not essentially under-identified due to correlation between the instruments. A scatterplot of the instruments confirmed that the instruments were practically uncorrelated. Additionally, when adjusted for the number of instruments and their intercorrelation, the partial correlations between the instruments and the corresponding treatments declined only slightly. However, no clear rule exists on how to evaluate instrument strength in studies with several treatments and instruments. We investigated several instruments over different lag time periods. We found that a more instantaneous preference of 3 months performed better with regard to correlation with the actual treatment and standard error of the risk difference than a preference definition of 6 or 12 months, which is in line with the findings from other IV studies using physician preference.3,4
The pseudo-observation approach to IVA used in the present study further assumes strongly independent censoring.5 We found that censoring was related to patient characteristics, which we could take into account by estimating stratified pseudo-observations.6 The results were largely unchanged, no matter which combination of confounders we stratified on.
  | Table S1 (A) Coding definition for treatments, (B) coding definition for composite outcome, and (C) coding definition for covariates Notes: aService codes for TT provided by primary care physicians have been identified by the Program for Clinical Research Infrastructure.7 bIdentification of deliberate self-harm follows the algorithm of Nordentoft et al.8 cClassification follows McGrath et al.9 Abbreviations: AD, antidepressants; ATC, Anatomical Therapeutic Chemical; CRS, Civil Registration System; ICD-8, International Classification of Diseases, 8th revision; ICD-10, International Classification of Diseases, 10th revision; NHSR, National Health Service Register; NPR, National Patient Register; PCRR, Psychiatric Central Research Register; RCD, Register of Causes of Death; RMPS, Register of Medicinal Product Statistics; TT, talk therapy. |
References
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. | ||
Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. New York: Springer; 2015. | ||
Hennessy S, Leonard CE, Palumbo CM, et al. Instantaneous preference was a stronger instrumental variable than 3- and 6-months prescribing preference for NSAIDs. J Clin Epidemiol. 2008;61(12):1285–1288. | ||
Ionescu-Ittu R, Abrahamowicz M, Pilote L. Treatment effect estimates varied depending on the definition of the provider prescribing preference-based instrumental variables. J Clin Epidemiol. 2012;65(2):155–162. | ||
Kjaersgaard MIS, Parner ET. Instrumental variable method for time-to-event data using a pseudo-observation approach. Biometrics. 2016;72(2):463–472. | ||
Andersen PK, Perme MP. Pseudo-observations in survival analysis. Stat Methods Med Res. 2010;19(1):71–99. | ||
Program for Clinical Research Infrastructure (PROCRIN). Samtaleterapi [Talk Therapy]. Available from: http://cap.au.dk/fileadmin/cap.au.dk/Documents/Samtaleterapi_v20160209.pdf. Accessed January 29, 2017. Danish. | ||
Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry. 2011;68(10):1058–1064. | ||
McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry. 2014;71(3):301–309. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






